Abstract
With age, there is a progressive loss of body balance function. Yet, the potential influence of osteoporosis on body balance is largely unknown. Dentin matrix protein 1 (DMP1) is highly expressed in bone and required for phosphate homeostasis and mineralization. Dmp1 null mice display striking defects in bone structure. In this study we reported circling behavior and hyper reaction to touching in Dmp1 null mice. Our histology, tartrate resistant acid phosphatase (TRAP) staining and µCT data showed dramatic changes, such as an expansion of poorly mineralized matrices, in the Dmp1 null porous bony structure in the vestibular apparatus. The targeted re-expression of DMP1 in the Dmp1 null bone fully rescued not only the bone phenotype, but also circling behavior and hyper reaction. Furthermore, X-gal stain and DMP1 immunohistochemistry assay showed that DMP1 was not expressed in neuron cells or balance related cells in the inner ear, suggesting that a defect in the bony labyrinth of the internal ear is indirectly responsible for the circling behavior and/or hyper reaction to touching. Finally, discovery of DMP1 lacZ signal in pericyte-like cells may suggest a new function of DMP1 in angiogenesis.
Keywords: Circling, vestibular function, DMP1, Bone, Pericyte.
INTRODUCTION
Body balance is a complex cognitive skill essential for daily life and vulnerable for the aging population. Balance disorder is directly linked to losses of vision, vestibular sense, proprioception, muscle strength and reaction time 1-3. As a result, falls in the senior population occur more often and approximately 10—15% of these falls are associated with serious injuries 1. Currently, most studies on these problems focus on the degenerative losses of neuronal functions.
Osteoporosis is another common disease closely associated with aging 4-6. One in two women and one in four men, age 50 and above, will have fracture experience due to osteoporosis if no intervention is made. Ironically, little attention has been focused on defects in the vestibular function, which could lead to more frequent falls in the senior population.
Body balance and motion are detected in the hair cells in the vestibular apparatus when position or gravity changes. The resulting distortion of the hair cells via fluid wave generates electrical signals, and finally transduces them to brain through nerve impulses. The type of motion or attitude detected by a hair cell is based on its associated mechanical structures, such as the curved tube of a semicircular canal or the calcium carbonate crystals (otolith) of the saccule and utricle located in the vestibule. Morphological changes in the vestibule or semicircular canal may induce balance problems.
An intact vestibular apparatus is essential for rodents, as circling behavior is very common in mice when there is a defect in vestibular function. In contrast, in humans balance dysfunction is an isolated event. The difference between humans and mice is that visual and proprioceptive information cannot be compensated in mice but can be compensated in humans. Therefore, dysfunction problem in human vestibular apparatus is unrecognized except that a vestibular function test is performed 7.
Dentin matrix protein 1 (DMP1) is highly expressed in bone and required for phosphate homeostasis and mineralization 8-9, although DMP1 is also expressed in soft tissues (salivary gland, kidney, lung, brain) or tumors 10-15. Deletion of murine Dmp1 causes striking defects in tooth and bone during postnatal development 16-17. Recently, we and others have demonstrated that DMP1 mutations result in Autosomal Recessive Hypophosphatemic Rickets in humans similar to the phenotype of Dmp1-null mice. This condition is characterized by rickets and the presence of large amounts of osteoid in bone (osteomalacia) and is accompanied by elevated circulating fibroblast growth factor 23 (FGF23) 18-21.
In this study, we attempted to address whether changes of bony surface in the vestibular apparatus could lead to dysfunction of balance and body motion in Dmp1 null mice. Second, we attempted to define expression of DMP1 in the brain. Our studies revealed that Dmp1 null mice developed circling and head shaking behavior, that the immunohistochemistry and X-gal stain assays showed no DMP1 signal in neuron cells, and that there are striking 'dents' on surfaces of Dmp1 null vestibular apparatus. These data seem support a concept that turbulence in fluid waves due to a rough surface is likely responsible for circling and head shaking behavior. This finding may have a potential relevance to osteoporosis patients whose defects in body balance may be caused in part by a change of the bony surface in degenerative vestibular apparatus. Next, the unexpected finding of DMP1-lacZ signal in pericyte-like cells of blood vessels may have a biological significance in angiogenesis.
MATERIALS AND METHODS
Mice
Dmp1-lacZ knock-in null mice where the lacZ reporter gene was inserted to replace Dmp1 gene were described previously 22. The rationale for using this null mouse model were 1) studies of effects of loss of DMP1 on control of body balance; and 2) the heterozygous (HET) mouse could be used for tracing an endogenous Dmp1 gene expression pattern via an x-gal stain assay which is very sensitive and easy to perform. These mice were initially generated in a 129 strain and back-crossed into the C57/B6 background 22. Later, these null mice were also crossed into CD-1 background for 5 generations 16. In this study, Dmp1-lacZ knock-in null mice in both C57/B6 and CD-1 strains were used.
Transgenic mice overexpressing a full-length Dmp1 under the control of a 3.6 kb Col 1 promoter, which is mainly expressed in osteoblast and odontoblast cells but not in brain, were generated as previously described 23. To determine the function of the full length DMP1 in bone, these transgenes were introduced into the Dmp1-null background, by breeding the transgenic mice with Dmp1-null mice. All mice were fed autoclaved Purina rodent chow (5010, Ralston Purina) containing calcium, 0.67% phosphorus and 4.4 international units (IU) of vitamin D/g. All animal protocols were approved by the Institutional Animal Care and Use Committee.
Microcomputed tomography (μCT)
The inner ears from the control, Dmp1 null and the rescued mice were dissected and scanned in a micro-CT imaging system (35, Scanco Medical, Bassersdorf, Switzerland) as previously described 16-17.
Histology
For paraffin block preparations, specimens were fixed in freshly prepared 4% paraformaldehyde (PFA) in phosphate-buffered saline (pH 7.4), decalcified, and embedded in paraffin by standard histological procedures as previously described 24. Five μm sections were cut and dried. Sections were used for immunohistochemistry, and TRAP staining as previously described 18. A polyclonal antibody against DMP1 was generated in our lab and described previously 25, and a polyclonal antibody against an α smooth muscle actin was used (Abcam, Cambridge, UK).
β-Galactosidase Expression Assay
β-galactosidase staining was assessed from a 3-week-old pup according to the method described previously 22, 26. A head with brain, bone, salivary glands and tongue plus a uterus was briefly fixed with ice-cold 4% PFA for 30 min, then stained overnight in freshly made X-Gal solution (1 mg/mL) at 37°C, followed by re-fixation, decalcification, wax embedding, sectioning, and counterstaining.
RESULTS AND DISCUSSION
Dmp1 null mice developed circling and head shaking behavior
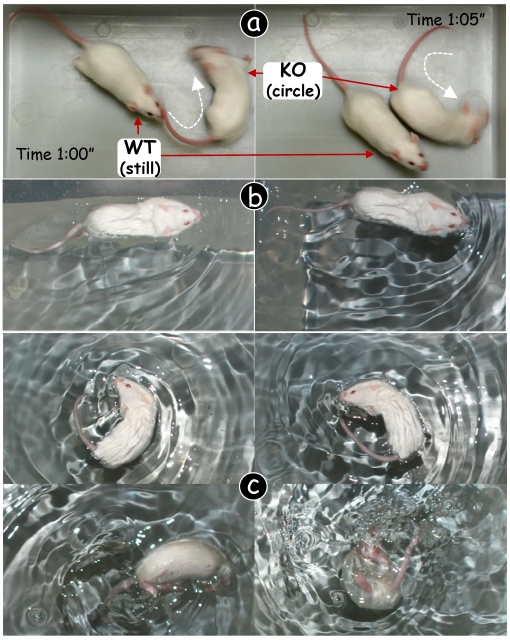
Circling and head shaking behavior in Dmp1 null (KO) mice was initially identified by accident (Fig 1a). To investigate this balance problem, three age groups (10-days, 3-weeks and 4-months, although only 4-month-group was shown in this report) of CD-1 Dmp1 KO mice were either housed alone or with wild type (WT) mice for photographing. The KO mice first shook their heads to either left or right, and then repeated again for several times followed by circling. Circling behavior lasted for up to 5 minutes randomly without a specific pattern. There is no a fixed circling direction, as they circled in both directions with a circling speed between 20 and 30 circle/min. When tested in the Porsolt forced swim test 27, the WT control mice swam straightly (Fig 1b), whereas the KO mice were unable to swim straightly. The KO mouse kept circling in the water (Fig 1c, upper panels). Note that some of the KO mice had to be taken out after awhile to prevent drowning (Fig 1c, lower panels).
Figure 1.
Circling behavior in Dmp1 null (KO) mice. (a) Dmp1-KO mouse (right) kept circling while the wild type (WT) littermate remained quiet most of the time (left). (b) In the forced swim test, the WT mouse swam straightly without circling. (c) The Dmp1-KO mouse swam circling (upper panels) but sank after a short period of time (lower panels).
The Dmp1 null mice in C57/B6 background were also tested, and showed an identical phenotype, although with a much lower incidence rate (<10%) in comparison with the Dmp1 null mice in CD-1 background (100%). This result is not expected, as we previously showed no difference in Dmp1 null bone and tooth phenotypes in both CD-1 and C57/B6 backgrounds 16-17, 28. Interestingly, Dmp1 null mice in both strains display a hyper reaction to touching or any physical stimulation.
Taken together, this behavior study showed clearly that Dmp1 null mice have a defect in body balance likely due to either a vestibular defect itself or a defect in neurons which are responsible for balancing behavior. However, our previous report on DMP1 mutation patients revealed no sign of balancing defects 18. This difference could be due to 1) the DMP1 mutant molecule retaining partial function whereas the Dmp1 null mice lost the whole gene; and 2) visual and proprioceptive information can be compensated in humans but less compensatory in mice for the balancing deficit 7. This result could be beneficial for DMP1 mutation patients for identifying this vestibular dysfunction through a vestibular test 29.
Dmp1 lacZ knock-in signal was detected in pericyte-like cells in blood vessels but not in neural cells
It was reported that DMP1 was expressed in the brain 11, suggesting that DMP1 may have a function in neurons. Likewise, the circling behavior and hyper reaction to physical stimulation might be linked to a possible defect in the brain of the Dmp1 KO mice. However, our DMP1 immunohistochemistry assay showed no signal in brain tissue, while it was detected in osteocytes, which accounts for over 95% of bone cells (Fig 2a). This failure to detect DMP1 in the brain could be due to a low expression level of this protein in neurons.
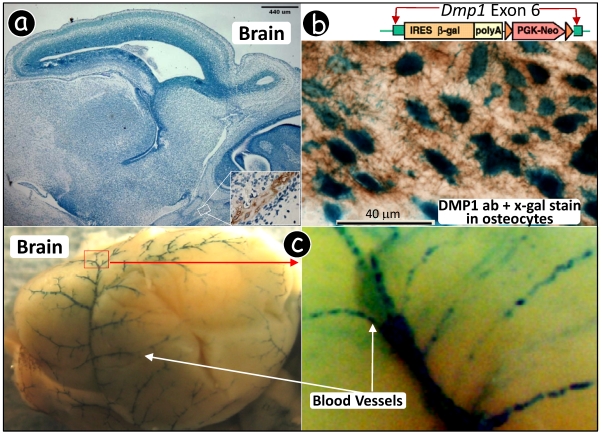
Figure 2.
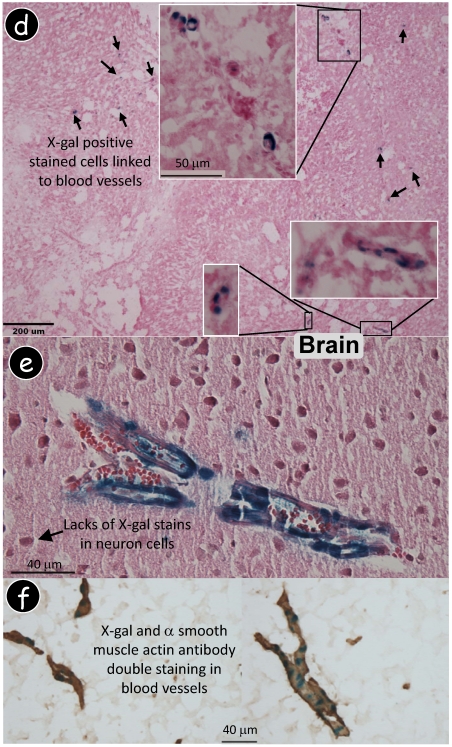
Dmp1 lacZ knock-in transgene is expressed in blood vessels. (a) Immunohistochemistry using a polyclonal antibody against DMP1 N-terminal showed DMP signal in bone only. (b) Dmp1 exon 6, containing 80% of most coding region, is replaced by a lacZ reporter gene whose expression reflects endogenous DMP1 expression pattern. A decalcified frozen section of alveolar bone was stained with X-gal followed by DMP1 antibody immunostaining, showing a blue stain in nuclei of osteocytes (lacZ expression) and brown stain in matrix (DMP1 expression). (c) Whole mount X-gal staining of a heterozygous Dmp1-lacZ knock-in brain overnight showed blue stain in blood vessels (3-wk old, left panel), and the signal appears in a particular group of muscle cells (right panel, the enlarged area in the left panel). (d) A frozen section of brain tissue was stained with X-gal, showing the blue stained cells which are similar to pericytes. (e) Whole mount X-gal stain of brain overnight was followed by paraffin section with an H&E count stain, showing blue stained cells in blood vessels but not in the neural cells. (f) A double stained image with an X-gal stain followed by an anti-muscle actin antibody stain showed that all muscle cells in the blood vessels were immunoreactive to the antibody but the blue-stained cells were limited in a certain type of cells.
Previously, we showed that an X-gal stain assay is a very sensitive approach for detection of DMP1 lacZ signal in the Dmp1-lacZ knock-in null mouse where the lacZ reporter gene, containing a nuclear signal peptide which leads to nucleus expression, was inserted for reflecting the endogenous Dmp1 signal 18. We first showed that both lacZ and DMP1 signals could be detected in the osteocytes where DMP1 is highly expressed 30. Specifically, a frozen section of a heterozygous (HET) Dmp1 lacZ alveolar bone was first stained with X-gal assay for an hour and then followed by a standard immunohistochemistry using the anti-DMP1 antibody. Fig 2b showed a very strong lacZ signal (blue in color) in osteocyte nuclei and a relative high DMP1 expression (brown in color) in the matrixes surrounding osteocytes, demonstrating that the lacZ stain assay is more sensitive than DMP1 immunostaining assay.
Next, a heterozygous (HET) Dmp1 lacZ knock-in brain was isolated and stained in X-gal solution overnight. Unexpectedly, 'blue' blood vessels noticeably displayed a discontinuous pattern in some muscle cells (Fig 2c). To confirm whether this expression pattern in the blood vessel is ubiquitous, a tongue and uterus were also stained and photographed, both showing a similar pattern as that in brain (Fig 2d). Note that the lacZ signal is much stronger in bone and incisor pulp. This could explain in part why the DMP1 immunohistochemistry assay only detected DMP1 signals in the cranial bone but not in brain.
In searching for the specific cells which expressed a Dmp1 lacZ signal, the above tissues were briefly fixed in 4% PFA on ice, followed by sucrose precipitation and frozen section preparations. The X-gal stain was performed with either H&E counter staining or an immunohistochemistry assay using a polyclonal antibody against an α smooth muscle actin. Interestingly, the X-gal stain positive blue cells were directly linked to the blood vessel in both the early and the late stages of angiogenesis (Fig 2e). Importantly, there were no neurons stained positive in the Dmp1-lacZ-knock-in mice (Fig 2f). Furthermore, in the brain blood vessel, all muscle cells were ubiquitously stained with an α smooth muscle actin antibody, whereas a lacZ signal was only detected in some of these muscle cells in a discontinuous pattern, suggesting that DMP1 is expressed in a subgroup of muscle cells (Fig 2g). This data seems supporting a ubiquitous expression pattern of the Dmp1 lacZ in a subgroup of muscle cells in the blood vessel, likely pericytes. Note that blood vessels are formed from endothelial cells and pericytes. The endothelial cells are well characterized but pericytes are not but now coming into focus as important regulators of angiogenesis and blood vessel function, and as potential drug targets. However, there is no good marker for pericytes. Dmp1-reporter mice might be developed to be used for cell sorting to isolate pure pericyte cells for angiogenesis in a future study.
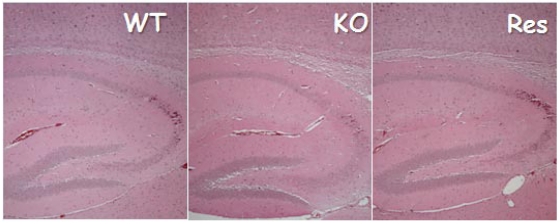
Although previous studies showed that DMP1 is present in the brain 11, the immunohistochemistry and X-gal stain assay performed in this current study failed to display DMP1 signals in neuron cells. Based on these results, a brain defect is an unlikely origin for the observed, abnormal balance behavior. Likewise, DMP1 null mice failed to show change in brain structure, which would have been linked to a change in body balance (see Fig. S1). Furthermore, DMP1 was only detected in bone cells (osteocytes) but not in any other cells of the vestibular apparatus (Fig 3). Finally, based on the data obtained, DMP1 was shown to be expressed in a subgroup of blood vessel muscle cells, including those found in the brain. It is still uncertain as to which specific muscle cells are positively stained with X-gal due to a lack of specific pericyte markers. The goal of future studies is to identify this unknown muscle group and understand its significance.
Fig S1.
There were no apparent changes in Dmp1 KO brain structures. H&E stained images showed no apparent difference in brain structure that is responsible for body balance in the WT control (left panel), KO (right panel) and Res group (right panel).
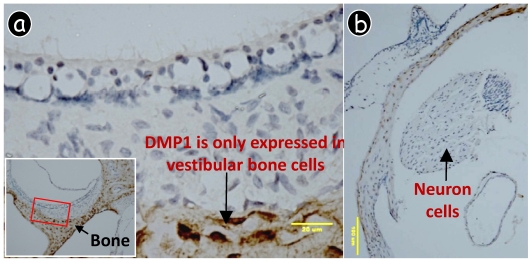
Fig 3.
DMP1 was only expressed in bone cells (osteocytes, brown color) but not in other cells of the vestibular apparatus. A polyclonal antibody against DMP1 was used for immunohistochemistry. DMP1 could not be detected in the vestibular hair or supporting cell (a) or the neuron cell (b).
Targeted expression of DMP1 fully rescued the defective balance behavior
The components responsible for conscious perception of the body's position are situated within the bony vestibule. This fluid-filled, vestibular apparatus contains sensory cells, the hair cells, that detect body movement. These cells, responding to changes of rapid linear accelerations and deceleration via an alteration of a fluid flow wave, create electrical action potentials. The signals are then transmitted to the brain for perception of the body's position. Dmp1-null mice displayed a striking change in both the bone morphology and bone mineralization 16, 18, 20. Thus, we hypothesize that the surface of the bony wall of the Dmp1-null vestibular apparatus is porous and rough, which may cause turbulent fluid movement when the body position is changed. As a result, the turbulent fluid wave affects the hair cells, and their transmission of motion signals to the brain, leading to circling behavior and hyper reaction.
To test this hypothesis, we compared the bony structures of the 4-month-old Dmp1 null, the WT and the rescued Dmp1 null mice where the full length DMP1 is mainly re-expressed in the Dmp1 null bone under control of a 3.6 kb Col 1 promoter which is not active in brain 31 (data not shown).
In the semicircular canal bones, DMP1 was expressed in the control and the rescued mice while no signal was detected in the pure Dmp1 null bone (Fig 4a). Second, the entire bony labyrinth, including semicircular canal and vestibule, was porous in the pure Dmp1 null group (Fig 4b-e, middle panels). The Dmp1 null bone was full of woven bone with few osteoclasts (Fig 4b, middle panel) compared to the age-matched control and the rescued mice where the semicircular canal is composed of both the lamella bone and woven bone. Particularly, this malformed bony labyrinth (Fig 4e, middle panel) was partly due to a defect in mineralization 16, 20 and bone remodeling (Zhang et al., manuscript in preparation), resulting in the rough expanded surface on the vestibular apparatus. Third, the rescued mice, like the WT control, swam straightly and showed no circling (data not shown).
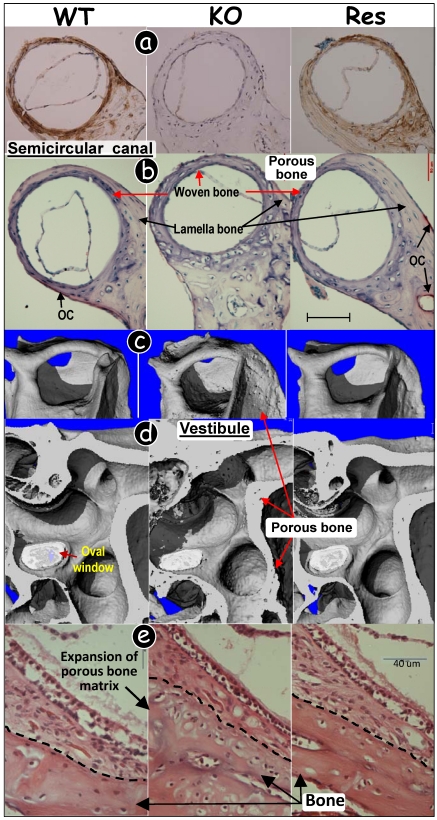
Fig 4.
Targeted expression of DMP1 rescued malformed inner ear bone in Dmp1-null mice. (a) Immunohistochemistry assay showed that DMP1 was highly expressed in 3-wk-old WT (left panel, brown color) and rescued (Res, targeted-reexpression of DMP1 in Dmp1 KO background, right panel) semicircular canal bones with no signal in KO bone (middle panel). (b) TRAP stained images showed few osteoclasts (OC) and a reduction of lamella bone volume in the KO semicircular canal (middle panel), and these pathological changes were restored in the Res canal (right panel) compared to the WT group (left panel). (c-d) μCT images showed porous bone in the KO semicircular canal (c), and vestibular organ (d)(middle panels); and this change was restored in all Res bones (right panels) compared to the WT control (left panels). (e) H&E stained images showed expanded bony mass in the KO vestibular apparatus, and this change was fully restored in the Res bone (right panel) compared to the WT control (left panel).
In summary, the Dmp1 null mice displayed circling behavior and hyper reaction to touching. Histology, TRAP staining and µCT data showed a dramatic change in the Dmp1 null bony structure in the vestibular apparatus. The targeted re-expression of DMP1 in the Dmp1 null bone fully rescued not only the bone phenotype, but also circling behavior and hyper reaction. Furthermore, X-gal stain and DMP1 immunohistochemistry assay showed that DMP1 is not expressed in neuron cells. Thus, we speculate that a rough and expanded bony surface in the Dmp1 null vestibular apparatus results in circling behavior or hyper reaction to touching via a change of the vestibular fluid flow, although more evidence is needed to confirm this hypothesis in future studies. Finally, discovery of DMP1 lacZ signal in pericyte-like cells may suggest a new function of DMP1 in angiogenesis.
Acknowledgments
This study was supported by NIH grants to JQF (DE015209, AR051587) and Genzyme Renal Innovations Program to JQF. Kun Lv was partly supported by The Chinese Scholarship Council.
References
- 1.Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin. 2008 Dec;38(6):467–78. doi: 10.1016/j.neucli.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Prasansuk S, Siriyananda C, Nakorn AN, Atipas S, Chongvisal S. Balance disorders in the elderly and the benefit of balance exercise. J Med Assoc Thai. 2004 Oct;87(10):1225–33. [PubMed] [Google Scholar]
- 3.Moffat SD. Aging and spatial navigation: what do we know and where do we go? Neuropsychol Rev. 2009 Dec;19(4):478–89. doi: 10.1007/s11065-009-9120-3. [DOI] [PubMed] [Google Scholar]
- 4.Christiansen C. Skeletal osteoporosis. J Bone Miner Res. 1993 Dec;8(Suppl 2):S475–80. doi: 10.1002/jbmr.5650081308. [DOI] [PubMed] [Google Scholar]
- 5.Smith SY, Jolette J, Turner CH. Skeletal health: primate model of postmenopausal osteoporosis. Am J Primatol. 2009 Sep;71(9):752–65. doi: 10.1002/ajp.20715. [DOI] [PubMed] [Google Scholar]
- 6.Gardner MJ, Demetrakopoulos D, Shindle MK, Griffith MH, Lane JM. Osteoporosis and skeletal fractures. HSS J. 2006 Feb;2(1):62–9. doi: 10.1007/s11420-005-0137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cryns K, van Alphen AM, van Spaendonck MP, van de Heyning PH, Timmermans JP, de Zeeuw CI, van Camp G. Circling behavior in the Ecl mouse is caused by lateral semicircular canal defects. J Comp Neurol. 2004 Jan 19;468(4):587–95. doi: 10.1002/cne.10975. [DOI] [PubMed] [Google Scholar]
- 8.Qin C, D'Souza R, Feng JQ. Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J Dent Res. 2007 Dec;86(12):1134–41. doi: 10.1177/154405910708601202. [DOI] [PubMed] [Google Scholar]
- 9.Toyosawa S, Shintani S, Fujiwara T, Ooshima T, Sato A, Ijuhin N, Komori T. Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J Bone Miner Res. 2001;16(11):2017–26. doi: 10.1359/jbmr.2001.16.11.2017. [DOI] [PubMed] [Google Scholar]
- 10.Toyosawa S, Tomita Y, Kishino M, Hashimoto J, Ueda T, Tsujimura T, Aozasa K, Ijuhin N, Komori T. Expression of dentin matrix protein 1 in tumors causing oncogenic osteomalacia. Mod Pathol. 2004 May;17(5):573–8. doi: 10.1038/modpathol.3800084. [DOI] [PubMed] [Google Scholar]
- 11.Terasawa M, Shimokawa R, Terashima T, Ohya K, Takagi Y, Shimokawa H. Expression of dentin matrix protein 1 (DMP1) in nonmineralized tissues. J Bone Miner Metab. 2004;22(5):430–8. doi: 10.1007/s00774-004-0504-4. [DOI] [PubMed] [Google Scholar]
- 12.Ogbureke KU, Abdelsayed RA, Kushner H, Li L, Fisher LW. Two members of the SIBLING family of proteins, DSPP and BSP, may predict the transition of oral epithelial dysplasia to oral squamous cell carcinoma. Cancer. 2010 Feb 22;116(7):1709–17. doi: 10.1002/cncr.24938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogbureke KU, Fisher LW. SIBLING expression patterns in duct epithelia reflect the degree of metabolic activity. J Histochem Cytochem. 2007 Apr;55(4):403–9. doi: 10.1369/jhc.6A7075.2007. [DOI] [PubMed] [Google Scholar]
- 14.Ogbureke KU, Fisher LW. Renal expression of SIBLING proteins and their partner matrix metalloproteinases (MMPs) Kidney Int. 2005 Jul;68(1):155–66. doi: 10.1111/j.1523-1755.2005.00389.x. [DOI] [PubMed] [Google Scholar]
- 15.Ogbureke KU, Fisher LW. Expression of SIBLINGs and their partner MMPs in salivary glands. J Dent Res. 2004 Sep;83(9):664–70. doi: 10.1177/154405910408300902. [DOI] [PubMed] [Google Scholar]
- 16.Ye L, Mishina Y, Chen D, Huang H, Dallas SL, Dallas MR, Sivakumar P, Kunieda T, Tsutsui TW, Boskey A, Bonewald LF, Feng JQ. Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype. J Biol Chem. 2005 Feb 18;280(7):6197–203. doi: 10.1074/jbc.M412911200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, Mishina Y, Feng JQ. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 2004 Apr 30;279(18):19141–8. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- 18.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006 Nov;38(11):1310–5. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Juppner H, Strom TM. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006 Nov;38(11):1248–50. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling Y, Rios HF, Myers ER, Lu Y, Feng JQ, Boskey AL. DMP1 depletion decreases bone mineralization in vivo: an FTIR imaging analysis. J Bone Miner Res. 2005 Dec;20(12):2169–77. doi: 10.1359/JBMR.050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrow EG, Davis SI, Ward LM, Summers LJ, Bubbear JS, Keen R, Stamp TC, Baker LR, Bonewald LF, White KE. Molecular analysis of DMP1 mutants causing autosomal recessive hypophosphatemic rickets. Bone. 2009 Feb;44(2):287–94. doi: 10.1016/j.bone.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng JQ, Huang H, Lu Y, Ye L, Xie Y, Tsutsui TW, Kunieda T, Castranio T, Scott G, Bonewald LB, Mishina Y. The Dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J Dent Res. 2003 Oct;82(10):776–80. doi: 10.1177/154405910308201003. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Ye L, Yu S, Zhang S, Xie Y, McKee MD, Li YC, Kong J, Eick JD, Dallas SL, Feng JQ. Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Dev Biol. 2007 Mar 1;303(1):191–201. doi: 10.1016/j.ydbio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fen JQ, Zhang J, Dallas SL, Lu Y, Chen S, Tan X, Owen M, Harris SE, MacDougall M. Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique bone marker gene. J Bone Miner Res. 2002 Oct;17(10):1822–31. doi: 10.1359/jbmr.2002.17.10.1822. [DOI] [PubMed] [Google Scholar]
- 25.Qin C, Brunn JC, Cook RG, Orkiszewski RS, Malone JP, Veis A, Butler WT. Evidence for the proteolytic processing of dentin matrix protein 1. Identification and characterization of processed fragments and cleavage sites. J Biol Chem. 2003 Sep 5;278(36):34700–8. doi: 10.1074/jbc.M305315200. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Tan X, Contag CH, Lu Y, Guo D, Harris SE, Feng JQ. Dissection of promoter control modules that direct Bmp4 expression in the epithelium-derived components of hair follicles. Biochem Biophys Res Commun. 2002;293(5):1412–9. doi: 10.1016/S0006-291X(02)00416-3. [DOI] [PubMed] [Google Scholar]
- 27.Porsolt RD. Animal model of depression. Biomedicine. 1979 Jul;30(3):139–40. [PubMed] [Google Scholar]
- 28.Ye L, Zhang S, Ke H, Bonewald LF, Feng JQ. Periodontal breakdown in the Dmp1 null mouse model of hypophosphatemic rickets. J Dent Res. 2008 Jul;87(7):624–9. doi: 10.1177/154405910808700708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janky KL, Shepard N. Vestibular evoked myogenic potential (VEMP) testing: normative threshold response curves and effects of age. J Am Acad Audiol. 2009 Sep;20(8):514–22. doi: 10.3766/jaaa.20.8.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng JQ, Zhang J, Dallas SL, Lu Y, Chen S, Tan X, Owen M, Harris SE, MacDougall M. Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique bone marker gene. J Bone Miner Res. 2002 Oct;17(10):1822–31. doi: 10.1359/jbmr.2002.17.10.1822. [DOI] [PubMed] [Google Scholar]
- 31.Bogdanovic Z, Bedalov A, Krebsbach PH, Pavlin D, Woody CO, Clark SH, Thomas HF, Rowe DW, Kream BE, Lichtler AC. Upstream regulatory elements necessary for expression of the rat COL1A1 promoter in transgenic mice. J Bone Miner Res. 1994 Feb;9(2):285–92. doi: 10.1002/jbmr.5650090218. [DOI] [PubMed] [Google Scholar]