Abstract
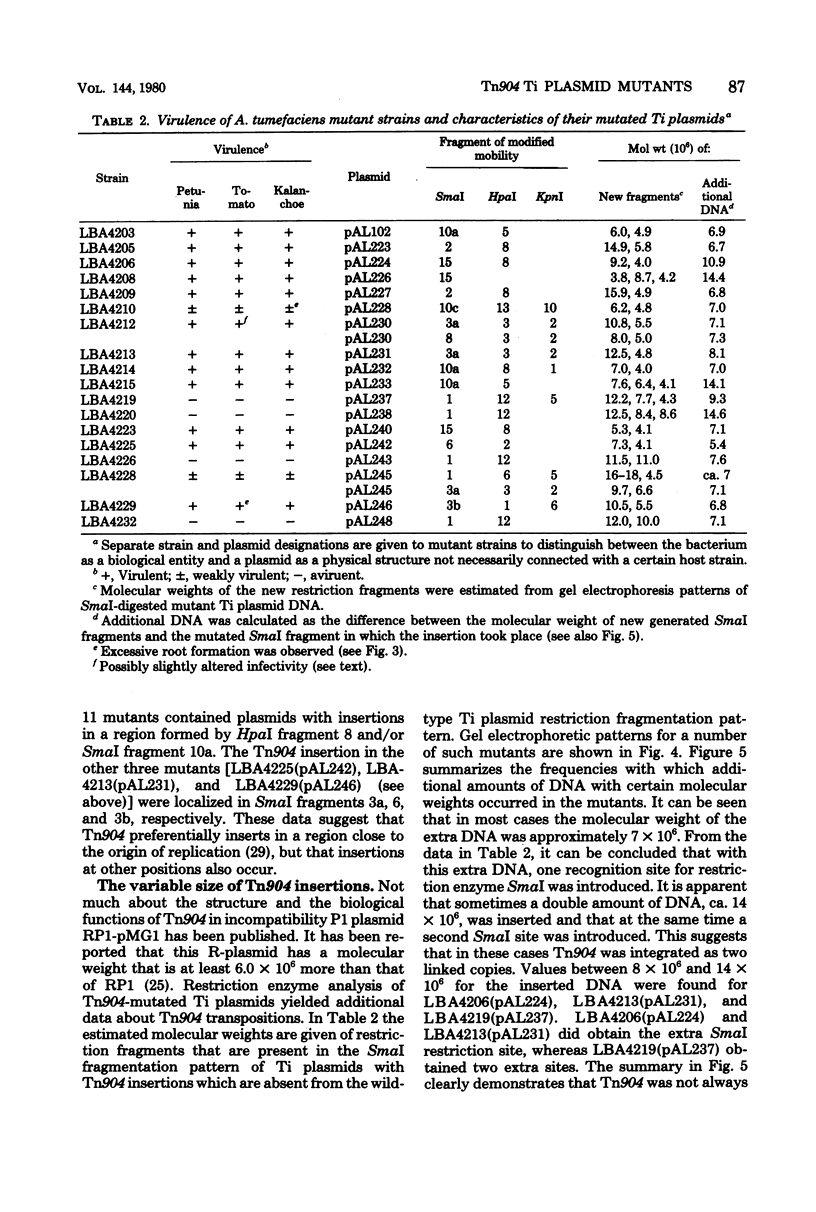
Seven Tn904 insertion mutants of pTi Ach5 affecting Agrobacterium tumefaciens virulence were studied. The mutant character was shown to be plasmid borne. Four of these mutants were avirulent and carried an insertion in restriction endonuclease HpaI fragment 12, a 3.3-megadalton fragment, which therefore appears to be a Ti plasmid region essential for virulence. Two mutants were attenuated in virulence. The inserts mapped close to HpaI fragment 12. One mutant giving rise to small tumors with excessive adventitious root formation on Kalanchoe daigremontiana carried an insertion in the right side of the common sequence in the deoxyribonucleic acid of the Ti plasmid detected in crown gall tumors. The insertion behavior of Tn904 was studied by analyzing 11 independently isolated and randomly chosen mutants. The Tn904 inserts did not affect oncogenicity, tumor morphology, bacterial transfer functions, octopine catabolism functions, or vital parts of the Ti plasmid, such as the origin of replication. Most of the Tn904 inserts were concentrated in a small part of the map. The size of additional deoxyribonucleic acid as a result of Tn904 inserts varied between 5 and 15 megadaltons. In two cases a Ti plasmid was found with two Tn904 insertions at different positions.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUN A. C. Conditioning of the host cell as a factor in the transformation process in crown gall. Growth. 1952 Jun;16(2):65–74. [PubMed] [Google Scholar]
- Braun A. C. A Physiological Basis for Autonomous Growth of the Crown-Gall Tumor Cell. Proc Natl Acad Sci U S A. 1958 Apr;44(4):344–349. doi: 10.1073/pnas.44.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A. C. Plant tumors. Biochim Biophys Acta. 1978 Oct 27;516(2):167–191. doi: 10.1016/0304-419x(78)90007-0. [DOI] [PubMed] [Google Scholar]
- Chilton M. D., Drummond M. H., Merio D. J., Sciaky D., Montoya A. L., Gordon M. P., Nester E. W. Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell. 1977 Jun;11(2):263–271. doi: 10.1016/0092-8674(77)90043-5. [DOI] [PubMed] [Google Scholar]
- Chilton M. D., Montoya A. L., Merlo D. J., Drummond M. H., Nutter R., Gordon M. P., Nester E. W. Restriction endonuclease mapping of a plasmid that confers oncogenicity upon Agrobacterium tumefaciens strain B6-806. Plasmid. 1978 Feb;1(2):254–269. doi: 10.1016/0147-619x(78)90043-4. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Bauer B. Plasmid-determined tetracycline resistance in Streptococcus faecalis: evidence for gene amplification during growth in presence of tetracycline. Proc Natl Acad Sci U S A. 1975 May;72(5):1720–1724. doi: 10.1073/pnas.72.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Evidence for diverse types of large plasmids in tumor-inducing strains of Agrobacterium. J Bacteriol. 1976 Apr;126(1):157–165. doi: 10.1128/jb.126.1.157-165.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Dobritsa A. P., Ksenzenko V. N., Fedoseeva V. B., Alexandrov A. A., Kamynina T. P., Khmelnitsky M. I. Isolation of transposon TnA from plasmid RP4 carrying two copies of this element. Gene. 1980 Jan;8(2):153–162. doi: 10.1016/0378-1119(80)90034-7. [DOI] [PubMed] [Google Scholar]
- Drummond M. H., Chilton M. D. Tumor-inducing (Ti) plasmids of Agrobacterium share extensive regions of DNA homology. J Bacteriol. 1978 Dec;136(3):1178–1183. doi: 10.1128/jb.136.3.1178-1183.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangman W. L. Separation of very large DNA molecules by gel electrophoresis. Nucleic Acids Res. 1978 Mar;5(3):653–665. doi: 10.1093/nar/5.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene P. J., Heyneker H. L., Bolivar F., Rodriguez R. L., Betlach M. C., Covarrubias A. A., Backman K., Russel D. J., Tait R., Boyer H. W. A general method for the purification of restriction enzymes. Nucleic Acids Res. 1978 Jul;5(7):2373–2380. doi: 10.1093/nar/5.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley W. B., Kemp J. D., Albert M. J., Sutton D. W., Callis J. Transcription of Ti plasmid-derived sequences in three octopine-type crown gall tumor lines. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2828–2832. doi: 10.1073/pnas.76.6.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Hernalsteens J. P., De Greve H., Van Montagu M., Schell J. Mutagenesis by insertion of the drug resistance transposon Tn7 applied to the Ti plasmid of Agrobacterium tumefaciens. Plasmid. 1978 Feb;1(2):218–225. doi: 10.1016/0147-619x(78)90040-9. [DOI] [PubMed] [Google Scholar]
- Holmans P. L., Clowes R. C. Transposition of a duplicate antibiotic resistance gene and generation of deletions in plasmid R6K. J Bacteriol. 1979 Feb;137(2):977–989. doi: 10.1128/jb.137.2.977-989.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsters M., Silva B., Van Vliet F., Genetello C., De Block M., Dhaese P., Depicker A., Inzé D., Engler G., Villarroel R. The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid. 1980 Mar;3(2):212–230. doi: 10.1016/0147-619x(80)90110-9. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Jacob A. E., Hedges R. W. Recombination between plasmids of incompatibility groups P-1 and P-2. J Bacteriol. 1976 Sep;127(3):1278–1285. doi: 10.1128/jb.127.3.1278-1285.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapwijk P. M., Schilperoort R. A. Negative control of octopine degradation and transfer genes of octopine Ti plasmids in Agrobacterium tumefaciens. J Bacteriol. 1979 Aug;139(2):424–431. doi: 10.1128/jb.139.2.424-431.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapwijk P. M., van Breukelen J., Korevaar K., Ooms G., Schilperoort R. A. Transposition of Tn904 encoding streptomycin resistance into the octopine Ti plasmid of Agrobacterium tumefaciens. J Bacteriol. 1980 Jan;141(1):129–136. doi: 10.1128/jb.141.1.129-136.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. Translocatable elements in procaryotes. Cell. 1977 May;11(1):11–23. doi: 10.1016/0092-8674(77)90313-0. [DOI] [PubMed] [Google Scholar]
- Koekman B. P., Ooms G., Klapwijk P. M., Schilperoort R. A. Genetic map of an octopine TI-plasmid. Plasmid. 1979 Jul;2(3):347–357. doi: 10.1016/0147-619x(79)90018-0. [DOI] [PubMed] [Google Scholar]
- Otten L. A., Schilperoort R. A. A rapid micro scale method for the detection of lysopine and nopaline dehydrogenase activities. Biochim Biophys Acta. 1978 Dec 8;527(2):497–500. doi: 10.1016/0005-2744(78)90363-7. [DOI] [PubMed] [Google Scholar]
- Robinson M. K., Bennett P. M., Grinsted J., Richmond M. H. The stable carriage of two TnA units on a single replicon. Mol Gen Genet. 1978 Apr 17;160(3):339–346. doi: 10.1007/BF00332978. [DOI] [PubMed] [Google Scholar]
- Robinson M. K., Bennett P. M., Richmond M. H. Inhibition of TnA translocation by TnA. J Bacteriol. 1977 Jan;129(1):407–414. doi: 10.1128/jb.129.1.407-414.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOOG F., MILLER C. O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–130. [PubMed] [Google Scholar]
- Schmitt R., Bernhard E., Mattes R. Characterisation of Tn1721, a new transposon containing tetracycline resistance genes capable of amplification. Mol Gen Genet. 1979 Apr 17;172(1):53–65. doi: 10.1007/BF00276215. [DOI] [PubMed] [Google Scholar]
- Schöffl F., Pühler A. Intramolecular amplification of the tetracycline resistance determinant of transposon Tn1771 in Escherichia coli. Genet Res. 1979 Jun;33(3):253–260. doi: 10.1017/s0016672300018395. [DOI] [PubMed] [Google Scholar]
- Smith E. F., Townsend C. O. A PLANT-TUMOR OF BACTERIAL ORIGIN. Science. 1907 Apr 26;25(643):671–673. doi: 10.1126/science.25.643.671. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Van Larebeke N., Genetello C., Schell J., Schilperoort R. A., Hermans A. K., Van Montagu M., Hernalsteens J. P. Acquisition of tumour-inducing ability by non-oncogenic agrobacteria as a result of plasmid transfer. Nature. 1975 Jun 26;255(5511):742–743. doi: 10.1038/255742a0. [DOI] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaenen I., Van Larebeke N., Van Montagu M., Schell J. Supercoiled circular DNA in crown-gall inducing Agrobacterium strains. J Mol Biol. 1974 Jun 15;86(1):109–127. doi: 10.1016/s0022-2836(74)80011-2. [DOI] [PubMed] [Google Scholar]