Abstract
Background:
Studies have demonstrated that flexor tendon repair strength fails to increase in the first three weeks following suturing of the tendon, a finding that correlates closely with the timing of many clinical failures. The application of growth factors holds promise for improving the tendon-repair response and obviating failure in the initial three weeks.
Methods:
The effects of basic fibroblast growth factor on flexor tendon healing were evaluated with use of a canine model. Operative repair followed by the sustained delivery of basic fibroblast growth factor, at two different doses, was compared with operative repair alone. Histological, biochemical, and biomechanical methods were used to evaluate the tendons twenty-one days after repair.
Results:
Vascularity, cellularity, and adhesion formation were increased in the tendons that received basic fibroblast growth factor as compared with the tendons that received operative repair alone. DNA concentration was increased in the tendons that received 1000 ng of basic fibroblast growth factor (mean and standard deviation, 5.7 ± 0.7 μg/mg) as compared with the tendons that received 500 ng of basic fibroblast growth factor (3.8 ± 0.7 μg/mg) and the matched control tendons that received operative repair alone (4.5 ± 0.9 μg/mg). Tendons that were treated with basic fibroblast growth factor had a lower ratio of type-I collagen to type-III collagen, indicating increased scar formation compared with that seen in tendons that received operative repair alone (3.0 ± 1.6 in the group that received 500-ng basic fibroblast growth factor compared with 4.3 ± 1.0 in the paired control group that received operative repair alone, and 3.4 ± 0.6 in the group that received 1000-ng basic fibroblast growth factor compared with 4.5 ± 1.9 in the paired control group that received operative repair alone). Consistent with the increases in adhesion formation that were seen in tendons treated with basic fibroblast growth factor, the range of motion was reduced in the group that received the higher dose of basic fibroblast growth factor than it was in the paired control group that received operative repair alone (16.6° ± 9.4° in the group that received 500 ng basic fibroblast growth factor, 13.4° ± 6.1° in the paired control group that received operative repair alone, and 29.2° ± 5.8° in the normal group [i.e., the group of corresponding, uninjured tendons from the contralateral forelimb]; and 15.0° ± 3.8° in the group that received 1000 ng basic fibroblast growth factor, 19.3° ± 5.5° in the paired control group that received operative repair alone, and 29.0° ± 8.8° in the normal group). There were no significant differences in tendon excursion or tensile mechanical properties between the groups that were treated with basic fibroblast growth factor and the groups that received operative repair alone.
Conclusions:
Although basic fibroblast growth factor accelerated the cell-proliferation phase of tendon healing, it also promoted neovascularization and inflammation in the earliest stages following the suturing of the tendon. Despite a substantial biologic response, the administration of basic fibroblast growth factor failed to produce improvements in either the mechanical or functional properties of the repair. Rather, increased cellular activity resulted in peritendinous scar formation and diminished range of motion.
Clinical Relevance:
Despite success in stimulating cellular proliferation and matrix synthesis in flexor tendons through the application of a growth factor in a clinically relevant animal model, no significant improvements were noted in either functional or structural properties. Therefore, as applied in this model, basic fibroblast growth factor is not recommended for intrasynovial flexor tendon repair.
Adhesion formation, repair-site gap formation, and catastrophic failure continue to be noted in the first twenty-one days after suture repair of the intrasynovial flexor tendon1. Although the outcomes of repair have improved over the last two decades, neither innovative suture techniques nor novel passive-motion rehabilitation methods have been entirely successful in preventing these complications1,2. Recently, new strategies for stimulating the repair response have been developed, including the sustained administration of selected growth factors at the site and time of tendon-suturing3-5. The application of exogenous heparin-binding growth factors, such as basic fibroblast growth factor (bFGF), is especially attractive for zone-II injuries because few native cells and only a minimal amount of matrix synthesis have been noted in the first three weeks following suturing of the tendon in this zone6.
In a prior study, it was observed that platelet-derived growth factor-BB (PDGF-BB) promoted modest but significant increases in cell proliferation, collagen synthesis, and collagen remodeling in a canine model of intrasynovial tendon injury and repair4,5. Although these changes in cellular activity were associated with improvements in digital range of motion, they did not lead to improvements in the strength or stiffness of the repair3. The growth factor bFGF has been shown to stimulate cell proliferation, neovascularization, and matrix synthesis in vivo and in vitro in a variety of tendon and ligament injury-and-repair models and thus holds promise for improved tendon healing7-10. Increases in cellularity and vascularity in the relatively acellular and hypovascular intrasynovial tendon could accelerate the healing response, with increased matrix synthesis substantially improving the strength of the repair. Supportive of this concept, a recent study showed that gene transfer of bFGF into repaired chicken intrasynovial tendons led to increased tensile strength9. Similarly, delivery of recombinant bFGF to repaired intrasynovial tendons through the use of coated sutures led to increased ultimate load values in a rabbit model7.
The goal of the current study was to determine whether or not the administration of bFGF could stimulate an early repair response in a canine model that included the use of multistrand sutures and passive mobilization rehabilitation. Our hypothesis was that the controlled delivery of bFGF would promote cellular proliferation and collagen synthesis, leading to an increase in the functional and structural properties of the tendon after intrasynovial tendon repair.
Materials and Methods
To determine the effects of bFGF on intrasynovial flexor tendon healing in vivo, fibrin matrices with a heparin-based delivery system loaded with either 500 ng (1.25 μg/mL) or 1000 ng (2.5 μg/mL) of bFGF were implanted between repaired flexor tendon stumps in either the second or fifth digit of the right forelimb (the bFGF group) in twenty dogs. The lower dose was chosen on the basis of a previous report7, and the higher dose was chosen on the basis of the maximum amount of bFGF that could be delivered with the heparin-based delivery system. The remaining second or fifth flexor tendon in the right forelimb of each dog was sharply transected and repaired, after which it received no additional treatment (the control group). The uninjured corresponding tendons from the left forelimb were also used for some assays. Animals were killed twenty-one days after surgical repair.
Fibrin-Heparin-Based Delivery System
The heparin-based delivery system used in this study included a bidomain peptide with a factor-XIIIa substrate derived from α2-plasmin inhibitor at the N-terminus, and a C-terminal heparin-binding domain derived from antithrombin III11. The peptide is covalently cross-linked to a fibrin matrix during coagulation by the transglutaminase activity of factor XIIIa. The peptide immobilizes heparin electrostatically to the matrix, which in turn immobilizes heparin-binding growth factors (e.g., bFGF, PDGF-BB), preventing their diffusion from the matrix. Fibrin matrices (30-μL matrix volume) were made with the following final component amounts: 500 ng or 1000 ng bFGF (R&D Systems, Minneapolis, Minnesota), 10 mg/mL fibrinogen concentration (preparation as previously described; Sigma-Aldrich, St. Louis, Missouri)11, 6.5 mM CaCl2, 13 units/mL thrombin (Sigma-Aldrich), 2.6 mM ATIII peptide11 (dLNQEQVSPK[βA]FAKLAARLYRKA-NH2, where dL denotes dansyl leucine), and heparin (627 μM, Sigma-Aldrich), in Tris-buffered saline solution (TBS; 137 mM NaCl, 2.7 mM KCl, 33 mM Tris, pH 7.4). Our prior in vitro studies in which this delivery system was used demonstrated that bFGF can be delivered in a controlled manner over a period of approximately ten days12.
Animal Model
All procedures were approved by the Washington University Animal Studies Committee. Forty flexor digitorum profundus tendons from the right forelimbs of twenty adult mongrel dogs (Covance, Denver, Pennsylvania) weighing 20 to 30 kg were treated surgically and used as either the bFGF groups (twenty tendons) or the control groups (twenty tendons). In addition, the forty uninjured corresponding flexor digitorum profundus tendons from the left forelimbs of the same animals were used as the normal groups. The animals were anesthetized, intubated, and maintained on anesthesia, and a tourniquet was applied. The sheaths of the second and fifth digits of the right forelimb in the region between the annular pulleys proximal and distal to the proximal interphalangeal joint were exposed through midlateral incisions. The sheaths were entered, and the flexor digitorum profundus tendons were cut transversely. To create space for the delivery system, transversely oriented defects were created in the free ends of both tendon stumps with a scalpel; this was also done for the tendons in the paired control groups. The tendons were repaired with an eight-strand core-suture technique consisting of a modified Kessler repair13 performed with use of a continuous loop of 4-0 Supramid suture (S. Jackson, Alexandria, Virginia). A sterile fibrin matrix with bFGF was incorporated into the repair site of the flexor digitorum profundus tendon of either the second or the fifth digit (selected randomly); the twenty tendons repaired in this manner composed the two bFGF groups (ten 500-ng bFGF tendons and ten 1000-ng bFGF tendons). The remaining (second or fifth) flexor digitorum profundus tendon was repaired without the fibrin matrix; the twenty tendons repaired in this manner served as the paired control groups. The sheaths were not repaired. After surgery, the right forelimb was immobilized with use of a fiberglass shoulder spica cast with the elbow flexed to 90° and the wrist flexed to 70°. Controlled passive motion exercise was applied to the digits during two five-minute rehabilitation sessions performed five days a week starting on the first postoperative day. The dogs were killed twenty-one days after surgical repair, and histologic, biochemical, or biomechanical assessment was carried out. Adhesion formation between the tendon and its sheath was qualitatively evaluated at the time of dissection as “none,” “slight,” “moderate,” or “severe.”
Histology
For all assays, each bFGF sample had a paired control (i.e., the paired control group). Samples for histologic analysis were fixed in 4% paraformaldehyde overnight, processed for paraffin-embedded sectioning, cut at 5 μm, and stained with hematoxylin and eosin (n = two samples per group; i.e., four samples from the bFGF groups, four samples from the paired control groups, and four samples from the uninjured group). Histologic sections were blinded to group and time point and evaluated under bright-field illumination for cellularity, acute inflammatory cells (i.e., neutrophils), chronic inflammatory cells (i.e., lymphocytes, macrophages, and plasma cells), and vascular response. Sections were then analyzed under polarized light illumination for collagen organization at the repair site.
Biomechanical Assays
The second and fifth digits from the normal (left) and surgically treated (right) forelimbs were disarticulated at the metacarpophalangeal joint, and the flexor digitorum profundus tendons were transected proximally at the musculotendinous junction. The range of motion of both the uninjured digits (i.e., the digits in the normal group) and the repaired digits (i.e., the digits in the control groups and the bFGF groups) was assessed with use of a motion-analysis system14 (PC-Reflex; Qualisys, Glastonbury, Connecticut) (n = eight samples per group; i.e., sixteen samples for the bFGF groups and sixteen samples for the paired control groups). Two pairs of reflective hemispherical markers were pinned to the middle and distal phalanges of each toenail. The proximal phalanx was clamped in a vertical orientation, and the coordinates of the markers were sampled first with the digit in a flexed position and then with the digit in an extended position, as previously described3,15. On the basis of the differences between the flexed and extended positions, we computed rotations of the proximal interphalangeal joint and linear displacement of the flexor tendon.
The flexor tendons with attached distal phalanges were then isolated from the repaired digits and tested to failure in tension with use of a servohydraulic materials testing system (8500R; Instron, Canton, Massachusetts), as previously described3,15 (n = eight samples for each group; i.e., sixteen samples for the bFGF groups and sixteen samples for the paired control groups). Prior to testing, the gap between repaired tendon ends was measured on digital images of each repair site. The distal phalanx was held rigidly in a custom fixture and the proximal tendon stump was held in a soft-tissue clamp such that the tendon-bone specimen was in approximately neutral alignment. A pair of reflective markers was attached to the tendon, one on each side of the repair site. The distal phalanx was then displaced vertically to apply a preload of 1 N. Five preconditioning cycles were applied in load control (triangle waveform, 1 to 5 N, 0.25 Hz). The specimens were then displaced in tension at a rate of 0.375 mm/s until failure. Using the marker positions, we calculated repair-site elongation-per-length (strain, mm/mm), where the unit length was the initial vertical distance between the markers. Using force-displacement and force-strain plots, we determined peak (ultimate) force, repair-site stiffness (slope of the linear portion of the force-elongation curve), repair-site rigidity (slope of the linear portion of the force-strain curve), and repair-site strain at a force of 20 N.
Biochemical Assays
After biomechanical testing, specimens (n = eight samples per group; i.e., sixteen samples from the bFGF groups, sixteen samples from the paired control groups, and sixteen samples from the uninjured group) were then processed for biochemical analysis. DNA content was determined fluorometrically with use of a PicoGreen assay kit (P7589; Invitrogen, Carlsbad, California) following digestion of the tissues with papain. Collagen-typing was carried out by gel-filtration high-performance liquid chromatography following partial digestion of the tissues with cyanogen bromide. Resulting peptide fragments unique to type-I and III collagens were then used to determine the ratio of these collagen types. Collagen concentration was determined with use of reverse-phase high-performance liquid chromatography quantitation of hydroxyproline following acid hydrolysis and derivitization with phenylisothiocyanate. Quantitation of reducible collagen cross-links was achieved using cation-exchange high-performance liquid chromatography following labeling and reduction of tissue collagen with [3H]-sodium borohydride. The reducible cross-links hydroxylysinonorleucine and dihydroxylysinonorleucine were isolated and quantitated by in-line liquid scintillation spectrometry.
Statistics
The repairs of the bFGF groups were compared with those of the paired control groups with use of paired t tests. Tendons treated with 1000 ng bFGF were compared with those treated with 500 ng bFGF with use of Student t tests. For range of motion, comparisons to normal tendons were made with use of an analysis of variance followed by a Fisher least-significant-difference post hoc test. An alpha level of p < 0.05 was considered significant. Results are presented as the mean and standard deviation.
Source of Funding
This study was supported by a grant from the National Institutes of Health (R01 AR033097).
Results
Gross Morphology and Histology
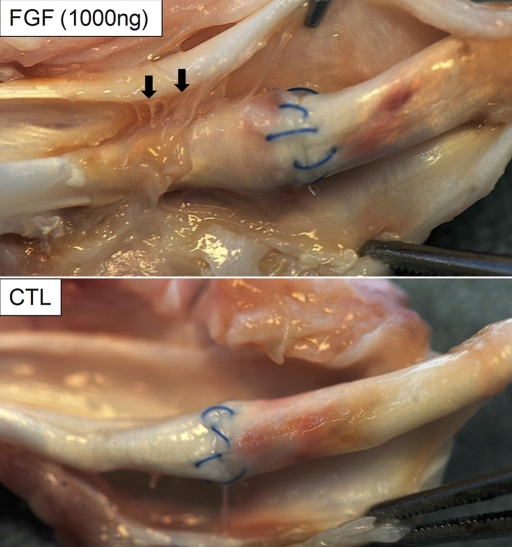
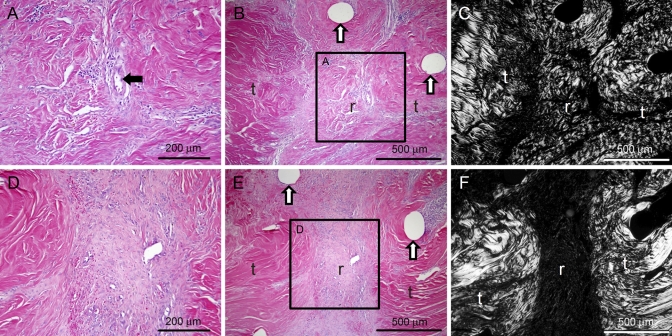
Of the forty repairs in a total of twenty animals, two repairs ruptured and four repairs had gaps of >2 mm. These failed repairs were distributed as follows: two in the 500-ng bFGF group, two in the 1000-ng bFGF group, and two in the paired control groups. These six samples were excluded from the final data set (Table I), leaving twenty-six of the thirty-two biomechanical samples for use in determining the mean and standard deviations. The average gap of these twenty-six samples was 0.34 ± 0.36 mm (Table I; there were no significant differences in gap size between groups). Grossly, there were more adhesions between the flexor tendon surface and its surrounding sheath in the bFGF-treated tendons than there were in the tendons in the control group (Fig. 1). These adhesions were most apparent in the 1000-ng bFGF group. Histologic evaluation revealed increased levels of fibroblasts, inflammatory cells, and vascular cells in all repaired tendons relative to normal, uninjured tendons. The vascular and cellular responses and the population of inflammatory cells were increased in the bFGF groups as compared with those in the control group (Table II, Fig. 2). Matrix production at the repair site was primarily scar tissue, as evidenced by the disorganized arrangement of collagen fibers, regardless of groups (Fig. 2, C and F).
TABLE I.
Biomechanical and Biochemical Measures*
| Group | Failures | Gap†(mm) | Cross-links†(DHLNL/HLNL) | Collagen†(%) | Rigidity†(N/mm/mm) | Strain at 20 N† |
| 500 ng bFGF | 2/10 | 0.28 ± 0.24 | 1.55 ± 0.41‡ | 72.2 ± 3.7 | 821.2 ± 183.0 | 0.075 ± 0.012 |
| Control | 1/10 | 0.56 ± 0.60 | 1.17 ± 0.08‡ | 73.9 ± 4.1 | 892.1 ± 320.9 | 0.073 ± 0.013 |
| 1000 ng bFGF | 2/10 | 0.17 ± 0.10 | 1.28 ± 0.23 | 75.3 ± 4.9 | 914.3 ± 188.6 | 0.073 ± 0.034 |
| Control | 1/10 | 0.38 ± 0.32 | 1.21 ± 0.33 | 74.8 ± 4.7 | 1255.1 ± 500.0 | 0.064 ± 0.025 |
bFGF = basic fibroblast growth factor, DHLNL = dihydroxylysinonorleucine, HLNL = hydroxylysinonorleucine.
Data are given as the mean and standard deviation.
p = 0.088.
Fig. 1.
There were more adhesions (arrows) between the flexor tendon surface and its surrounding sheath in the bFGF-treated tendons (top panel) than there were in the non-bFGF-treated (control group [CTL]) tendons (bottom panel). Arrows indicate adhesions proximal to the repair site in a tendon treated with 1000 ng bFGF. The paired control tendon did not have any apparent adhesions.
TABLE II.
Histologic Grades*
| Group | Fibroblast Quantity | Polymorphonuclear Leukocytes | Mononuclear Cells (Histiocytes, Lymphocytes, and Plasma Cells) | Vascular Repair |
| Normal | — | — | — | — |
| Control | ++ | +/++ | +/++ | +/++ |
| 500 ng bFGF | +++ | +/++ | +++ | +++ |
| 1000 ng bFGF | +++ | + | +++ | +++ |
Dash indicates normal levels, plus sign indicates slightly elevated levels, two plus signs indicate an elevated level, and three plus signs indicate a dramatically elevated level (levels were defined on the basis of standard micrographs for each level). Vascular repair indicates vascular prominence and angiogenesis at the repair site. bFGF = basic fibroblast growth factor.
Fig. 2.
Vascularity, cellularity, and inflammation were substantially increased in the bFGF groups as compared with the responses in the paired control groups. Representative micrographs for 1000-ng bFGF-treated tendons (panels A, B, and C) and tendons in the control group (panels D, E, and F) are shown under bright-field (panels A, B, D, and E) and polarized (panels C and F) light. A large blood vessel can be seen in the bFGF-treated tendon (black arrow in panel A). Increased levels of disorganized collagen can be seen at the repair site of the bFGF-treated tendon (panel C). White arrows indicate suture tracks, t = tendon, r = repair site. (A and D: hematoxylin and eosin stain, ×20; B, C, E, and F: hematoxylin and eosin stain, ×10; regions shown in A and D are indicated by black boxes in B and E, respectively.)
Biochemistry
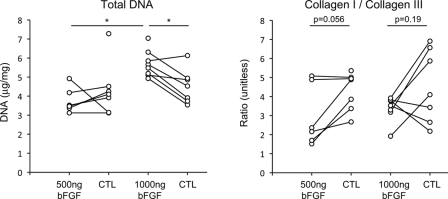
DNA concentration was significantly increased in tendons treated with 1000 ng bFGF compared with that in the tendons treated with 500 ng bFGF (p = 0.0002) or with that in the control group tendons (p = 0.011) (3.8 ± 0.7 μg/mg in the group treated with 500 ng bFGF compared with 4.3 ± 1.4 μg/mg in the paired control group; and 5.7 ± 0.7 μg/mg in the group treated with 1000 ng bFGF compared with 4.5 ± 0.9 μg/mg in the paired control group) (Fig. 3). DNA concentration in the 500-ng group was not significantly different from that in the paired control group (p = 0.85). These results indicate enhanced cellularity secondary to high-dose bFGF treatment during the early phase following injury and repair. However, bFGF-treated tendons had a lower ratio of type-I collagen to type-III collagen than the control tendons had, indicating increased scar-tissue formation due to the growth factor, but this difference did not reach significance (p = 0.056 for the group treated with 500 ng bFGF, and p = 0.19 for the 1000 ng bFGF group; 3.0 ± 1.6 in the group treated with 500 ng bFGF compared with 4.3 ± 1.0 in the paired control group, and 3.4 ± 0.6 in the 1000 ng bFGF group compared with 4.5 ± 1.9 in the paired control group; Fig. 3). There was no effect of bFGF dose on the ratio of type-I collagen to type-III collagen (p = 0.51). Cross-link maturity (i.e., the ratio of dihydroxylysinonorleucine to hydroxylysinonorleucine) was higher in the bFGF-treated tendons than it was in the control tendons, indicating enhanced remodeling due to bFGF, but this difference did not reach significance (p = 0.088 for the group treated with 500 ng bFGF and p = 0.34 for the group treated with 1000 ng bFGF: 1.6 ± 0.4 in the group treated with 500 ng bFGF compared with 1.2 ± 0.08 in the paired control group; and 1.3 ± 0.2 in the group treated with 1000 ng bFGF compared with 1.2 ± 0.3 in the paired control group). Cross-link maturity was higher in the group treated with 500 ng bFGF than it was in the group treated with 1000 ng bFGF, but this difference did not reach significance (p = 0.15). There were no apparent differences between groups when examining the percentage of collagen per dry weight of tissue (p = 0.51 for the group treated with 500 ng bFGF when compared with the paired control group, p = 0.32 for the group treated with 1000 ng bFGF when compared with the paired control group, and p = 0.21 for the group treated with 500 ng bFGF when compared with the group treated with 1000 ng bFGF: 72.2% ± 3.7% for the group treated with 500-ng bFGF compared with 73.9% ± 4.1% for the paired control group; 75.3% ± 4.9% for the group treated with 1000 ng bFGF compared with 74.8% ± 4.7% for the paired control group) (Table I).
Fig. 3.
DNA concentration was significantly increased in tendons that were treated with 1000 ng bFGF as compared with tendons treated with 500 ng bFGF and non-bFGF-treated (control group [CTL]) tendons (left plot). The bFGF-treated tendons had a lower (but not significantly different) ratio of type-I collagen to type-III collagen, suggesting increased scar formation due to the growth factor (right plot). All data are shown; paired data points are indicated by points connected with a line. Asterisks indicate a p value of <0.05.
Biomechanics
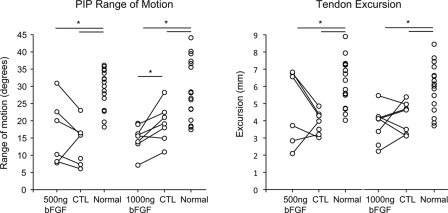
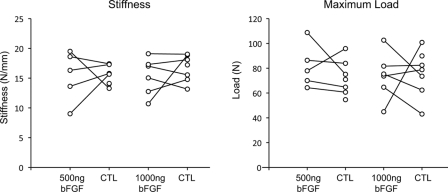
Proximal interphalangeal joint rotation and tendon excursion were significantly reduced in surgically repaired tendons as compared with normal tendons (p < 0.001 for the group treated with 500 ng bFGF and p < 0.001 for the group treated with 1000 ng bFGF). Proximal interphalangeal joint rotation was 16.6° ± 9.4° in the group treated with 500 ng bFGF, 13.4° ± 6.1° in the paired control group, and 29.2° ± 5.8° in the normal group; whereas it was 15.0° ± 3.8° in the group treated with 1000 ng bFGF, 19.3° ± 5.5° in the paired control group, and 29.0° ± 8.8° in the normal group. Tendon excursion was 4.8 ± 2.2 mm in the group treated with 500 ng bFGF, 3.9 ± 0.7 mm in the paired control group, and 6.2 ± 1.4 mm in the normal group; whereas it was 3.8 ± 1.0 mm in the group treated with 1000 ng bFGF, 4.2 ± 1.9 mm in the paired control group, and 5.9 ± 1.4 mm in the normal group (Fig. 4). Moreover, consistent with the increase in adhesions that was noted at the time of dissection, proximal interphalangeal joint rotation was significantly lower in the tendons treated with 1000 ng bFGF than it was in the tendons in the paired control group (p = 0.017) (Fig. 4). Proximal interphalangeal joint rotation in the group treated with 500 ng bFGF was not significantly different from that in the paired control group (p = 0.32) (Fig. 4). Dose had no significant effect on proximal interphalangeal joint rotation (p = 0.67) or tendon excursion (p = 0.26). There was no significant difference in tensile mechanical properties between the bFGF groups and their respective control groups (for the group treated with 500 ng bFGF, the p value was 0.84 for stiffness, 0.55 for maximum load, 0.61 for rigidity, and 0.99 for strain at 20 N; and for the group treated with 1000 ng bFGF, the p value was 0.44 for stiffness, 0.98 for maximum load, 0.30 for rigidity, and 0.67 for strain at 20 N), nor was there a significant difference in tensile mechanical properties between the group treated with 500 ng bFGF and the group treated with 1000 ng bFGF (p = 0.97 for stiffness, p = 0.51 for maximum load, p = 0.43 for rigidity, and p = 0.91 for strain at 20 N) (Fig. 5, Table I). Thus, the higher dose of bFGF was associated with impaired range of motion (a functional property), but neither dose of bFGF was associated with enhanced tendon strength.
Fig. 4.
Proximal interphalangeal (PIP) joint rotation was significantly lower in the tendons treated with 1000 ng bFGF as compared with that seen in the control (CTL) group (left plot). When compared with the normal (uninjured) group, all repair groups had a significantly lower range of motion (left plot) and tendon excursion (right plot). All data are shown; paired data points are indicated by points connected with a line. Asterisks indicate a p value of <0.05.
Fig. 5.
The results for stiffness (left plot) and maximum load (right plot) were not significantly different between the bFGF groups and their respective control (CTL) groups or between the low-dose and high-dose bFGF groups. All data are shown; paired data points are indicated by points connected with a line.
Discussion
Recent experimental studies have indicated that the flexor tendon repair site fails to accrue strength and stiffness in the early stages following suture repair1,16. Despite the use of multistrand suture repair and early controlled motion, a rupture rate of 13% and a repair-site elongation (gap) rate of 48% were noted in a recent experimental study16. These rates of failure are similar to those reported in clinical studies in which controlled passive-and-active-motion rehabilitation have been used1,17,18. Reasons for the delayed repair response include a relative paucity of tendon fibroblasts and minimal vascularization in zone II, which is a common region of tendon injury19,20. In an effort to stimulate collagen synthesis and to increase the strength and stiffness of the repair site in the early period after suturing, recent studies have explored the utility of the administration of exogenous growth factor, with PDGF-BB, bFGF, and transforming growth factor (TGF)-β showing the greatest promise for enhanced healing3-5,8,10. In experiments on dense, regular connective-tissue healing, bFGF was found to be particularly effective in promoting cellular proliferation, neovascularization, collagen production, and collagen organization in both tendon7,9,21 and ligament22.
Our choice of bFGF was further supported by studies that demonstrated that bFGF improves the repair process in operatively repaired flexor tendons. Tang et al. reported that tendon fibroblasts that were transfected with bFGF stimulated repair in sutured flexor tendons in chickens, with increased tensile strength values noted at two and four weeks after suturing the tendon9. Similarly, a study by Hamada et al. showed that the delivery of bFGF with use of coated sutures led to improved mechanical properties in a rabbit intrasynovial tendon repair model7. A different report noted increased cell proliferation and collagen production after administration of bFGF, but these effects were not associated with improved mechanical properties21. These divergent findings indicate that either the animal model or the delivery method, or both, significantly influence the effectiveness of bFGF treatment. Alternatively, bFGF may be too nonspecific or may have additional untoward effects (e.g., matrix metalloproteinase stimulation) that alter its impact on intrasynovial tendon repair. The goal of this study was to administer bFGF in a clinically applicable model of tendon repair with a well-characterized sustained delivery system with the aim of accelerating the healing process. Our hypothesis was only partly supported by our results; controlled delivery of bFGF promoted biologic activity, but this activity did not result in improvements in either the functional or structural properties of the repair.
In the current experiment, we used a clinically appropriate suture repair method, a controlled method of bFGF administration, and early passive mobilization in a canine model. The canine model was chosen because of its clinical relevance23 and for ease of comparison with our previous studies3-5. In this setting, although we found significant increases in biologic activity with bFGF treatment, we saw no improvements in tensile mechanical properties. Further, a reduction in digital range of motion was observed in the group that received the higher dose (1000 ng) of growth-factor treatment as compared with the group that received repair alone (the paired control group). In addition, notable increases in adhesions and vascularity were associated with either the high or the low dose of bFGF treatment. Evidence of neovascularization of the proximal tendon stump was apparent on blinded histologic evaluation of bFGF-treated tendons as compared with the evidence found in control tendons. This observation is consistent with the previously reported role of bFGF in the promotion of angiogenic activity in vivo and in vitro through a direct action on endothelial cells24,25. In prior studies, endothelial cells treated with bFGF showed increased cell proliferation, protease production, chemotaxis26, and the formation of capillary-like structures in collagen gels27. In our model, it is likely that, while bFGF promoted fibroblast proliferation and matrix synthesis, it also stimulated neovascularization in the region of the repair. Increased vascularity in this hypovascular region may be beneficial to the healing process; however, peritendinous endothelial cell stimulation may have contributed to adhesion formation and a subsequent decrease in digital range of motion.
The central objectives of the current study were to stimulate cellular proliferation and matrix production within the first few weeks following repair. Significant increases in cellularity and total DNA were observed, and these findings are consistent with the results of prior experimental studies7,21,28. Other research in which canine intrasynovial flexor tendon fibroblasts were used has shown that bFGF promotes cell proliferation in a dose-dependent manner29. Due to the paucity of cells in the uninjured flexor tendon, increased cell proliferation could enhance the healing process. However, the increases in cellularity that we observed did not facilitate repair, as evidenced by a failure to enhance mechanical properties and a deleterious effect on range of motion in the group that received the high dose of bFGF.
In addition to its positive effect on cellular replication, bFGF has been shown to enhance matrix production in tendon fibroblasts21,29. In one prior study, increases in collagen production by intrasynovial flexor tendon fibroblasts in vitro were seen with administration of bFGF29. However, recent data indicate that bFGF may downregulate collagen expression and may promote the secretion of matrix metalloproteinases (i.e., increase matrix degradation) in tendon and ligament fibroblasts30-32. In addition, evidence from a prior report21 and data from the current study indicate that the matrix that is produced as a result of bFGF administration has structural and compositional properties that are closer to scar tissue than to normal tendon tissue. Specifically, bFGF in the current study led to an increased ratio of type-III collagen to type-I collagen, which is indicative of lower-quality scar tissue. The organization of this collagen, as observed with the aid of polarized light microscopy, was haphazard and not well aligned, likely contributing little to the mechanical properties of the repair.
Two doses of bFGF were examined in this study. The lower dose promoted a small but measurable increase in biologic activity at the repair site. This increase in biologic activity, however, had no significant effect on the tensile mechanical properties of the tendon or the range of motion of the digit. A more substantial increase in biologic activity was promoted with the higher dose than with the lower dose. This increase in activity did not have a significant effect on the tensile mechanical properties and had a detrimental effect on the range of motion of the treated digit. Therefore, caution must be used when applying growth-factor treatments to intrasynovial tendon repair. Increased biologic activity may not necessarily improve the repair; on the contrary, increased activity may increase adhesions and reduce the range of motion of the treated digit.
One limitation of our study was that the comparison groups did not include all possible controls. We compared operative repair without treatment to operative repair in addition to treatment that included a heparin-based delivery of a growth factor. However, we showed in a previous study that the healing process in tendons containing a heparin-based delivery system does not differ from the healing process in tendons that are treated with repair alone5. Therefore, it is unlikely that there was an effect of the delivery system alone in the current study. A second limitation of our study is that the small sample size may have been insufficient to detect significant differences for some comparisons. Specifically, we observed an increase in range of motion, tendon excursion, and cross-link maturity in the low-dose bFGF group in comparison with controls, but these changes were not significant. It is possible that increasing the power of the statistical comparisons may reveal a significant beneficial effect of bFGF. However, even if the effects are statistically significant, the small increases in these measures are unlikely to be clinically relevant.
Important findings of this study include evidence of a profound biologic response that can be attributed to bFGF treatment in a clinically relevant canine model of intrasynovial flexor tendon injury and repair. While this finding supports our premise that bFGF administration leads to increased tendon cellularity and matrix synthesis, the effects were not associated with improved functional and mechanical properties of the repair. The results from this study imply the need for a growth factor that promotes a more specific response (e.g., an increase in type-I collagen without an increase in type-III collagen) than that promoted by bFGF. Furthermore, due to the paucity of cells in the healing flexor tendon, in addition to growth-factor treatment, a cell-based treatment strategy may be necessary to enhance the healing process in the early period after repair.
Supplementary Material
Acknowledgments
Note: The authors thank Dr. Necat Havlioglu for analyzing histologic sections, Mr. Fredrick Harwood for performing biochemical assays, and Ms. Cionne Manning for assisting in the biomechanical testing.
Footnotes
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the National Institutes of Health (R01 AR033097). In addition, one or more of the authors or a member of his or her immediate family received, in any one year, payments or other benefits of less than $10,000 (Wright Medical) and in excess of $10,000 or a commitment or agreement to provide such benefits from commercial entities (Medartis and Lippincott Williams & Wilkins).
References
- 1.Boyer MI, Goldfarb CA, Gelberman RH. Recent progress in flexor tendon healing. The modulation of tendon healing with rehabilitation variables. J Hand Ther. 2005;18:80-5 [DOI] [PubMed] [Google Scholar]
- 2.Khanna A, Friel M, Gougoulias N, Longo UG, Maffulli N. Prevention of adhesions in surgery of the flexor tendons of the hand: what is the evidence? Br Med Bull. 2009;90:85-109 [DOI] [PubMed] [Google Scholar]
- 3.Gelberman RH, Thomopoulos S, Sakiyama-Elbert SE, Das R, Silva MJ. The early effects of sustained platelet-derived growth factor administration on the functional and structural properties of repaired intrasynovial flexor tendons: an in vivo biomechanic study at 3 weeks in canines. J Hand Surg Am. 2007;32:373-9 [DOI] [PubMed] [Google Scholar]
- 4.Thomopoulos S, Zaegel M, Das R, Harwood FL, Silva MJ, Amiel D, Sakiyama-Elbert S, Gelberman RH. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25:1358-68 [DOI] [PubMed] [Google Scholar]
- 5.Thomopoulos S, Das R, Silva MJ, Sakiyama-Elbert S, Harwood FL, Zampiakis E, Kim HM, Amiel D, Gelberman RH. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. J Orthop Res. 2009;27:1209-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelberman RH, Amiel D, Harwood F. Genetic expression for type I procollagen in the early stages of flexor tendon healing. J Hand Surg Am. 1992;17:551-8 [DOI] [PubMed] [Google Scholar]
- 7.Hamada Y, Katoh S, Hibino N, Kosaka H, Hamada D, Yasui N. Effects of monofilament nylon coated with basic fibroblast growth factor on endogenous intrasynovial flexor tendon healing. J Hand Surg Am. 2006;31:530-40 [DOI] [PubMed] [Google Scholar]
- 8.Letson AK, Dahners LE. The effect of combinations of growth factors on ligament healing. Clin Orthop Relat Res. 1994;308:207-12 [PubMed] [Google Scholar]
- 9.Tang JB, Cao Y, Zhu B, Xin KQ, Wang XT, Liu PY. Adeno-associated virus-2-mediated bFGF gene transfer to digital flexor tendons significantly increases healing strength. An in vivo study. J Bone Joint Surg Am. 2008;90:1078-89 [DOI] [PubMed] [Google Scholar]
- 10.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381-94 [DOI] [PubMed] [Google Scholar]
- 11.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65:389-402 [DOI] [PubMed] [Google Scholar]
- 12.Thomopoulos S, Das R, Sakiyama-Elbert S, Silva MJ, Charlton N, Gelberman RH. bFGF and PDGF-BB for tendon repair: controlled release and biologic activity by tendon fibroblasts in vitro. Ann Biomed Eng. 2010;38:225-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winters SC, Gelberman RH, Woo SL, Chan SS, Grewal R, Seiler JG., 3rd The effects of multiple-strand suture methods on the strength and excursion of repaired intrasynovial flexor tendons: a biomechanical study in dogs. J Hand Surg Am. 1998;23:97-104 [DOI] [PubMed] [Google Scholar]
- 14.Silva MJ, Boyer MI, Ditsios K, Burns ME, Harwood FL, Amiel D, Gelberman RH. The insertion site of the canine flexor digitorum profundus tendon heals slowly following injury and suture repair. J Orthop Res. 2002;20:447-53 [DOI] [PubMed] [Google Scholar]
- 15.Boyer MI, Gelberman RH, Burns ME, Dinopoulos H, Hofem R, Silva MJ. Intrasynovial flexor tendon repair. An experimental study comparing low and high levels of in vivo force during rehabilitation in canines. J Bone Joint Surg Am. 2001;83:891-9 [PubMed] [Google Scholar]
- 16.Gelberman RH, Boyer MI, Brodt MD, Winters SC, Silva MJ. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. An experimental study on the early stages of tendon-healing in dogs. J Bone Joint Surg Am. 1999;81:975-82 [DOI] [PubMed] [Google Scholar]
- 17.May EJ, Silfverskiöld KL. Rate of recovery after flexor tendon repair in zone II. A prospective longitudinal study of 145 digits. Scand J Plast Reconstr Surg Hand Surg. 1993;27:89-94 [DOI] [PubMed] [Google Scholar]
- 18.Silfverskiöld KL, May EJ. Gap formation after flexor tendon repair in zone II. Results with a new controlled motion programme. Scand J Plast Reconstr Surg Hand Surg. 1993;27:263-8 [PubMed] [Google Scholar]
- 19.Gelberman RH, Khabie V, Cahill CJ. The revascularization of healing flexor tendons in the digital sheath. A vascular injection study in dogs. J Bone Joint Surg Am. 1991;73:868-81 [PubMed] [Google Scholar]
- 20.Lundborg G, Myrhage R, Rydevik B. The vascularization of human flexor tendons within the digital synovial sheath region–structural and functional aspects. J Hand Surg Am. 1997;2:417-27 [DOI] [PubMed] [Google Scholar]
- 21.Chan BP, Fu S, Qin L, Lee K, Rolf CG, Chan K. Effects of basic fibroblast growth factor (bFGF) on early stages of tendon healing: a rat patellar tendon model. Acta Orthop Scand. 2000;71:513-8 [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi D, Kurosaka M, Yoshiya S, Mizuno K. Effect of basic fibroblast growth factor on the healing of defects in the canine anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 1997;5:189-94 [DOI] [PubMed] [Google Scholar]
- 23.Potenza AD. Tendon healing within the flexor digital sheath in the dog. J Bone Joint Surg Am. 1962;44:49-64 [PubMed] [Google Scholar]
- 24.Friesel RE, Maciag T. Molecular mechanisms of angiogenesis: fibroblast growth factor signal transduction. FASEB J. 1995;9:919-25 [DOI] [PubMed] [Google Scholar]
- 25.Poole TJ, Finkelstein EB, Cox CM. The role of FGF and VEGF in angioblast induction and migration during vascular development. Dev Dyn. 2001;220:1-17 [DOI] [PubMed] [Google Scholar]
- 26.Gualandris A, Urbinati C, Rusnati M, Ziche M, Presta M. Interaction of high-molecular-weight basic fibroblast growth factor with endothelium: biological activity and intracellular fate of human recombinant M(r) 24,000 bFGF. J Cell Physiol. 1994;161:149-59 [DOI] [PubMed] [Google Scholar]
- 27.Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci USA. 1986;83:7297-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takayama S, Murakami S, Miki Y, Ikezawa K, Tasaka S, Terashima A, Asano T, Okada H. Effects of basic fibroblast growth factor on human periodontal ligament cells. J Periodontal Res. 1997;32:667-75 [DOI] [PubMed] [Google Scholar]
- 29.Thomopoulos S, Harwood FL, Silva MJ, Amiel D, Gelberman RH. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg Am. 2005;30:441-7 [DOI] [PubMed] [Google Scholar]
- 30.Fukui N, Katsuragawa Y, Sakai H, Oda H, Nakamura K. Effect of local application of basic fibroblast growth factor on ligament healing in rabbits. Rhum Engl Rev Ed. 1998;65:406-14 [PubMed] [Google Scholar]
- 31.Palmon A, Roos H, Edel J, Zax B, Savion N, Grosskop A, Pitaru S. Inverse dose- and time-dependent effect of basic fibroblast growth factor on the gene expression of collagen type I and matrix metalloproteinase-1 by periodontal ligament cells in culture. J Periodontol. 2000;71:974-80 [DOI] [PubMed] [Google Scholar]
- 32.Silverio-Ruiz KG, Martinez AE, Garlet GP, Barbosa CF, Silva JS, Cicarelli RM, Valentini SR, Abi-Rached RS, Junior CR. Opposite effects of bFGF and TGF-beta on collagen metabolism by human periodontal ligament fibroblasts. Cytokine. 2007;39:130-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.