Abstract
Objective
A range of behavioral and psychosocial factors may contribute to a chronically stimulated hypo-thalamic-pituitary-adrenal (HPA) axis and subsequently altered diurnal patterns. The goal of this cross-sectional study was to examine associations among diurnal cortisol levels, perceived stress, and obesity patterns.
Methods
Seventy-eight women (aged 24–72 years) employed in a rural public school system completed the perceived stress scale, collected diurnal saliva samples, and underwent anthropometric assessments. Reduced peak-to-nadir cortisol values across the day were considered a sign of impairment in HPA function. A series of linear regression models determined the best predictors of diurnal cortisol variation.
Results
There was a marginal linear trend in stress levels across body mass index (BMI) categories, with obese women reporting the highest levels of stress (p = 0.07). Perceived stress was the only significant predictor of the degree of flattening of the diurnal cortisol curve in the sample as a whole (β = −0.042, R2 = 0.11, F = 8.6, p = 0.005), indicating reduction in the normal diurnal pattern. Among overweight women (BMI = 25–29.9 kg/m2), stress and waist circumference combined predicted 35% of the variability in diurnal cortisol. In contrast, among obese women (BMI ≥ 30 kg/m2), BMI predicted 31% of the variability in diurnal cortisol (F = 13.8, p = 0.001), but stress was no longer significantly related to diurnal cortisol.
Conclusions
Psychological stress predicts a significant portion of HPA axis functioning. In overweight women, perceived stress and waist circumference were of approximately equal importance in predicting adrenal cortisol secretion. However, among obese women, a major portion of the diurnal cortisol variation was predicted by BMI alone, not stress or waist circumference. This may help elucidate the mechanisms linking obesity to increased risk of cardiovascular disease (CVD).
Introduction
Approximately 300,000 adults die each year of obesity-related causes in the United States.1 Accordingly, it became one of the leading health indicators used to measure progress toward the Healthy People 2010 goals.2 Obesity is a major preventable cardiovascular disease (CVD) risk factor, as it contributes to increases in blood pressure (BP), glucose, triglycerides, and low-density lipoprotein cholesterol (LDL-C) and decreases in high-density lipoprotein (HDL-C).3 The pervasive increase in the prevalence of obesity, both in the United States and worldwide, makes it imperative that we understand the connection between obesity and physiological dysregulation.
The hypothalamic-pituitary-adrenal (HPA) axis plays a central role in the regulation of energy metabolism through the actions of the glucocorticoids.4 Cortisol has a circadian rhythm, with a pronounced peak around the time of awakening and a nadir during the first half of the sleep cycle. This morning peak acts as a “master clock”5 that provides a circadian signal to the cells of the body, allowing cortisol to regulate gene expression in many cell types and to entrain their activity. A reduction in the quality of this signal, seen as a smaller peak-nadir difference, represents a degraded diurnal signal to the body that may indicate a reduction in integrated systems function. Disturbances in this circadian rhythm are seen in diabetes and hypertension, and they are detectable before clinical manifestations of these disorders.6,7
Obesity itself is associated with disturbances in HPA axis function, leading to lower morning plasma levels of cortisol and, hence, a blunted diurnal variation.8,9 Although obesity may contribute to cortisol dysregulation, it is also likely that stress-induced elevations of cortisol may contribute to a tendency to overeat, which in turn contributes to a cascade starting with obesity and ending with type 2 diabetes and CVD. Dallman et al.10 proposed a mechanism by which food intake is rewarded during periods of stress because eating becomes associated, through negative feedback, with a reduction of central corticotropin-releasing factor (CRF) activation, a core component of the stress response. Under Dallman's model, excessive cortisol secretion resulting from frequent activation of the HPA axis, either by extrinsic stressors or intrinsic factors may contribute to obesity and type 2 diabetes.11
Dysregulation of the HPA axis has been documented in individuals exposed to chronic stress.12 The Whitehall II Study found a higher incidence of obesity in people reporting higher levels of job stress.13 The majority of studies examining stress-HPA axis relationships have either focused on job stress alone13 or treated body mass index (BMI) as a confounding variable and controlled for it rather than examining its independent relationship to stress and HPA axis function. In a review of the findings from several of their studies, Bjorntorp and Rosmond14 describe the neuroendocrine abnormalities associated with visceral obesity and report a decreased cortisol variability in relation to increased abdominal obesity. Accordingly, we collected data on perceived stress, obesity, and diurnal cortisol levels in a sample of women employed at a rural public school system. We hypothesized that obese women would report higher perceived stress levels, would have worse hemodynamic and lipid profiles, and would have a smaller peak-nadir cortisol difference on a workday compared with normal weight individuals.
Materials and Methods
Study sample
This study was performed as part of a larger community-based research study that involved a worksite wellness program in a rural public school system, in which 202 employees participated. There were no inclusion/exclusion criteria, and all school employees were offered the opportunity to participate. The wellness program consisted of a biannual screening for CVD risk factors with an intervening physical activity promotion program. Stress and cortisol assessments were offered to program participants during baseline screening. Seventy-eight women (average age 46 years) agreed to complete the stress and cortisol assessments. Compared with the 202 participants in the larger study, responders to the stress portion were not significantly different in BMI or age. Eighty-five percent of the 78 women who participated in the stress portion of the study were Caucasian, and 70% had at least a college level education. Sixty percent of participants were teachers, and 79% were married. Only 5 women reported being smokers. Data on medication use and comor-bidities were collected as present vs. absent without details on formulation or dosage of medications. All assessments took place on a workday after an overnight fast. The study was approved by the University of Oklahoma Institutional Review Board.
Salivary cortisol assessment
Participants received Salivette devices (Sarstedt, Rommeldorf, Germany) composed of cotton swabs in a plastic holder fitted inside a centrifuge tube, as well as detailed instructions for producing samples. Participants collected seven saliva samples on a regular workday. The first two samples were morning samples (wakeup, wakeup + 40 minutes), two samples were collected during school hours (11:00 am and 2:00 pm), and the last three samples were collected at home (6:00 pm, 9:00 pm, and bedtime). After completion of saliva collection, participants hand-delivered the Salivettes to the study staff. Participants were not required to refrigerate the samples during collection but rather to keep them at room temperature. Salivary cortisol levels have been shown to be stable at room temperature and under stored, mailed, and frozen conditions.15 The Salivettes were centrifuged at 3000 rpm at +4°C for 15 minutes when received, and the filtrates were collected in prelabeled tubes and stored at −80°C until assayed.
Commercial salivary cortisol (enhanced range) enzyme immunoassay kits were purchased from Salimetrics LLC (State College, PA). Samples were thawed, vortexed, and centrifuged at 1500g or 3000 rpm for 15 minutes to sediment particulate matter. Free cortisol in saliva was measured by a competitive protein-binding enzyme immunoassay. The intraassay and interassay coefficients of variation (CVs) were 0.02%–2.6% and 3.1%–7.6%, respectively.
Anthropometric assessments
Subjects stood barefoot during all anthropometric assessments. Body weight was measured to the nearest 0.1 kg on a digital scale. Height was measured to the nearest 0.1 cm using a portable stadiometer (Perspective Enterprises, Portage, MI). BMI was calculated as weight in kg divided by the square of height in meters. Participants were classified as normal weight if 18.5 ≤ BMI ≤ 24.9, as overweight if 25 ≤ BMI ≤ 29.9, and as obese if BMI ≥ 30 kg/m2. Waist circumference was measured by a tape measure at the midpoint between the upper iliac crest and lower costal margin in the midaxillary line.
Blood pressure and lipid assessments
BP and heart rate were measured using an automated device (HEM-907, Omron Healthcare Inc., Vernon Hills, IL). Two measurements were taken, using an appropriate size cuff, 1-minute apart after a 3-minute seated rest. After an overnight fast, capillary whole blood specimens were obtained by fingerstick method using heparin-coated capillary tubes. The samples were analyzed using the Cholestech LDX system (Cholestech, Hayward, CA) immediately after sample collection. Samples were analyzed for total cholesterol, HDL-C, LDL-C, VLDL-C, triglyceride, and glucose levels.
Psychosocial assessments
Levels of psychological stress were assessed using the perceived stress scale (PSS).16 The 10-item scale assesses feelings and thoughts during the last month. The PSS measures the degree to which situations in one's life are perceived as stressful. It is more strongly related to life event impact scores as opposed to the number of stressful events, thereby representing one's appraisal of the events as being stressful.17 Coefficient alpha reliability for the PSS ranged from 0.84 to 0.86, and test-retest correlation is 0.85.17 The participants were asked to rate their feelings on a scale of 0–4, with 0 indicating never and 4 indicating very often. The PSS scores range from 0 to 40, with higher scores indicating higher levels of stress. The Hollingshead four-factor index was used to assess socioeconomic status (SES).18 Participants are assigned an education score of 1–7, with 1 equal to less than a seventh grade education and 7 equal to graduate training, and an occupation score of 1–9 based on occupation category. Education and occupation scores are then weighted to obtain a single score that ranges from 8 to 66.18
Statistical analyses
Because it was not possible to sample the absolute daily cortisol nadir using the Salivette device, we represented an evening level by averaging the 9:00 pm and bedtime values. Diurnal cortisol variation was calculated by subtracting the nadir cortisol values (average of 9 pm and bedtime values) from the peak cortisol values (awake + 40 min). Cortisol values were skewed, and accordingly the log transformed cortisol values were used in all analyses. Initial analyses included one-way ANOVA with linear contrasts to examine differences in sociodemographic and physiological variables by BMI category. Bonferroni corrections were used to adjust for multiple comparisons.
Loess fitting was used to examine the relationship between diurnal cortisol variation and BMI. We generated a series of univariate and multivariate linear regression models using the independent variables to determine the model that best predicts diurnal cortisol variation. These independent variables were used in the larger model that included all BMI categories as well as in the separate models stratified by BMI category. Distance weighted least squares was used to illustrate the 3-dimensional relationship among BMI, perceived stress, and diurnal cortisol variation. Two-tailed tests were used for all analyses, and significance level was set at α < 0.05. Data were analyzed using SPSS (SPSS for Windows, rel.10.1.0, SPSS, Chicago, IL) and STATISTICA (StatSoft Inc., version 6, Tulsa, OK).
Results
Differences in sociodemographic and physiological variables by BMI category are displayed in Table 1. There was a significant linear trend in triglycerides (p = 0.006), HDL-C (p < 0.0001), VLDL-C (p = 0.03), glucose (p = 0.003), and diastolic BP (p = 0.05). Obese individuals had the highest triglycerides, VLDL-C, glucose, and diastolic BP and the lowest HDL-C levels. There was a marginal linear trend in perceived stress levels by BMI category (p = 0.07), with obese women reporting the highest levels of stress.
Table 1.
Differences in Sociodemographic and Physiological Variables by BMI Category
| Mean ± SD | Normal weight n = 29 | Overweight n = 26 | Obese n = 23 | pa |
|---|---|---|---|---|
| Age (years) | 45 ± 10 | 46 ± 9 | 46 ± 9 | 0.6 |
| Triglycerides (mg/dL)b | 95 ± 68 | 116 ± 65 | 154 ± 90 | 0.006 |
| Total cholesterol (mg/dL) | 193 ± 28 | 181 ± 29 | 195 ± 34 | 0.9 |
| HDL-C (mg/dL)b | 67 ± 13 | 59 ± 13 | 45 ± 9 | <0.0001 |
| LDL-C (mg/dL) | 107 ± 27 | 101 ± 26 | 120 ± 29 | 0.1 |
| VLDL-C (mg/dL) | 21 ± 14 | 25 ± 13 | 29 ± 10 | 0.03 |
| Glucose (mg/dL)b | 84 ± 10 | 86 ± 11 | 98 ± 25 | 0.003 |
| Systolic BP (mm Hg) | 122 ± 16 | 126 ± 171 | 122 ± 17 | 0.8 |
| Diastolic BP (mm Hg) | 74 ± 8 | 78 ± 13 | 80 ± 9 | 0.05 |
| Heart rate (beats/min) | 71 ± 11 | 76 ± 12 | 77 ± 11 | 0.1 |
| Perceived stress | 16.9 ± 6.1 | 16.7 ± 6.3 | 20.0 ± 6.1 | 0.07 |
| Socioeconomic status | 49 ± 11.1 | 51 ± 10.3 | 50 ± 11.3 | 0.7 |
| Diurnal cortisol variation (nmol/L)c | 2.5 ± .74 | 2.4 ± .66 | 2.2 ± .88 | 0.2 |
p for linear trend.
p < 0.05 for between-subjects F statistic (one-way ANOVA) with Bonferroni adjustment for multiple comparisons.
Cortisol values are log transformed.
Predictors of diurnal cortisol variation
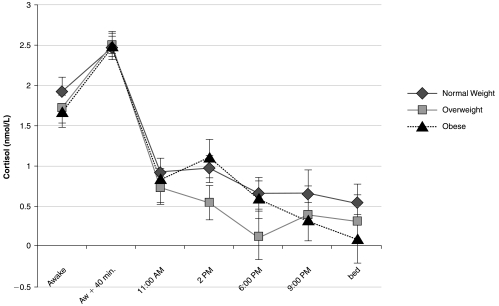
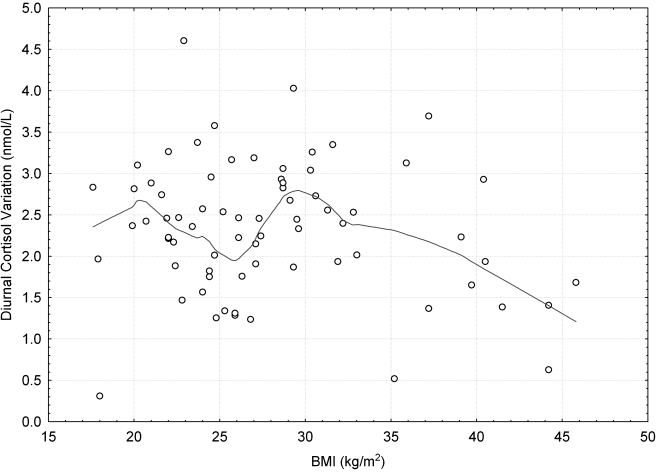
The diurnal pattern of cortisol by BMI category is shown in Figure 1. There were no significant differences in cortisol levels by BMI category at any of the seven time points. A series of linear regression models was used to determine the predictors of diurnal cortisol variation. Univariate and multivariate regression were used to model the independent variables—perceived stress, BMI, waist circumference, age, and SES—singly and in combination on the dependent variable diurnal cortisol variation. We were interested in identifying the model that best predicted diurnal cortisol variation. The final models are presented in Table 2. Perceived stress was the only significant predictor of diurnal cortisol variation. Waist circumference, BMI, age, and SES were not significant predictors of diurnal cortisol variation (Table 3). None of the interaction effects were significant. Loess fitting (Fig. 2) identified a nonlinear relationship between BMI and diurnal cortisol variation, but a threshold effect was observed at BMI = 30. Consequently, we stratified by BMI and used separate regression models to predict diurnal cortisol variation within each BMI category, as subsequently described.
FIG. 1.
Mean ± SEM of diurnal cortisol levels across the workday by BMI category. One-way ANOVA showed no statistically. significant differences in cortisol levels by BMI category at any of the points across the workday.
Table 2.
Predictors of Diurnal Cortisol Variationa
| |
|
Stress |
BMI |
Waist |
|
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Intercept | β | SE | β | SE | β | SE | R2 | p | |
| All subjects | 3.079 | −0.042 | 0.014 | — | — | — | — | 0.11 | 0.005 |
| Normal weight | 3.047 | −0.033 | 0.024 | — | — | — | — | 0.8 | 0.17 |
| Overweight | 0.488 | −0.045 | 0.023 | — | — | 0.029 | 0.016 | 0.35 | 0.02 |
| Obese | 5.861 | — | — | −0.099 | 0.027 | — | — | 0.31 | 0.011 |
The dependent variable was diurnal cortisol variation. The independent variables (stress, BMI, waist circumference, age, and SES) were tested in univariate and multivariate models, and the final models that provided the best fit are presented here.
Table 3.
Univariate Relationships between Age and Socioeconomic Status (SES) and Diurnal Cortisol Variation
| |
Age |
SES |
||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | R2 | p | β | SE | R2 | p | |
| All subjects | −0.003 | 0.008 | 0.001 | 0.8 | −0.002 | 0.008 | 0.001 | 0.8 |
| Normal weight | 0.002 | 0.015 | 0.001 | 0.9 | −0.023 | 0.02 | 0.06 | 0.2 |
| Overweight | −0.015 | 0.015 | 0.04 | 0.3 | 0.019 | 0.01 | 0.1 | 0.09 |
| Obese | 0.009 | 0.02 | 0.013 | 0.5 | −0.018 | 0.014 | 0.06 | 0.19 |
FIG. 2.
Loess fitted relationship between BMI and diurnal cortisol variation across BMI categories.
Predictors of diurnal cortisol variation by BMI category
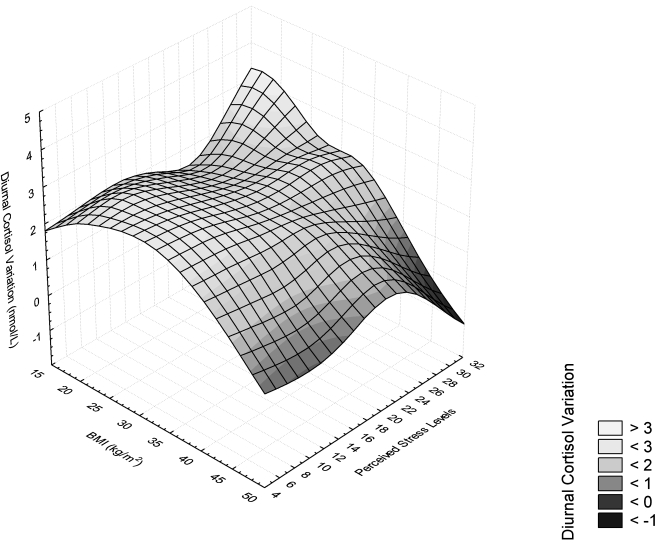
Among normal weight women, the only predictor variable that approached significance was perceived stress (p = 0.17) (Table 2). Among overweight women, perceived stress was a significant predictor of diurnal cortisol variation (R2 = 0.20, β = −0.053, t = −2.252, p = 0.04). However, waist circumference increased the ability of the model to predict diurnal cortisol variation (adjusted model R2 = 0.35). In contrast, among obese women, there was no effect of perceived stress on diurnal cortisol variation, and BMI predicted a significant portion of the variability in diurnal cortisol variation (R2 = 0.31). In a univariate model in the obese group, waist circumference predicted diurnal cortisol variation (R2 = 0.15, β = −0.028, t = −2.24, p = 0.03). When waist circumference was included in the same model with BMI, however, only BMI remained as a significant predictor of diurnal cortisol variation, and waist circumference did not contribute significantly to the model (adjusted model R2 = 0.33). In addition, there was no BMI × waist circumference interaction. The relationship among BMI, perceived stress, and diurnal cortisol variation is shown in Figure 3. There were no significant effects of age or SES on diurnal cortisol variation among any of the BMI categories.
FIG. 3.
Distance weighted least squares relationship among perceived stress, BMI, and diurnal cortisol variation.
Effect of age and medications
Because of the wide range of ages (24–72 years), we explored the effect of age on our models. We also explored the effect of SES on diurnal cortisol variation. Neither age nor SES predicted diurnal cortisol variation in univariate or multivariate models. The univariate associations between age and SES are displayed in Table 3. It is worth noting that only 6 women were above 55 years in this sample. We also considered the effect of medications and existing comorbidities, such as high blood pressure and hypertriglyceridemia. None of these factors were significant predictors of diurnal cortisol variation, individually or with any other variables.
Discussion
Perceived stress predicted impairment of normal diurnal cortisol rhythm, seen in a reduced morning to evening cortisol difference in the sample as a whole. Stress predicted approximately 11% of the variability in diurnal cortisol variation in this sample of working women across the range of BMIs. This is consistent with findings from other studies that have demonstrated an association between stress and diurnal cortisol levels.19,20 A normal HPA axis function is characterized by high morning and low evening values. Cortisol levels during the waking hours are regulated primarily by diurnal and metabolic factors. On the other hand, the peak and nadir cortisol levels are hypothalamically driven; hence, the value of examining peak-nadir difference as an index of the functional status of the HPA axis.21 Chronic stress, with its characteristic repeated and prolonged cortisol peaks, causes a rigid cortisol secretion pattern with reduced daily variation. Thus, the peak-nadir difference in cortisol levels is smaller in individuals exposed to high levels of chronic stress.22 The resultant loss of the magnitude of the peak-nadir variation is seen as a degraded signal and an indicator of HPA axis dysfunction. This dysregulated HPA axis pattern has been implicated in the observed relationship between cortisol secretion and risk factors for CVD and type 2 diabetes.23,24
Low SES has been found to be associated with chronically elevated cortisol levels.25,26 This social gradient may be a result of chronic stress associated with difficult living conditions or lifestyle behaviors, such as diet and physical inactivity.27,28 In this study, SES was not related to diurnal cortisol variation. However, approximately 70% of our sample had at least a college education, and this restricted range of social gradient may have limited our ability to detect differences in obesity and cortisol measures by SES.
Predictors of diurnal cortisol variation differed by BMI category. Perceived stress accounted for 20% of the variability in diurnal cortisol variation in overweight women. None of the variables examined in this study were significant predictors of diurnal cortisol variation in normal weight women. In overweight women, waist circumference predicted an additional 15% of the variability in diurnal cortisol variation over and above the effect of perceived stress. Similar to normal weight women, BMI was not a significant predictor of diurnal cortisol variation in overweight women. In contrast, despite higher reported levels of perceived stress, HPA axis function in obese women was more strongly related to BMI. Approximately 31% of the variability in diurnal cortisol variation in obese women was accounted for by BMI. It is possible that the metabolic consequences of obesity outweigh neuroendocrine changes associated with stress and are thus stronger predictors of HPA axis function in obese women. High job stress levels have been linked to subsequent increases in BMI in the Whitehall II study.13 This may be mediated by HPA functioning, which is known to be altered in relation to chronic stress.20,29 Although we cannot establish causal pathways from this cross-sectional analysis, it is possible that higher stress levels caused altered HPA axis function, which in turn contributed to increases in BMI. Once the BMI threshold of obesity is reached, however, HPA axis function appears to be primarily driven by the metabolic and neuroendocrine consequences of obesity. These findings should be interpreted with caution because of the bidirectional nature of the relationship between BMI and HPA axis function. It is also plausible that a dysfunctional HPA axis, perhaps genetically mediated,30 caused obesity and that higher levels of stress are a consequence of social stigma associated with obesity.
A key component of obesity is intra-abdominal accumulation of fat, which is responsible for a great portion of the increased CVD risk associated with obesity.31 The high concentration of glucocorticoid receptors in the abdominal region32 may be responsible for the observed association between high cortisol levels and abdominal obesity. Waist circumference is a convenient surrogate for abdominal obesity. In this study, waist circumference accounted for 15% of the variability in diurnal cortisol variation in overweight and obese women alike. However, this relationship did not persist in obese women when waist circumference was included with BMI in the same model. Only BMI remained as a significant predictor of cortisol variation in obese women. Janssen et al.33 reported that BMI and waist circumference independently predict intra-abdominal obesity, with BMI being more strongly related to abdominal subcutaneous fat. Abdominal subcutaneous fat is a significant predictor of insulin resistance,34 a condition associated with HPA axis dysfunction. Therefore, although we were unable to detect an association between waist circumference and cortisol variation in obese women, this does not negate a relationship between abdominal obesity and HPA dysfunction because BMI is a useful surrogate for subcutaneous abdominal fat. Although the National Institutes of Health guidelines on the evaluation of obesity recommend measurement of waist circumference in overweight individuals, in obese individuals with a BMI ≥ 35 kg/m2, waist circumference is found to lose its disease predictive power.35 This is in line with our findings of a relationship between HPA function and waist circumference in overweight women only, whereas only BMI was related to HPA axis function in obese women.
The regulation of cortisol levels in obesity may result from either increased secretion10 or prereceptor metabolism by 11β-hydroxysteroid dehydrogenase type 1 (HSD-1).36 The activity of HSD-1 is increased in abdominal fat depots of obese individuals.37 HSD-1 converts cortisone to active cortisol, which results in detection of increased glucocorticoid levels by the liver, which in turn causes feedback inhibition of adrenal cortisol secretion.38 This, in addition to increased cortisol excretion in obesity,39 may explain the lower absolute levels of cortisol seen in obese people.
Study limitations
A few limitations of this study are worth noting. The cross-sectional design limits our ability to ascribe any causal relationships to the observed associations. To address this, we are proposing a follow-up study of this sample to determine the prospective relationships among stress, obesity, and HPA axis dysfunction. In addition, the small sample sizes in subgroup analyses may have reduced the power to detect differences; thus, the current results should be interpreted with caution and replicated in larger studies. We did not observe an interaction between BMI and stress on diurnal cortisol in the overall model. This may be due to the lack of power caused by the large number of variables in the interaction model. The large difference in BMI range across the BMI categories is worth noting. The range of BMI in the obese category is large compared to the normal and overweight categories. However, when we truncated the obese BMI range so that it is equal to the range for the normal/overweight BMI category (range of 12.5), there still existed a stronger linear relationship with cortisol variation than the normal/overweight class (R2 = 0.24, t = −2.9, p = .008). Figure 2 provides evidence of this relationship. There seems to be a graphic absence of a linear relationship between BMI and cortisol within the normal through overweight categories, although it becomes quite apparent in the obese category even with a BMI range restriction of 30–42.5 kg/m2.
Our ability to generalize these findings across racial and sex groups is limited by the fact that our sample consisted of primarily Caucasian women. Other studies have documented clear sex differences in perceptions of stress,40 neuroendocrine responses to stress,29,41 and neuroendocrine effects of differential body fat distribution.42 Sex and racial differences in stress responses,12,43 diurnal cortisol rhythm,25 and CVD mortality44 have also been documented. Future studies should contrast the effects of stress and obesity on HPA axis function in men and women, taking into account the racial distribution of disease outcomes, such as insulin resistance and type 2 diabetes, which are more common in African Americans and Native Americans than in Caucasians.45,46 Psychological comorbidities have been shown to affect cortisol secretion patterns.47,48 We cannot address this issue in our study because we did not collect data on psychological comorbidities. Another possible limitation of this study is that salivary cortisol measures were taken on a single workday. It has been shown that several daily measurements of cortisol are needed to reliably assess cortisol secretion patterns.49 Although we instructed our participants to collect samples on what they considered to be a typical workday, repeated saliva sampling over several days may have reduced random error in the change scores and increased the reliability of cortisol assessments.
Conclusions
Psychological stress predicted a significant portion of HPA axis function. The nature of this relationship appears to be altered by indicators of overweight and obesity. In overweight women, perceived stress and waist circumference were approximately of equal importance in predicting diurnal patterns in cortisol secretion. In contrast, among obese women, a major portion of the diurnal cortisol variation was predicted by BMI alone, not stress or waist circumference. This may help elucidate the mechanisms linking obesity to increased risk of CVD. The causal relationships among perceived stress, obesity, and HPA function remain to be elucidated.
Disclosure Statement
No competing financial interests exist.
References
- 1.Allison DB. Fontaine KR. Manson JE. Stevens J. VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530–1538. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- 2.Healthy people 2010. Leading health indicators. www.healthypeople.gov/LHI/Priorities.htm www.healthypeople.gov/LHI/Priorities.htm
- 3.Van Gaal LF. Mertens IL. De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 4.Hiller-Sturmhofel S. Bartke A. The endocrine system: An overview. Alcohol Health Res World. 1998;22:153–164. [PMC free article] [PubMed] [Google Scholar]
- 5.Buijs RM. van Eden CG. Goncharuk VD. Kalsbeek A. The biological clock tunes the organs of the body: Timing by hormones and the autonomic nervous system. J Endocrinol. 2003;177:17–26. doi: 10.1677/joe.0.1770017. [DOI] [PubMed] [Google Scholar]
- 6.Borghi C. Costa FV. Boschi S. Mussi A. Ambrosioni E. Predictors of stable hypertension in young borderline subjects: A five-year follow-up study. J Cardiovasc Pharmacol. 1986;8(Suppl 5):S138–S141. doi: 10.1097/00005344-198608005-00030. [DOI] [PubMed] [Google Scholar]
- 7.Eilam R. Malach R. Bergmann F. Segal M. Hypertension induced by hypothalamic transplantation from genetically hypertensive to normotensive rats. J Neurosci. 1991;11:401–411. doi: 10.1523/JNEUROSCI.11-02-00401.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosmond R. Dallman MF. Bjorntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998;83:1853–1859. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- 9.Walker BR. Soderberg S. Lindahl B. Olsson T. Independent effects of obesity and cortisol in predicting cardiovascular risk factors in men and women. J Intern Med. 2000;247:198–204. doi: 10.1046/j.1365-2796.2000.00609.x. [DOI] [PubMed] [Google Scholar]
- 10.Dallman MF. la Fleur SE. Pecoraro NC. Gomez F. Houshyar H. Akana SF. Minireview: Glucocorticoids—Food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145:2633–2638. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]
- 11.Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30:1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Kudielka BM. Kirschbaum C. Sex differences in HPA axis responses to stress: A review. Biol Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Brunner EJ. Chandola T. Marmot MG. Prospective effect of job strain on general and central obesity in the Whitehall II Study. Am J Epidemiol. 2007;165:828–837. doi: 10.1093/aje/kwk058. [DOI] [PubMed] [Google Scholar]
- 14.Bjorntorp P. Rosmond R. Neuroendocrine abnormalities in visceral obesity. Int J Obes Rel Metab Disord. 2000;24(Suppl 2):S80–S85. doi: 10.1038/sj.ijo.0801285. [DOI] [PubMed] [Google Scholar]
- 15.Clements AD. Parker CR. The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- 16.Cohen S. Williamson GM. Perceived stress in a probability sample of the United States. In: Spacapan S, editor; Oskamp S, editor. The social psychology of health. Beverly Hills, CA: Sage Publications; 1998. pp. 31–67. [Google Scholar]
- 17.Cohen S. Kamarck T. Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 18.Cirino PT. Chin CE. Sevcik RA. Wolf M. Lovett M. Morris RD. Measuring socioeconomic status: Reliability and preliminary validity for different approaches. Assessment. 2002;9:145–155. doi: 10.1177/10791102009002005. [DOI] [PubMed] [Google Scholar]
- 19.Powell LH. Lovallo WR. Matthews KA, et al. Physiologic markers of chronic stress in premenopausal, middle-aged women. Psychosom Med. 2002;64:502–509. doi: 10.1097/00006842-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Sjogren E. Leanderson P. Kristenson M. Diurnal saliva cortisol levels and relations to psychosocial factors in a population sample of middle-aged Swedish men and women. Int J Behav Med. 2006;13:193–200. doi: 10.1207/s15327558ijbm1303_2. [DOI] [PubMed] [Google Scholar]
- 21.Lovallo WR. Stress and health: Biological and psychological interactions. Thousand Oaks: Sage Publications; 2005. [Google Scholar]
- 22.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 23.Rosmond R. Bjorntorp P. The interactions between hypothalamic-pituitary-adrenal axis activity, testosterone, insulin-like growth factor I and abdominal obesity with metabolism and blood pressure in men. Int J Obes Rel Metab Disord. 1998;22:1184–1196. doi: 10.1038/sj.ijo.0800745. [DOI] [PubMed] [Google Scholar]
- 24.Rosmond R. Stress induced disturbances of the HPA axis: A pathway to type 2 diabetes? Med Sci Monit. 2003;9:RA35–39. [PubMed] [Google Scholar]
- 25.Cohen S. Schwartz JE. Epel E. Kirschbaum C. Sidney S. Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- 26.Wright CE. Steptoe A. Subjective socioeconomic position, gender and cortisol responses to waking in an elderly population. Psychoneuroendocrinology. 2005;30:582–590. doi: 10.1016/j.psyneuen.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Adler NE. Boyce T. Chesney MA, et al. Socioeconomic status and health. The challenge of the gradient. Am Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 28.Feldman PJ. Steptoe A. How neighborhoods and physical functioning are related: The roles of neighborhood socioeconomic status, perceived neighborhood strain, and individual health risk factors. Ann Behav Med. 2004;27:91–99. doi: 10.1207/s15324796abm2702_3. [DOI] [PubMed] [Google Scholar]
- 29.Takai N. Yamaguchi M. Aragaki T. Eto K. Uchihashi K. Nishikawa Y. Gender-specific differences in salivary bio-marker responses to acute psychological stress. Ann NY Acad Sci. 2007;1098:510–515. doi: 10.1196/annals.1384.014. [DOI] [PubMed] [Google Scholar]
- 30.Rosmond R. Chagnon YC. Chagnon M. Perusse L. Bouchard C. Bjorntorp P. A polymorphism of the 5′-flanking region of the glucocorticoid receptor gene locus is associated with basal cortisol secretion in men. Metabolism. 2000;49:1197–1199. doi: 10.1053/meta.2000.7712. [DOI] [PubMed] [Google Scholar]
- 31.Sharma AM. Adipose tissue: A mediator of cardiovascular risk. Int J Obes Rel Metab Disord. 2002;26(Suppl 4):S5–7. doi: 10.1038/sj.ijo.0802210. [DOI] [PubMed] [Google Scholar]
- 32.Rebuffe-Scrive M. Bronnegard M. Nilsson A. Eldh J. Gustafsson JA. Bjorntorp P. Steroid hormone receptors in human adipose tissues. J Clin Endocrinol Metab. 1990;71:1215–1219. doi: 10.1210/jcem-71-5-1215. [DOI] [PubMed] [Google Scholar]
- 33.Janssen I. Heymsfield SB. Allison DB. Kotler DP. Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr. 2002;75:683–688. doi: 10.1093/ajcn/75.4.683. [DOI] [PubMed] [Google Scholar]
- 34.Abate N. Garg A. Peshock RM. Stray-Gundersen J. Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest. 1995;96:88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clinical guidelines on the identification, evaluation, treatment of overweight, obesity in adults: Executive summary. Expert panel on the identification, evaluation, and treatment of overweight in adults. Am J Clin Nutr. 1998;68:899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 36.Rask E. Olsson T. Soderberg S, et al. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab. 2001;86:1418–1421. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- 37.Stewart PM. Boulton A. Kumar S. Clark PM. Shackleton CH. Cortisol metabolism in human obesity: Impaired cortisone → cortisol conversion in subjects with central adiposity. J Clin Endocrinol Metab. 1999;84:1022–1027. doi: 10.1210/jcem.84.3.5538. [DOI] [PubMed] [Google Scholar]
- 38.Stulnig TM. Waldhausl W. 11Beta-hydroxysteroid dehydrogenase type 1 in obesity and type 2 diabetes. Diabetologia. 2004;47:1–11. doi: 10.1007/s00125-003-1284-4. [DOI] [PubMed] [Google Scholar]
- 39.Strain GW. Zumoff B. Kream J. Strain JJ. Levin J. Fukushima D. Sex difference in the influence of obesity on the 24 hr mean plasma concentration of cortisol. Metabolism. 1982;31:209–212. doi: 10.1016/0026-0495(82)90054-3. [DOI] [PubMed] [Google Scholar]
- 40.Hall EM. Gender, work control, and stress: A theoretical discussion and an empirical test. Int J Health Serv. 1989;19:725–745. doi: 10.2190/5MYW-PGP9-4M72-TPXF. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein IB. Shapiro D. Chicz-DeMet A. Guthrie D. Ambulatory blood pressure, heart rate, and neuroendocrine responses in women nurses during work and off work days. Psychosom Med. 1999;61:387–396. doi: 10.1097/00006842-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 42.Therrien F. Drapeau V. Lalonde J, et al. Awakening cortisol response in lean, obese, and reduced obese individuals: Effect of gender and fat distribution. Obesity (Silver Spring) 2007;15:377–385. doi: 10.1038/oby.2007.509. [DOI] [PubMed] [Google Scholar]
- 43.Fredrikson M. Racial differences in cardiovascular reactivity to mental stress in essential hypertension. J Hypertens. 1986;4:325–331. doi: 10.1097/00004872-198606000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Thomas AJ. Eberly LE. Davey Smith G. Neaton JD. Stamler J. Race/ethnicity, income, major risk factors, and cardiovascular disease mortality. Am J Public Health. 2005;95:1417–1423. doi: 10.2105/AJPH.2004.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brancati FL. Kao WH. Folsom AR. Watson RL. Szklo M. Incident type 2 diabetes mellitus in African American and white adults: The Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 46.Abate N. Chandalia M. The impact of ethnicity on type 2 diabetes. J Diabetes Complications. 2003;17:39–58. doi: 10.1016/s1056-8727(02)00190-3. [DOI] [PubMed] [Google Scholar]
- 47.Simeon D. Knutelska M. Yehuda R. Putnam F. Schmeidler J. Smith LM. Hypothalamic-pituitary-adrenal axis function in dissociative disorders, post-traumatic stress disorder, and healthy volunteers. Biol Psychiatry. 2007;61:966–973. doi: 10.1016/j.biopsych.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meewisse ML. Reitsma JB. de Vries GJ. Gersons BP. Olff M. Cortisol and post-traumatic stress disorder in adults: Systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- 49.Hellhammer J. Fries E. Schweisthal OW. Schlotz W. Stone AA. Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait components. Psychoneuroendocrinology. 2007;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]