Abstract
Background
The two-component systems of Mycobacterium tuberculosis are apparently required for its growth and resistance in hostile host environments. In such environments, MtrAB has been reported to regulate the expression of the M. tuberculosis replication initiator gene, dnaA. However, the dnaA promoter binding sites and many potential target genes for MtrA have yet to be precisely characterized.
Results
In this study, a 7 bp sequence motif in the dnaA promoter region was identified for MtrA binding using DNaseI footprinting assays and surface plasmon resonance (SPR) analysis. Approximately 420 target genes potentially regulated by MtrA, including the isoniazid inducible gene iniB, were further characterized from M. tuberculosis and M. smegmatis genomes. When assayed using quantitative real-time PCR (qRT-PCR), many of the target genes demonstrated significant expression changes when the antisense mRNA of the mtrA gene was expressed in M. smegmatis. The recombinant mycobacteria grew in length and were more sensitive to two anti-tuberculosis drugs, isoniazid and streptomycin.
Conclusions
These findings yield critical information about the regulatory mechanisms of the MtrAB two-component system and its role in the drug resistance of M. smegmatis.
Background
M. tuberculosis is one of the most devastating human pathogens, and its threat to human health has intensified with the emergence of multidrug-resistant tuberculosis (TB) and the worldwide prevalence of co-infection with HIV [1,2]. Two-component regulatory systems (TCRs) are widely distributed among bacteria and plants and enable organisms to regulate gene expression in response to a variety of environmental stimuli [3,4]. Some TCRs are clearly involved in regulating the virulence of pathogenic bacteria [3].
The M. tuberculosis genome contains 11 paired TCRs and several orphan kinases and regulators [5]. Several TCRs are apparently required for the growth of M. tuberculosis under specific conditions [6-8]; for example, mprA-mprB is important for the maintenance of persistence [6]. Of the 11 M. tuberculosis TCRs, only the mtrA-mtrB system has been confirmed as essential for in vitro and in vivo survival of M. tuberculosis [9-11]. Several recent reports show that the regulator MtrA modulates M. tuberculosis proliferation by regulating dnaA expression and binding the origin of replication [12,13]. In Mycobacterium avium, morphotypic multidrug resistance requires the presence of an MtrA homologue [14].
The mtrAB system has been successfully deleted in Corynebacterium glutamicum, an industrial amino acid production strain [15]. Mutant cells lacking mtrAB showed a different cell morphology and were more sensitive to penicillin, vancomycin, and lysozyme, however, they were more resistant to ethambutol [15]. The expression of some genes involved in both peptidoglycan metabolism and osmoprotection was also substantially changed [15]. Therefore, MtrAB in C. glutamicum is thought to be involved in regulating cell wall metabolism and osmoprotection.
The M. tuberculosis MtrAB system is thought to be involved in the expression of many target genes and contributes to the pathogen survival and resistance within its host tissue. However, these target genes and their MtrA binding sites have not been clearly established. In the current study, we have identified conserved sites for the recognition of MtrA in the dnaA promoter, as well as approximately 420 potential target genes. Further in vivo studies concerning a related organism, M. smegmatis, reveal changes in both cell morphology and drug resistance when MtrA gene expression is inhibited. The data presented here significantly enhance our understanding of the regulatory mechanisms of the essential two-component MtrAB system and its role in mycobacterial drug resistance.
Results
MtrA interacted with the regulatory region of the M. tuberculosis dnaA gene
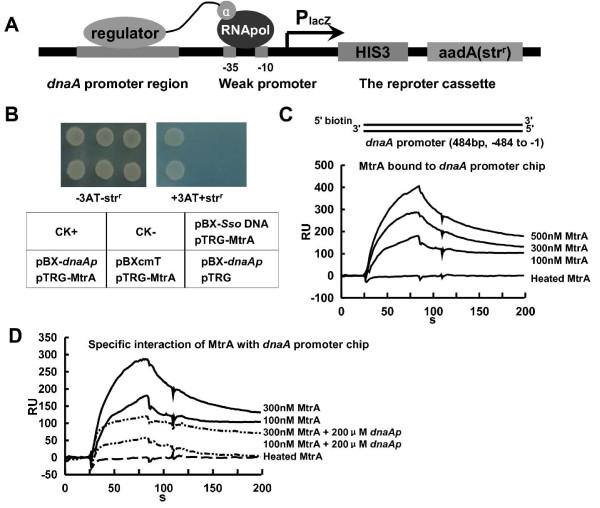
Bacterial one-hybrid assays confirmed the interaction between MtrA and the regulatory sequence of the dnaA initiator gene. The dnaA promoter region was cloned into the reporter genes upstream of HIS3-aadA and the reporter vector pBXcmT (Fig. 1A). As shown in Fig. 1B, the co-transformant strain with the dnaA promoter and MtrA was observed to grow well on the screening medium. In contrast, there was no growth for the strain containing either MtrA or the dnaA promoter alone. In addition, neither the co-transformant strain containing an unrelated DNA, SsoDNA (Additional file 1), nor MtrA did grew, indicating that this DNA cannot interact with MtrA (Fig. 1B). Thus, MtrA specifically interacted with the dnaA gene promoter.
Figure 1.
Two-component regulator MtrA interacts with the regulatory region of dnaA. (A) The regulatory sequence of the dnaA initiator gene was cloned into the reporter genes upstream of HIS3-aadA of the reporter vector pBXcmT (24). (B) The interaction between MtrA and the promoter region of dnaA was measured by bacterial one-hybrid analysis. Upper panel: bacterial two-hybrid plates. Lower panel: an outline of the plates in the upper panel. Each unit represents the corresponding co-transformant in the plates. CK+: co-transformant containing pBX-Rv2031p and pTRG-Rv3133c as a positive control (24). CK-: co-transformant containing pBX-Rv2031p and pTRG-Rv3133c-deltaC as a negative control (24). SsoDNA, an unrelated archaeal DNA sequence, was also used a negative control. (C) SPR assays for the binding of dnaA promoter chip by MtrA. (D) The specific interaction between the regulatory region of the M. tuberculosis dnaA gene was assayed by SPR. Unlabeled promoter DNA was used as competition for the binding of MtrA with DNA on chip. An overlay plot was produced to show the interactions.
The interaction of the purified MtrA protein with the dnaA promoter was confirmed by the interaction with the DNA on the chip. As shown in Fig. 1C, the biotinylated promoter DNA was first associated with the streptavidin (SA) chip (GE Healthcare). When an increasing concentration of MtrA protein (100-500 nM) was passed over the chip surface, a corresponding increasing response value was observed. This again indicated that the MtrA protein could bind with the dnaA promoter DNA (Fig. 1C). In contrast, heated inactive protein showed no response when it was passed over the chip (Fig. 1C). When an unspecific DNA, the promoter of Rv0467, was coated on the chip, no significant association for MtrA was observed (Additional file 2). In a further confirmatory experiment, 200 μM unlabeled promoter DNA was also added along with the MtrA protein. This DNA competed with that on the chip for the available MtrA; here, a significantly lower response was observed compared to a control with no competition (Fig. 1D).
Characterization of the DNA-box motif in the dnaA promoter that allows MtrA binding
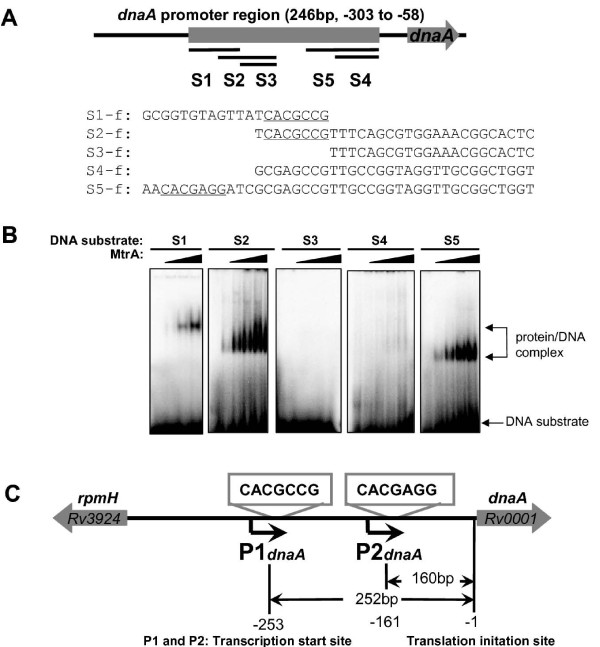
Several short DNA fragments (S1-S5) were used to precisely determine the DNA-box motif for the MtrA in this promoter region (Fig. 2A). As shown in Fig. 2B, a specific protein/DNA complex was observed on S1, S2, and S5, indicating that MtrA could recognize these DNA substrates. In contrast, no binding activity was observed for substrates S3 and S4, both of which lacked the 5-CACGCCG-3 or 5-CACGAGG-3 sequence box (Fig. 2A). Further confirmation of the specific interaction was obtained by conducting the competing surface plasmon resonance (SPR) assay with the unlabeled DNA fragments. As shown in Additional file 3, a significantly lower response was observed when either the unlabeled S2 or S5 was added together with MtrA, which indicated that they could compete the binding of MtrA with the promoter DNA on the chip. Therefore, these two sequence motifs appeared to be essential for the MtrA binding with the dnaA regulatory region.
Figure 2.
Characterization of the sequence motifs for MtrA in the M. tuberculosis dnaA gene promoter region. The DNA-binding assays of M. tuberculosis MtrA were performed using modified EMSA and SPR assays, as described in "Materials and Methods". (A) Several short DNA fragments were synthesized and used as DNA substrates, which covered a different dnaA gene promoter region. The sequences of their positive strands are indicated, and two 7 bp motifs are underlined. (B) The DNA-binding assays for MtrA on different DNA substrates. The EMSA reactions (10 μl) for measuring the mobility shift contained 200 fmol 32P-labeled DNA and increasing amounts of MtrA proteins (100 nM-600 nM). The protein/DNA complex is indicated by arrows on the right of the panels. (C) Schematic representation of conserved motifs located downstream of two dnaA promoters. The base-pair numbers far from the start codon of the dnaA gene are indicated.
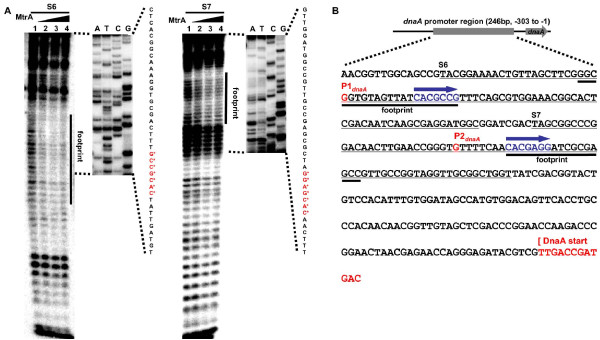
The interaction between MtrA and these two sequence boxes was further confirmed by DNase I footprinting assays (Fig. 3). Regions that contain these two boxes were significantly protected when MtrA was present. Protection at S6 occurred at all MtrA concentrations while the protection of S7 was dependent on the concentration of MtrA. This suggests that MtrA has different binding affinities with these regions.
Figure 3.
MtrA footprinting analysis in the M. tuberculosis dnaA promoter region. (A) DNase I footprinting assay of the protection of two short dnaA promoter regions (S6 and S7) against DNase I digestion by MtrA. The substrate S6 contains S1 and S2 sequences, and the substrate S7 contains S5 sequences. The ladders are shown in the right panel and the obtained nucleotide sequences are listed. The protected regions are indicated. The two specific sequence boxes are indicated by "*". (B) Summary of MtrA footprinting analysis in the M. tuberculosis dnaA promoter region. The DNA sequence correspond with the dnaA promoter region from -303 to -1. The position of two transcription start sites (P1dnaA and P2dnaA), two footprint regions, and two MtrA binding boxes are indicated.
We characterized two sequence boxes for the recognition of MtrA within the dnaA promoter, situated immediately downstream of promoters P1 and P2. The binding sequence boxes and their situation within the dnaA promoter are summarized in Fig. 2C.
Characterization of potential target genes regulated by MtrA in mycobacterial genomes
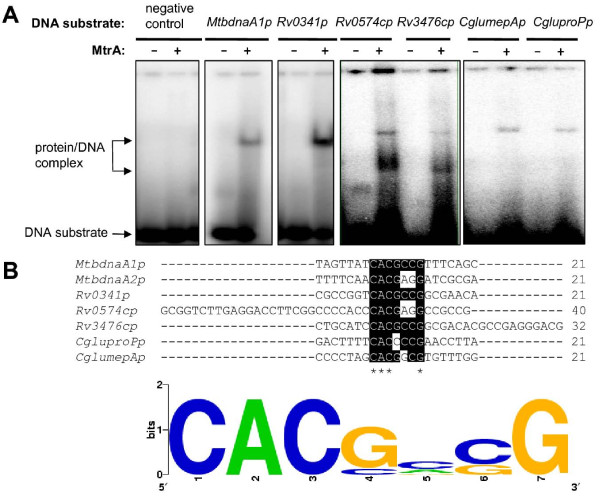
We searched the intergenic regions of the M. tuberculosis and M. smegmatis genomes extensively based on the two sequence motifs for MtrA in the dnaA gene promoter region. To validate the target genes, several regulatory regions of the genes were amplified. The DNA-binding activities of MtrA were examined using EMSA assays. As shown in Fig. 4, the regulatory sequence of a predicted target gene, isoniazid inducible gene iniB (rv0341), could be recognized by MtrA. A specific DNA/protein complex band was also observed. In addition, MtrA was able to bind with two target promoter DNA sequences of Rv0574 (a hypothetical protein) and Rv3476 (KgtP), producing a corresponding DNA/protein band (Fig. 4A). The positive target DNA was shown to bind with MtrA, while the negative DNA was not. The 7 bp sequence motif could also be found in the promoter regions of two previously characterized target genes, CgmepA and CgproP, in C. glutamicum. Interestingly, M. tuberculosis MtrA bound with the regulatory sequences and produced specific shifted bands (Fig. 4A).
Figure 4.
Characterization of the conserved sequence motif for MtrA in mycobacteria and C. glutamicum. (A) EMSA assays for validating the binding of MtrA with regulatory sequences of several potential target genes from M. tuberculosis. The promoter DNA of M. tuberculosis dnaA gene was used as positive control. An unrelated DNA was used as negative control. Several DNA substrates, namely, Rv0341_up, Rv0574c_up, and Rv3476c_up, were amplified from their promoter regions using specific primers. Several regulatory sequences of potential target genes from C. glutamicum including CglumepAp and CgluproPp, were amplified and used as DNA substrates. (B) A blast assay for the conserved sequence motif recognition by MtrA. Sequence alignment was carried and visualized by local BioEdit software. The complete consensus sequence is indicated by the stars under the base in the upper panel. Sequence logo was generated by WebLogo tool.
A further logo assay for the consensus sequence was conducted using the WebLogo tool [16]. A more general conserved motif for MtrA recognition was mapped out (Fig. 4B). In all, 155 potential target genes were characterized from the M. tuberculosis genome (Additional file 4), and 264 genes were characterized from the M. smegmatis genome (Additional file 5).
Effects of mtrA gene expression level on mycobacterial drug resistance and cell morphology
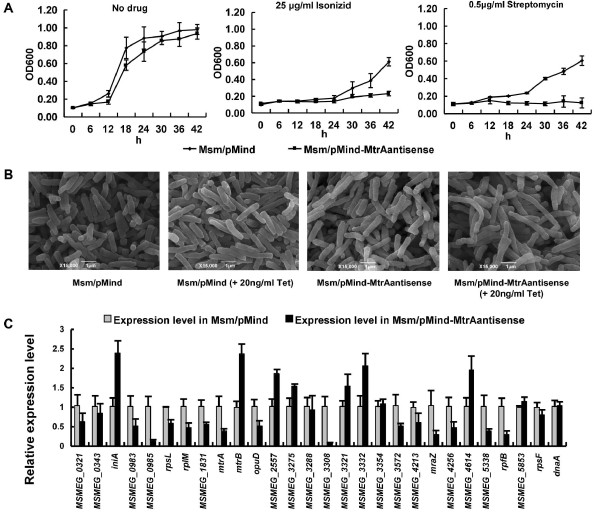
The mRNA antisense expression of the mtrA gene in M. smegmatis showed a regulatory effect of mtrA on mycobacterial drug resistance and cell morphology [17]. No substantial change was observed for the general growth of the recombinant mycobacterial strains. However, as shown in Fig. 5A, the recombinant mycobacterial cells became sensitive to the anti-TB drugs isoniazid and streptomycin, as evidenced by their inhibited growth in the presence of 25 μg/mL of isoniazid or 0.5 μg/mL of streptomycin in the medium. In contrast, no noticeable inhibition was observed for two other drugs, ethambutol and rifampicinB (data not shown). With a general growth of the recombinant mycobacterial strains resulting in minimal change, the cell morphology was further examined using the scanning electron microscopy (SEM) technique. As shown in Fig. 5B, the cell lengthened when 20 ng/mL tetracycline was added to the medium to induce expression of the antisense mtrA mRNA (right panel).
Figure 5.
Effects of the expression level of mtrA gene on target genes and cell growth in M. smegmatis. (A) Drug resistance assays. The antimicrobial activity of four first-line anti-tuberculosis drugs against M. smegmatis was determined as described under "Materials and Methods". Representative growth curves for isonizid and streptomycin are shown. (B) Scanning electron microscopy assay of cell morphology. The experiment was carried out as described in the "Materials and Methods". Representative images are shown. The images were taken at 15,000× magnification. Bars, 1 μm. (C) qRT-PCR assays for the gene expression of M. smegmatis. The experiment was carried out as described in the "Materials and Methods". 16S rRNA gene, rrs, was used as control. All target genes were amplified using specific primers. Different gene expressions were normalized to the levels of 16S rRNA gene transcripts, and the folds of expression change were calculated. Representative data are shown.
When relative gene expression was measured via qRT-PCR as shown in Fig. 5C, the mtrA gene was only 0.38-fold that of the wild-type strain, indicating that the expression of the mtrA gene in recombinant M. smegmatis was greatly inhibited. The expression of the dnaA gene in the recombinant strain basically remained constant when compared with that in the wild-type strain. This was consistent with the fact that no conserved sequence motif existed within the regulatory region of this gene in M. smegmatis. Another approximately 26 potential target genes were randomly chosen to measure the expression change in the recombinant M. smegmatis strain (Fig. 5C). The expression levels of these genes clearly changed; iniA and mtrB gene expression increased 2.5-fold expression (Fig. 5C), while mraZ (Msmeg_4236) and rpfB (Msmeg_5439) gene expression decreased by about 0.2-fold (Fig. 5C).
Therefore, the inhibition of the mtrA gene resulted in corresponding expression changes in many predicted target genes in M. smegmatis. The expression level of the mtrA gene consequently affected the drug resistance and cell morphology of M. smegmatis.
Discussion
MtrAB has been reported to regulate the expression of the M. tuberculosis replication initiator gene, dnaA [12]. However, potential binding sites for MtrA have not been clearly characterized. In addition, there are many potential target genes that also appear to be regulated by MtrA. In the current study, we identified a 7 bp conserved sequence motif for the recognition of MtrA within the dnaA promoter. About 420 potential target genes regulated by MtrAB were predicted from the M. tuberculosis and M. smegmatis genomes upon searching their promoter databases. Many predicted target genes showed significant expression changes when the mtrA homologue of M. smegmatis was partially inhibited. The recombinant M. smegmatis cells increased in length and became sensitive to the anti-TB drugs isoniazid and streptomycin.
The transcription of dnaA starts essentially at P1dnaA, which is conserved in all mycobacterial species [18]. The analysis of the sequence in the upstream region of dnaA revealed a second promoter, P2dnaA, in M. tuberculosis [18]. In previous in vivo experiments, MtrA bound with the regulatory region of the dnaA gene [12]. In the current study, two binding motifs for MtrA were located immediately downstream from the two promoters (Fig. 2C). Therefore, MtrA can apparently interfere with the promoter activity and thus regulate the expression of the replication initiator gene. The promoter P2dnaA only exists in a viral strain or derivative strains such as M. tuberculosis, M. bovis and BCG, but not in M. avium or M. smegmatis [18]. This suggests that the two-component system MtrAB might contribute to the virulence of the M. tuberculosis complex through selective regulation of dnaA gene expression. A parallel study [13] has identified a "GTCACAgcg" motif for the recognition of MtrA in the fbpB promoter and the origin of replication. Interestingly, there exists a common conserved core sequence between the 9 bp motif and the motif identified within the dnaA promoter in the current study. Using a MalE-EnvZ kinase, but not the cognate partner kinase of MtrB, Rajagopalan et al suggested that the phosphorylation of MtrA had distinct regulation capacities. However, only 5% of the MtrA protein was shown to be phosphorylated [13]. In the present study, efforts to phosphorylate MtrA using the partner kinase of MtrB failed (data not shown). Importantly, using several different methods, we showed that the nonphosphorylated MtrA could bind to the target DNA very well, suggesting that the form of MtrA might be involved in regulating the expression of its target genes. Obviously, the regulation mechanism of MtrAB needs to be further addressed in the future.
Attempts to disrupt the mtrA gene in M. tuberculosis have been unsuccessful; thus, mtrA seems to be an essential gene for M. tuberculosis proliferation [11]. The genes encoding the MtrAB two-component system of C. glutamicum were deleted successfully, and this deletion strongly influenced the cell morphology, antibiotic susceptibility, and expression of genes involved in osmoprotection [15]. In the current study, a large group of target genes for MtrA was characterized from the genomes of both M. tuberculosis and M. smegmatis, including multiple transcriptional factors such as TetR family regulators, stress gene family protein (MSMEG_3308), and the isoniazid inducible protein IniA (MSMEG_0695). Inhibition of the mtrA gene, therefore, resulted in corresponding expression changes in many or all of these target genes in M. smegmatis (Fig. 5C).
In the current study, we found the conserved motifs of the MtrAB two-component system upstream of a large list of genes that have several different functions, including cell cycle progression regulation, secreted antigen, and drug resistance. Interestingly, as shown in Additional file 6, there are 42 genes (10%) that were found in both mycobacterial species. MtrA was reported to be involved in the transcriptional regulation of dnaA in M. tuberculosis; this provides the first direct evidence of its role in cell cycle progression [12]. In M. avium, MtrAB could respond to general stresses and ultimately inhibit cell division [14]. A recent study found that the promoter for immunodominant secreted antigen 85B was also characterized as the targets of MtrA [13]. Therefore, our findings were consistent with these previous studies.
Among the many predicted target genes, there are drug resistance and transport-related genes (including the isoniazid inducible gene iniB), a probable transport integral membrane gene, and osmoprotection-related genes. The operon iniBAC was previously found to confer multidrug tolerance to M. bovis BCG through an associated pump-like activity, and was induced by isoniazid and ethambutol [19,20]. These findings suggest that the mtrA gene might be involved in drug resistance. In the current study, we have confirmed that MtrA could bind the iniB promoter region. The recombinant M. smegmatis strain was found to become sensitive to the anti-TB drugs, isoniazid and streptomycin, when mtrA gene expression was inhibited by an antisense mRNA technique (Fig. 5A). In M. avium, mtrAB was shown to play a role in regulating the composition and permeability of mycobacterial cell walls and was required for morphotypic multidrug resistance [14]. In the current study, the recombinant M. smegmatis cells were observed to increase in length. This is most likely due to the changes of the mycobacterial cell wall, which would contribute to mycobacterial sensitivity to anti-TB drugs. All evidence makes MtrA a good target candidate for drug design.
Conclusions
The two-component systems of M. tuberculosis are apparently required for its growth and resistance in hostile host environments, in which MtrAB has been reported to regulate the expression of the M. tuberculosis replication initiator gene, dnaA. In the current study, we have identified the conserved sites for the recognition of MtrA in the dnaA promoter as well as approximately 420 potential target genes. Further in vivo studies about a related organism, M. smegmatis, reveal changes in both cell morphology and drug resistance when MtrA gene expression is inhibited. The data presented here significantly enhance our understanding of the regulatory mechanisms of the essential two-component MtrAB system and its role in the drug resistance of M. smegmatis.
Methods
Cloning, expression and purification of recombinant proteins
All DNA primers (Additional file 7) and oligonucleotides (Additional file 8) were synthesized by Invitrogen. M. tuberculosis mtrA was amplified using primers from genomic DNA. The MtrA genes were cloned into the overexpression vectors pET28a or pGEX-4T-1 to produce recombinant plasmids (Additional file 1). E. coli BL21(DE3) cells that were transformed with the recombinant plasmid were grown at 37°C in 1 L of LB medium containing 30 μg/mL kanamycin or 100 μg/mL ampicillin, respectively. Protein purification was carried out as described in earlier reports [21-24].
Bacterial one-hybrid analysis
The interaction between the regulatory region of the M. tuberculosis dnaA gene and MtrA was assayed using the bacterial one-hybrid technique [24]. The reporter vector pBXcmT and pTRG vectors containing MtrA were generated (Additional file 1). The bacterial one-hybrid assays were carried out as described in a previous study [24]. Positive growth co-transformants were selected on the screening medium plate containing 15 mM 3-AT, 8 μg/ml streptomycin, 15 μg/ml tetracycline, 34 μg/ml chloramphenicol, and 50 μg/ml kanamycin.
Electrophoretic Mobility Shift Assay (EMSA)
The double-stranded substrates were prepared according to a previously published procedure [21]. DNA-binding assays of M. tuberculosis MtrA and its mutant proteins were performed using a modified electrophoretic mobility shift assay (EMSA), as previously described [21-23] but with several changes. The reactions (10 μL) for measuring the mobility shift contained 200 fmol 32P-labeled DNA and various amounts of MtrA diluted in a buffer containing 20 mM Tris-HCl (pH 7.5), 100 mM NaCl, 0.5 mM MgCl2, 10 μg/ml sonicated salmon sperm DNA, 0.7 mM 2-mercaptoethanol and 5% glycerol. Reactions were performed and gels were exposed to a storage-phosphor screen overnight at room temperature. The images were acquired using a Typhoon scanner (GE Healthcare).
Surface Plasmon Resonance (SPR) analysis
The interaction between the regulatory region of the M. tuberculosis dnaA gene was assayed using SPR. Biotin-labeled promoter DNA was immobilized onto a SA chip (BIAcore), based on a previously published procedure [24]. The purified MtrA protein was passed over the chip. DNA-protein interaction assays were performed at 25°C. Each analysis was performed in triplicate. An overlay plot was generated to illustrate the interactions.
Scanning Electron Microscopy (SEM) observation
M. smegmatis cells prepared for scanning electron microscopy (SEM) observation were grown in LB for 24 hours in the presence of 20 ng·mL-1 tetracycline. Cells were harvested by centrifugation. The bacterial pellets were resuspended and incubated at 4°C for 24 hours in 2.5% glutardialdehyde solution. The cells were washed twice in double distilled water and then dehydrated by 15 min treatments in 30, 50, 75, 85, 95 and 100% ethanol. The incubation in 100% ethanol was repeated to ensure complete dehydration. Samples were critical-point dried, sputter-coated with gold, and observed using a scanning electron microscope (S570; Hitachi, Tokyo, Japan). Representative images are shown.
Quantitative real-time PCR (qRT-PCR)
For real-time PCR analysis, gene-specific primers (Additional file 9) were used and first-strand cDNAs were synthesized using SuperScript II reverse transcriptase (Invitrogen), according to the manufacturer's instructions. Each PCR reaction (10 μl) contained 10 μl of 2× SYBR Green Master Mix Reagent (Applied Biosystems), 1.0 μl of cDNA samples, and 200 nM gene-specific primers. The thermocycling conditions were 95°C for 5 min, and 40 cycles at 95°C for 30 s, 60°C for 30 s and 72°C for 30 s. Amplification specificity was assessed using melting curve analysis. Different gene expressions were normalized to the levels of 16S rRNA gene transcripts [15]. The degrees of expression change were calculated using the 2-ΔΔCt method [25].
Drug resistance assays
The antimicrobial activity of four first-line anti-tuberculosis drugs against M. smegmatis was determined using a modified bacterial growth time course assay. M. smegmatis was grown in LB at 37°C overnight. This culture was then diluted (1:100) in 5 ml of fresh LB broth containing the indicated concentration of each drug, and the culture was again incubated at 37°C with shaking at 220 rpm for two days. Samples were taken at various time points (0, 6, 12, 18, 24, 30, 36, 42, and 48 h). Optical density was measured at 600 nm (OD600) using a Beckman DU650 spectrophotometer. All assays were performed three times. Representative growth curves are shown.
DNase I footprinting assays
The 84 bp (S6) and 75 bp (S7) dnaA promoter regions were amplified (dnaAf1 and dnaAr2 were used to amplify S6 from genomic DNA, while dnaAf3 and dnaAr4 were used to amplify S7) (Additional file 7) and cleaved by endonuclease EcoRI, leaving a sticky 5' end that was five nucleotides from the original end. The recessive 3' end was labeled with [α-32P] dATP (Furui Biotech, Beijing, China) by the Klenow fragment, and then subjected to the same binding reaction as in the electrophoretic mobility shift assay. DNase I footprinting was performed as previously described [26]. The ladders were produced using the Sanger dideoxy method and dnaAf1 and dnaAf3 primers that were end-labeled by T4 polynucleotide kinase and [γ-32P] ATP (Furui Biotech, Beijing, China), respectively.
Bioinformatics assays on the distribution of the identified 7 bp motif within mycobacterial genomes
The regulatory sequences were collected from the complete genomes of M. tuberculosis and M. smegmatis and the database of intergenic regions of ORFs (from stop codon to start codon) were constructed. The exact motifs (CACGCCG or CACGAGG) were then used to search for the distribution of the identified 7 bp motifs in the M. tuberculosis H37Rv and the M. smegmatis genomes. The identified target genes are listed (Additional file 10 and Additional file 11).
Abbreviations
3-AT: 3-amino-1, 2, 4-triazole; EMSA: electrophoretic mobility shift assay; PCR: polymerase chain reaction; qRT-PCR: Quantitative real-time PCR; SA chips: Streptavidin chip; SEM: scanning electron microscopy; SPR: surface plasmon resonance; TCRs: two-component systems
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
YL and ZGH designed the experiments. YL and JZ performed the experiments. YL HZ and ZGH analyzed the data. ZGH contributed reagents/materials/analysis tools. ZGH and YL wrote the paper. All authors have read and approved the final manuscript.
Supplementary Material
Plasmids and recombinant vectors used in this study. The data present plasmids and recombinant vectors used in this study.
SPR assays for the binding of unspecific promoter chip by MtrA. The data present SPR assays for the binding of unspecific promoter chip by MtrA.
Competing SPR assay with the unlabeled DNA fragments for the binding of the promoter chip by MtrA. The data present the competing SPR assay with the unlabeled DNA fragments for the binding of the promoter chip by MtrA.
Potential target genes for MtrA in M. tuberculosis. The data provided potential target genes for MtrA in M. tuberculosis.
Potential target genes for MtrA in M.smegmatis. The data present Potential target genes for MtrA in M.smegmatis.
Homologous target genes recognized by MtrA in M. tuberculosis and M. smegmatis. The data present homologous target genes recognized by MtrA in M. tuberculosis and M. smegmatis.
Primers used in this study. The data provided primers used in this study.
Sequences of the DNA substrates used in this study. The data provided sequences of the DNA substrates used in this study.
Primers used for quantitative real time PCR in this study. The data present the primers used for quantitative real time PCR in this study.
Classification and percentage of the target genes containing the 7-bp motif recognized by MtrA in M. smegmatis. The data present the categories and percentage of the target genes containing the 7-bp motif recognized by MtrA in M. smegmatis.
The data present the categories and percentage of the target genes containing the 7-bp motif recognized by MtrA in M. tuberculosis. The data present the categories and percentage of the target genes containing the 7-bp motif recognized by MtrA in M. tuberculosis.
Contributor Information
Yuqing Li, Email: liyuqing13@163.com.
Jumei Zeng, Email: xuewawa@webmail.hzau.edu.cn.
Hua Zhang, Email: zhanghua421@163.com.
Zheng-Guo He, Email: hezhengguo@mail.hzau.edu.cn.
Acknowledgements
We thank Prof. Yi Zhang and her group members for help with footprinting assays. This work was supported by the National Natural Science Foundation of China (30930003) and 973 Program (2006CB504402).
References
- Johnson R, Streicher EM, Louw GE, Warren RM, van Helden PD, Victor TC. Drug resistance in Mycobacterium tuberculosis. Curr Issues Mol Biol. 2006;8(2):97–111. [PubMed] [Google Scholar]
- Wright A, Zignol M, Van Deun A, Falzon D, Gerdes SR, Feldman K, Hoffner S, Drobniewski F, Barrera L, van Soolingen D, Boulabhal F, Paramasivan CN, Kam KM, Mitarai S, Nunn P, Raviglione M. Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Epidemiology of antituberculosis drug resistance 2002-07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet. 2009;373(9678):1861–1873. doi: 10.1016/S0140-6736(09)60331-7. [DOI] [PubMed] [Google Scholar]
- Beier D, Gross R. Regulation of bacterial virulence by two-component systems. Curr Opin Microbiol. 2006;9(2):143–152. doi: 10.1016/j.mib.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J. et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393(6685):537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- Zahrt TC, Deretic V. Mycobacterium tuberculosis signal transduction system required for persistent infections. Proc Natl Acad Sci USA. 2001;98(22):12706–12711. doi: 10.1073/pnas.221272198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewann F, Jackson M, Pethe K, Cooper A, Mielcarek N, Ensergueix D, Gicquel B, Locht C, Supply P. Transient requirement of the PrrA-PrrB two-component system for early intracellular multiplication of Mycobacterium tuberculosis. Infect Immun. 2002;70(5):2256–2263. doi: 10.1128/IAI.70.5.2256-2263.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HD, Guinn KM, Harrell MI, Liao R, Voskuil MI, Tompa M, Schoolnik GK, Sherman DR. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol. 2003;48(3):833–843. doi: 10.1046/j.1365-2958.2003.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish T, Smith DA, Kendall S, Casali N, Bancroft GJ, Stoker NG. Deletion of two-component regulatory systems increases the virulence of Mycobacterium tuberculosis. Infect Immun. 2003;71(3):1134–1140. doi: 10.1128/IAI.71.3.1134-1140.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via LE, Curcic R, Mudd MH, Dhandayuthapani S, Ulmer RJ, Deretic V. Elements of signal transduction in Mycobacterium tuberculosis: in vitro phosphorylation and in vivo expression of the response regulator MtrA. J Bacteriol. 1996;178(11):3314–3321. doi: 10.1128/jb.178.11.3314-3321.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt TC, Deretic V. An essential two-component signal transduction system in Mycobacterium tuberculosis. J Bacteriol. 2000;182(13):3832–3838. doi: 10.1128/JB.182.13.3832-3838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fol M, Chauhan A, Nair NK, Maloney E, Moomey M, Jagannath C, Madiraju MV, Rajagopalan M. Modulation of Mycobacterium tuberculosis proliferation by MtrA, an essential two-component response regulator. Mol Microbiol. 2006;60(3):643–657. doi: 10.1111/j.1365-2958.2006.05137.x. [DOI] [PubMed] [Google Scholar]
- Rajagopalan M, Dziedzic R, Al Zayer M, Stankowska D, Ouimet MC, Bastedo DP, Marczynski GT, Madiraju MV. The Mycobacterium tuberculosis origin of replication and the promoter for immunodominant secreted antigen 85B are the targets of MtrA, the essential response regulator. J Biol Chem. 2010;285(21):15816–15827. doi: 10.1074/jbc.M109.040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangelosi GA, Do JS, Freeman R, Bennett JG, Semret M, Behr MA. The two-component regulatory system mtrAB is required for morphotypic multidrug resistance in Mycobacterium avium. Antimicrob Agents Chemother. 2006;50(2):461–468. doi: 10.1128/AAC.50.2.461-468.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möker N, Brocker M, Schaffer S, Krämer R, Morbach S, Bott M. Deletion of the genes encoding the MtrA-MtrB two-component system of Corynebacterium glutamicum has a strong influence on cell morphology, antibiotics susceptibility and expression of genes involved in osmoprotection. Mol Microbiol. 2004;54(2):420–438. doi: 10.1111/j.1365-2958.2004.04249.x. [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: A sequence logo generator. Genome Res. 2004;14(6):1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokpoel MC, Murphy HN, O'Toole R, Wiles S, Runn ES, Stewart GR, Young DB, Robertson BD. Tetracycline-inducible gene regulation in mycobacteria. Nucleic Acids Res. 2005;33(2):e22. doi: 10.1093/nar/gni023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar L, Guerrero E, Casart Y, Turcios L, Bartoli F. Transcription analysis of the dnaA gene and oriC region of the chromosome of Mycobacterium smegmatis and Mycobacterium bovis BCG, and its regulation by the DnaA protein. Microbiology. 2003;149(Pt 3):773–784. doi: 10.1099/mic.0.25832-0. [DOI] [PubMed] [Google Scholar]
- Colangeli R, Helb D, Sridharan S, Sun J, Varma-Basil M, Hazbón MH, Harbacheuski R, Megjugorac NJ, Jacobs WR Jr, Holzenburg A, Sacchettini JC, Alland D. The Mycobacterium tuberculosis iniA gene is essential for activity of an efflux pump that confers drug tolerance to both isoniazid and ethambutol. Mol Microbiol. 2005;55(6):1829–1840. doi: 10.1111/j.1365-2958.2005.04510.x. [DOI] [PubMed] [Google Scholar]
- Alland D, Steyn AJ, Weisbrod T, Aldrich K, Jacobs WR Jr. Characterization of the Mycobacterium tuberculosis iniBAC promoter, a promoter that responds to cell wall biosynthesis inhibition. J Bacteriol. 2000;182(7):1802–1811. doi: 10.1128/JB.182.7.1802-1811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He ZG, Rezende LF, Willcox S, Griffith JD, Richardson CC. The carboxyl-terminal domain of bacteriophage T7 single-stranded DNA-binding protein modulates DNA binding and interaction with T7 DNA polymerase. J Biol Chem. 2003;278(32):29538–29545. doi: 10.1074/jbc.M304318200. [DOI] [PubMed] [Google Scholar]
- Jiang PX, Wang J, Feng Y, He ZG. Divergent functions of multiple eukaryote-like Orc1/Cdc6 proteins on modulating the loading of the MCM helicase onto the origins of the hyperthermophilic archaeon Sulfolobus solfataricus P2. Biochem Biophys Res Commun. 2007;361(3):651–658. doi: 10.1016/j.bbrc.2007.07.073. [DOI] [PubMed] [Google Scholar]
- Wang J, Jiang PX, Feng H, Feng Y, He ZG. Three eukaryote-like Orc1/Cdc6 proteins functionally interact and mutually regulate their activities of binding to the replication origin in the hyperthermophilic archaeon Sulfolobus solfataricus P2. Biochem Biophys Res Commun. 2007;363(1):63–70. doi: 10.1016/j.bbrc.2007.08.125. [DOI] [PubMed] [Google Scholar]
- Guo M, Feng H, Zhang J, Wang W, Wang Y, Li Y, Gao C, Chen H, Feng Y, He ZG. Dissecting transcription regulatory pathways through a new bacterial one-hybrid reporter system. Genome Res. 2009;19(7):1301–1308. doi: 10.1101/gr.086595.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Yin P, Li TY, Xie MH, Jiang L, Zhang Y. A type Ib ParB protein involved in plasmid partitioning in a Gram-positive bacterium. J Bacteriol. 2006;188(23):8103–8108. doi: 10.1128/JB.01232-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasmids and recombinant vectors used in this study. The data present plasmids and recombinant vectors used in this study.
SPR assays for the binding of unspecific promoter chip by MtrA. The data present SPR assays for the binding of unspecific promoter chip by MtrA.
Competing SPR assay with the unlabeled DNA fragments for the binding of the promoter chip by MtrA. The data present the competing SPR assay with the unlabeled DNA fragments for the binding of the promoter chip by MtrA.
Potential target genes for MtrA in M. tuberculosis. The data provided potential target genes for MtrA in M. tuberculosis.
Potential target genes for MtrA in M.smegmatis. The data present Potential target genes for MtrA in M.smegmatis.
Homologous target genes recognized by MtrA in M. tuberculosis and M. smegmatis. The data present homologous target genes recognized by MtrA in M. tuberculosis and M. smegmatis.
Primers used in this study. The data provided primers used in this study.
Sequences of the DNA substrates used in this study. The data provided sequences of the DNA substrates used in this study.
Primers used for quantitative real time PCR in this study. The data present the primers used for quantitative real time PCR in this study.
Classification and percentage of the target genes containing the 7-bp motif recognized by MtrA in M. smegmatis. The data present the categories and percentage of the target genes containing the 7-bp motif recognized by MtrA in M. smegmatis.
The data present the categories and percentage of the target genes containing the 7-bp motif recognized by MtrA in M. tuberculosis. The data present the categories and percentage of the target genes containing the 7-bp motif recognized by MtrA in M. tuberculosis.