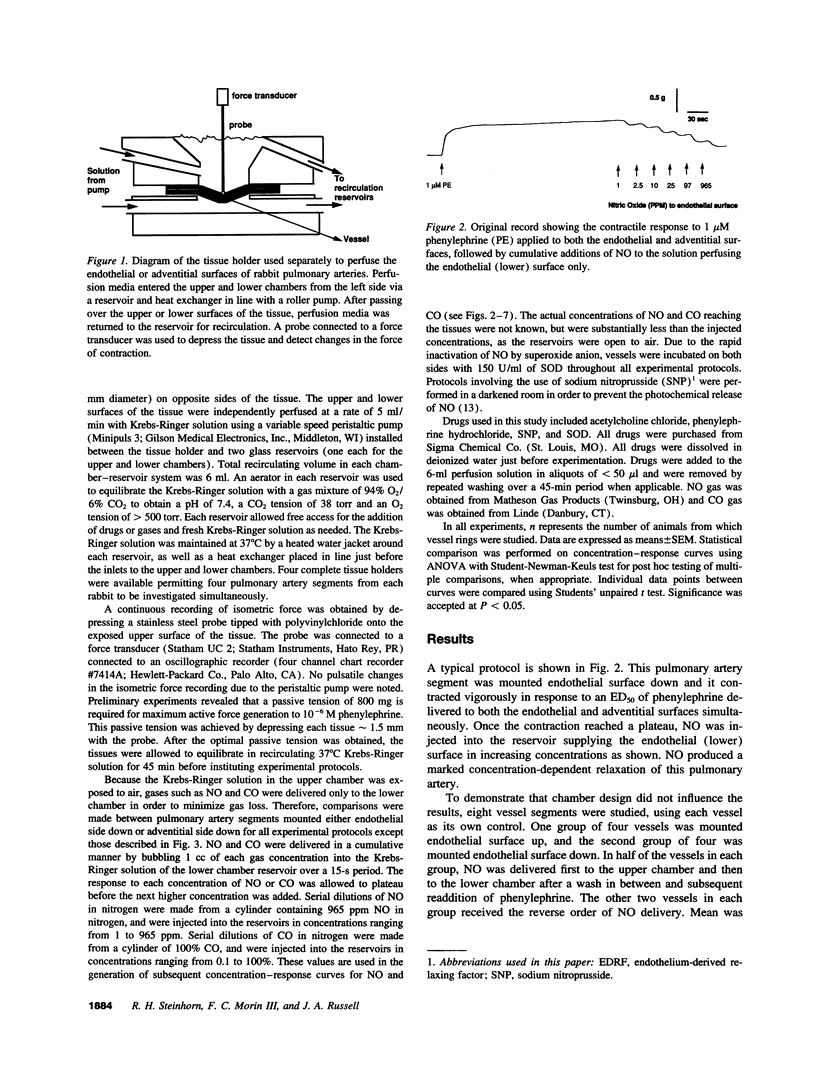
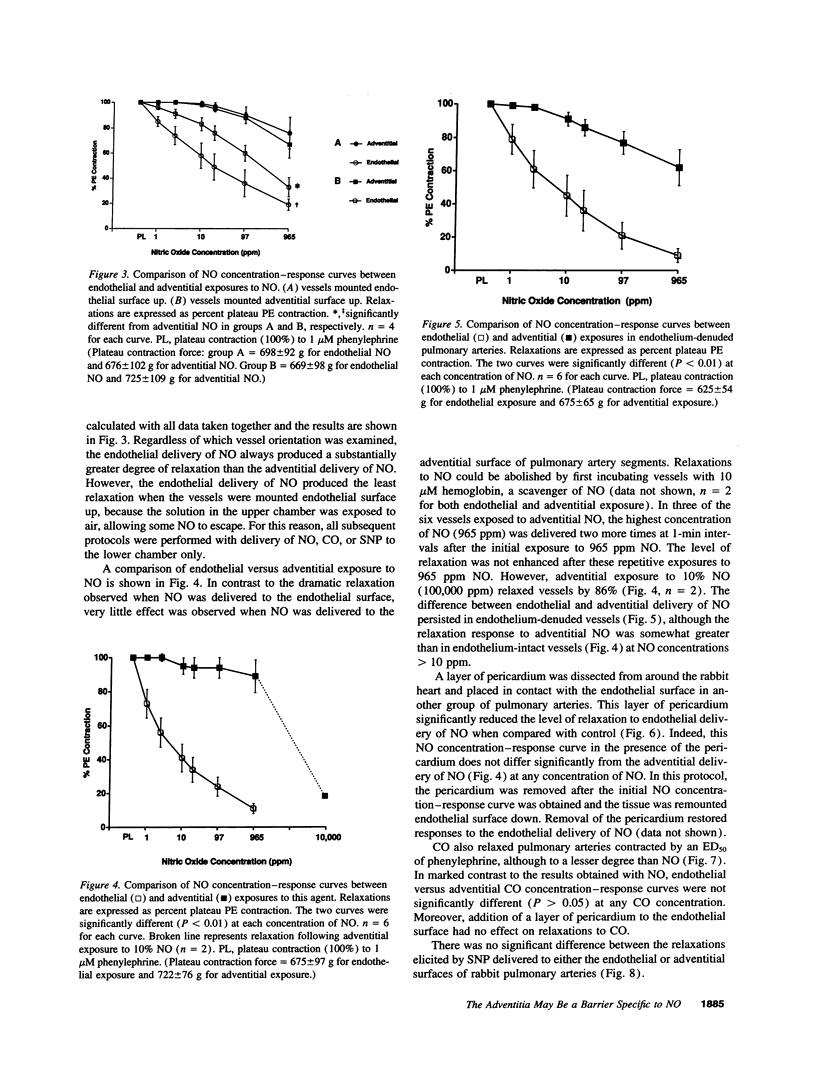
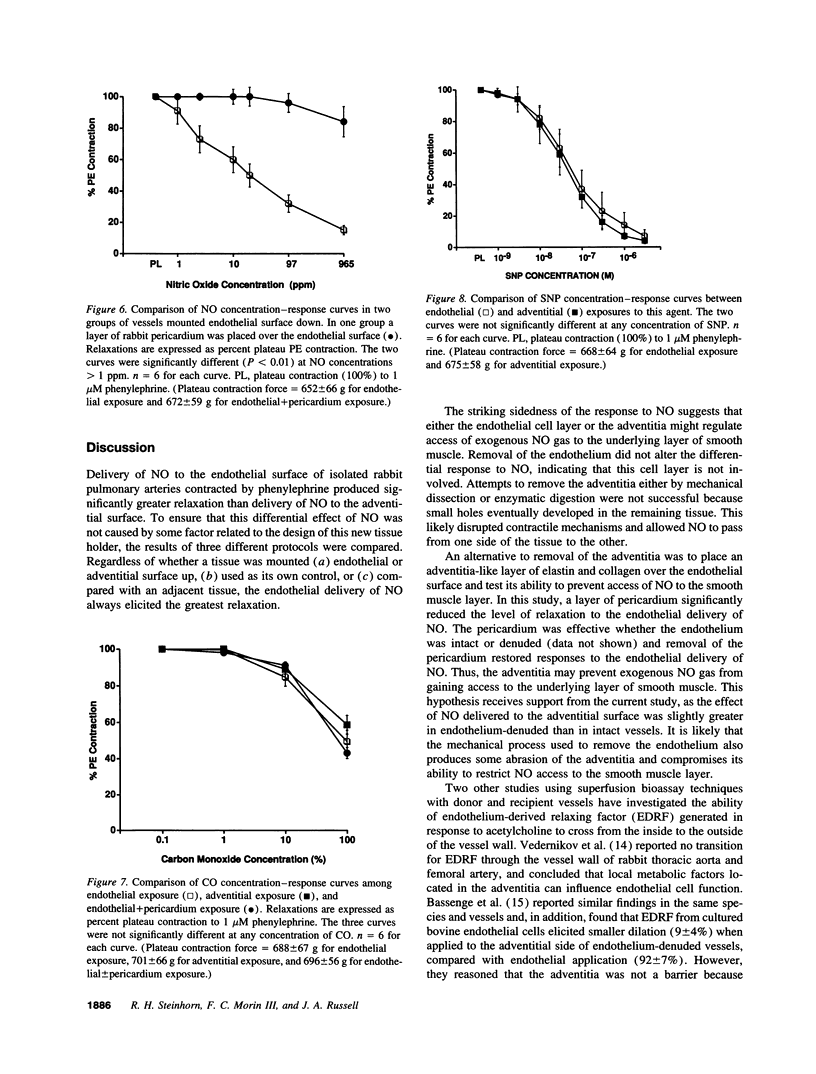
Abstract
To determine whether the adventitia that surrounds pulmonary vessels acts as a barrier specific to nitric oxide, special lucite chambers were constructed to measure the force of contraction of rabbit pulmonary artery rings in which the endothelial or adventitial surfaces could be preferentially exposed to nitric oxide (NO), carbon monoxide (CO), or sodium nitroprusside (SNP). Delivery of NO to the endothelial and adventitial surfaces of preconstricted vessels produced markedly different concentration-response curves with maximal relaxations of 89 +/- 3 and 11 +/- 9%, respectively. In contrast, relaxations induced by both CO and SNP did not differ significantly between endothelial and adventitial exposure to these agents. Placement of a layer of pericardium onto the endothelial surface eliminated relaxation to the endothelial delivery of NO but not to CO. We conclude that the pulmonary vascular response to NO displays a striking sidedness which is not observed either with CO, another gas of similar molecular weight, or with SNP, both of which cause relaxation by stimulating guanylate cyclase. The elimination of NO but not CO relaxations with a layer of pericardium may indicate that the adventitia acts as a barrier specific to NO. This directionality of effect provides evidence for a highly localized regulation of pulmonary vascular tone by endothelial cell NO and also indicates that extravascular NO may have limited access to pulmonary vascular smooth muscle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates J. N., Baker M. T., Guerra R., Jr, Harrison D. G. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochem Pharmacol. 1991 Dec 11;42 (Suppl):S157–S165. doi: 10.1016/0006-2952(91)90406-u. [DOI] [PubMed] [Google Scholar]
- Bernard C., Szekely B., Philip I., Wollman E., Payen D., Tedgui A. Activated macrophages depress the contractility of rabbit carotids via an L-arginine/nitric oxide-dependent effector mechanism. Connection with amplified cytokine release. J Clin Invest. 1992 Mar;89(3):851–860. doi: 10.1172/JCI115664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette E. Y., Hogaboam C. M., Wallace J. L., Befus A. D. Potentiation of tumor necrosis factor-alpha-mediated cytotoxicity of mast cells by their production of nitric oxide. J Immunol. 1991 Nov 1;147(9):3060–3065. [PubMed] [Google Scholar]
- Brüne B., Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 1987 Oct;32(4):497–504. [PubMed] [Google Scholar]
- Busse R., Mülsch A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990 Nov 26;275(1-2):87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- Demling R. H. Adult respiratory distress syndrome: current concepts. New Horiz. 1993 Aug;1(3):388–401. [PubMed] [Google Scholar]
- Fratacci M. D., Frostell C. G., Chen T. Y., Wain J. C., Jr, Robinson D. R., Zapol W. M. Inhaled nitric oxide. A selective pulmonary vasodilator of heparin-protamine vasoconstriction in sheep. Anesthesiology. 1991 Dec;75(6):990–999. doi: 10.1097/00000542-199112000-00011. [DOI] [PubMed] [Google Scholar]
- Gaston B., Reilly J., Drazen J. M., Fackler J., Ramdev P., Arnelle D., Mullins M. E., Sugarbaker D. J., Chee C., Singel D. J. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10957–10961. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. B., Tod M. L. Effects of N omega-nitro-L-arginine on total and segmental vascular resistances in developing lamb lungs. J Appl Physiol (1985) 1993 Jul;75(1):76–85. doi: 10.1152/jappl.1993.75.1.76. [DOI] [PubMed] [Google Scholar]
- Gräser T., Vedernikov Y. P., Li D. S. Study on the mechanism of carbon monoxide induced endothelium-independent relaxation in porcine coronary artery and vein. Biomed Biochim Acta. 1990;49(4):293–296. [PubMed] [Google Scholar]
- Henry Y., Lepoivre M., Drapier J. C., Ducrocq C., Boucher J. L., Guissani A. EPR characterization of molecular targets for NO in mammalian cells and organelles. FASEB J. 1993 Sep;7(12):1124–1134. doi: 10.1096/fasebj.7.12.8397130. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Signal transduction mechanisms involving nitric oxide. Biochem Pharmacol. 1991 Feb 15;41(4):485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- Iwamoto J., Morin F. C., 3rd Nitric oxide inhibition varies with hemoglobin saturation. J Appl Physiol (1985) 1993 Nov;75(5):2332–2336. doi: 10.1152/jappl.1993.75.5.2332. [DOI] [PubMed] [Google Scholar]
- LaBourene J. I., Coles J. G., Johnson D. J., Mehra A., Keeley F. W., Rabinovitch M. Alterations in elastin and collagen related to the mechanism of progressive pulmonary venous obstruction in a piglet model. A hemodynamic, ultrastructural, and biochemical study. Circ Res. 1990 Feb;66(2):438–456. doi: 10.1161/01.res.66.2.438. [DOI] [PubMed] [Google Scholar]
- Lew M. J., Rivers R. J., Duling B. R. Arteriolar smooth muscle responses are modulated by an intramural diffusion barrier. Am J Physiol. 1989 Jul;257(1 Pt 2):H10–H16. doi: 10.1152/ajpheart.1989.257.1.H10. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- McCall T. B., Boughton-Smith N. K., Palmer R. M., Whittle B. J., Moncada S. Synthesis of nitric oxide from L-arginine by neutrophils. Release and interaction with superoxide anion. Biochem J. 1989 Jul 1;261(1):293–296. doi: 10.1042/bj2610293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Munakata M., Masaki Y., Sakuma I., Ukita H., Otsuka Y., Homma Y., Kawakami Y. Pharmacological differentiation of epithelium-derived relaxing factor from nitric oxide. J Appl Physiol (1985) 1990 Aug;69(2):665–670. doi: 10.1152/jappl.1990.69.2.665. [DOI] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nelin L. D., Dawson C. A. The effect of N omega-nitro-L-arginine methylester on hypoxic vasoconstriction in the neonatal pig lung. Pediatr Res. 1993 Sep;34(3):349–353. doi: 10.1203/00006450-199309000-00022. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Persson M. G., Wiklund N. P., Gustafsson L. E. Endogenous nitric oxide in single exhalations and the change during exercise. Am Rev Respir Dis. 1993 Nov;148(5):1210–1214. doi: 10.1164/ajrccm/148.5.1210. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H. NO., CO and .OH. Endogenous soluble guanylyl cyclase-activating factors. FEBS Lett. 1992 Jul 27;307(1):102–107. doi: 10.1016/0014-5793(92)80910-9. [DOI] [PubMed] [Google Scholar]
- Steinhorn R. H., Morin F. C., 3rd, Gugino S. F., Giese E. C., Russell J. A. Developmental differences in endothelium-dependent responses in isolated ovine pulmonary arteries and veins. Am J Physiol. 1993 Jun;264(6 Pt 2):H2162–H2167. doi: 10.1152/ajpheart.1993.264.6.H2162. [DOI] [PubMed] [Google Scholar]
- Vedernikov Y. P., Gräser T., Tiedt N. Is there an abluminal release of endothelium-derived relaxing factor (EDRF)? Basic Res Cardiol. 1987 Mar-Apr;82(2):172–177. doi: 10.1007/BF01907064. [DOI] [PubMed] [Google Scholar]
- Vodovotz Y., Kwon N. S., Pospischil M., Manning J., Paik J., Nathan C. Inactivation of nitric oxide synthase after prolonged incubation of mouse macrophages with IFN-gamma and bacterial lipopolysaccharide. J Immunol. 1994 Apr 15;152(8):4110–4118. [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]
- Wennmalm A., Benthin G., Petersson A. S. Dependence of the metabolism of nitric oxide (NO) in healthy human whole blood on the oxygenation of its red cell haemoglobin. Br J Pharmacol. 1992 Jul;106(3):507–508. doi: 10.1111/j.1476-5381.1992.tb14365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapol W. M., Hurford W. E. Inhaled nitric oxide in the adult respiratory distress syndrome and other lung diseases. New Horiz. 1993 Nov;1(4):638–650. [PubMed] [Google Scholar]
- Zayek M., Cleveland D., Morin F. C., 3rd Treatment of persistent pulmonary hypertension in the newborn lamb by inhaled nitric oxide. J Pediatr. 1993 May;122(5 Pt 1):743–750. doi: 10.1016/s0022-3476(06)80020-x. [DOI] [PubMed] [Google Scholar]
- Zayek M., Wild L., Roberts J. D., Morin F. C., 3rd Effect of nitric oxide on the survival rate and incidence of lung injury in newborn lambs with persistent pulmonary hypertension. J Pediatr. 1993 Dec;123(6):947–952. doi: 10.1016/s0022-3476(05)80393-2. [DOI] [PubMed] [Google Scholar]


