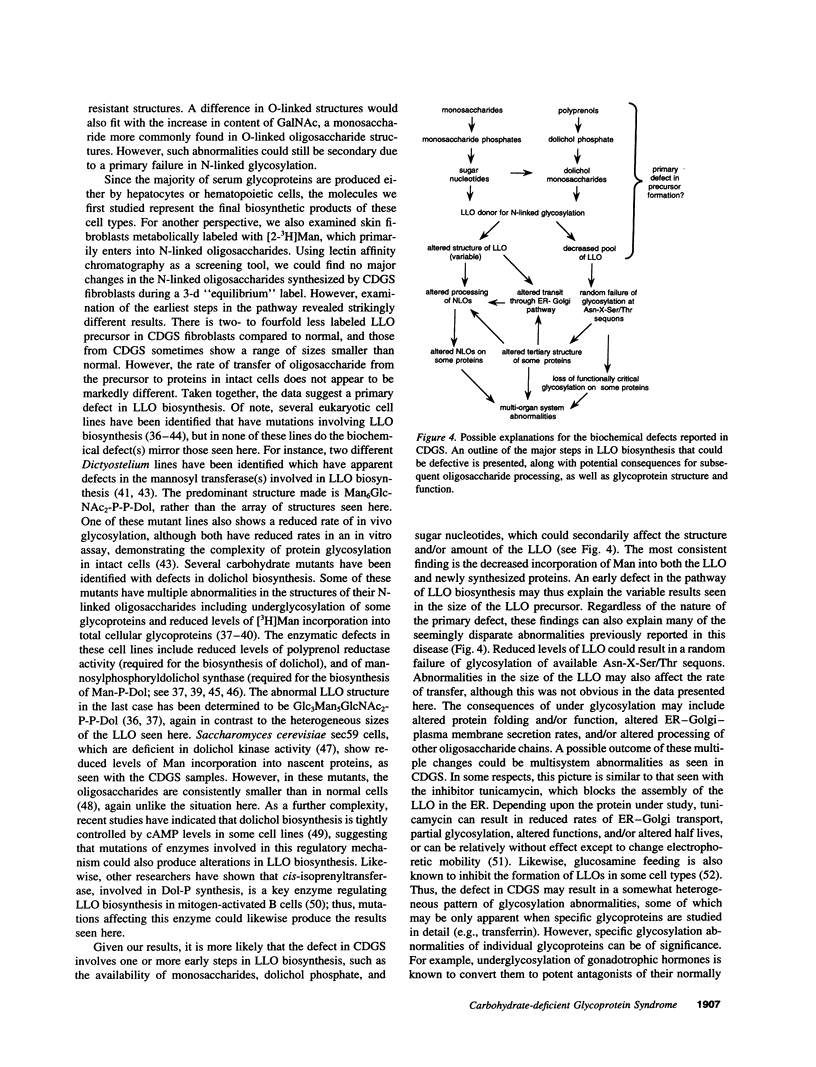
Abstract
The carbohydrate-deficient glycoprotein syndrome (CDGS) is a developmental disease associated with an abnormally high isoelectric point of serum transferrin. Carbohydrate analyses of this glycoprotein initially suggested a defect in N-linked oligosaccharide processing, although more recent studies indicate a defect in the attachment of these sugar chains to the protein. We studied both serum glycoproteins and fibroblast-derived [2-3H]mannose-labeled oligosaccharides from CDGS patients and normal controls. While there was a decrease in the glycosylation of serum glycoproteins of affected individuals, differences were not seen in either monosaccharide composition or oligosaccharide structures. The lectin-binding profiles of glycopeptides from [2-3H]-mannose-labeled fibroblasts were likewise indistinguishable. However, the incorporation of [2-3H]mannose into both glycoproteins and the dolichol-linked oligosaccharide precursor was significantly reduced. Thus, at least in some patients, CDGS is not due to a defect in processing of N-linked oligosaccharides, but rather to defective synthesis and transfer of nascent dolichol-linked oligosaccharide precursors. This abnormality could result in both a failure to glycosylate some sites on some proteins, as well as secondary abnormalities in overall glycoprotein processing and/or function.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein M., Kepes F., Schekman R. Sec59 encodes a membrane protein required for core glycosylation in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Mar;9(3):1191–1199. doi: 10.1128/mcb.9.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A., Fujimoto K., Kornfeld S. The primary glycosylation defect in class E Thy-1-negative mutant mouse lymphoma cells is an inability to synthesize dolichol-P-mannose. J Biol Chem. 1980 May 25;255(10):4441–4446. [PubMed] [Google Scholar]
- Crick D. C., Scocca J. R., Rush J. S., Frank D. W., Krag S. S., Waechter C. J. Induction of dolichyl-saccharide intermediate biosynthesis corresponds to increased long chain cis-isoprenyltransferase activity during the mitogenic response in mouse B cells. J Biol Chem. 1994 Apr 8;269(14):10559–10565. [PubMed] [Google Scholar]
- Elbein A. D. Glycosylation inhibitors for N-linked glycoproteins. Methods Enzymol. 1987;138:661–709. doi: 10.1016/0076-6879(87)38060-7. [DOI] [PubMed] [Google Scholar]
- Freeze H. H., Koza-Taylor P., Jones J. A., Loomis W. F. Cell-free N-glycosylation in Dictyostelium discoideum: analysis of wild-type and mutants defective in lipid-linked oligosaccharide biosynthesis. J Cell Biochem. 1990 May;43(1):27–42. doi: 10.1002/jcb.240430104. [DOI] [PubMed] [Google Scholar]
- Freeze H. H., Willies L., Hamilton S., Koza-Taylor P. Two mutants of Dictyostelium discoideum that lack a sulfated carbohydrate antigenic determinant synthesize a truncated lipid-linked precursor of N-linked oligosaccharides. J Biol Chem. 1989 Apr 5;264(10):5653–5659. [PubMed] [Google Scholar]
- Fukuda M. N. HEMPAS disease: genetic defect of glycosylation. Glycobiology. 1990 Sep;1(1):9–15. doi: 10.1093/glycob/1.1.9. [DOI] [PubMed] [Google Scholar]
- Hagberg B. A., Blennow G., Kristiansson B., Stibler H. Carbohydrate-deficient glycoprotein syndromes: peculiar group of new disorders. Pediatr Neurol. 1993 Jul-Aug;9(4):255–262. doi: 10.1016/0887-8994(93)90060-p. [DOI] [PubMed] [Google Scholar]
- Heller L., Orlean P., Adair W. L., Jr Saccharomyces cerevisiae sec59 cells are deficient in dolichol kinase activity. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7013–7016. doi: 10.1073/pnas.89.15.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herscovics A., Orlean P. Glycoprotein biosynthesis in yeast. FASEB J. 1993 Apr 1;7(6):540–550. doi: 10.1096/fasebj.7.6.8472892. [DOI] [PubMed] [Google Scholar]
- Hirschberg C. B., Snider M. D. Topography of glycosylation in the rough endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1987;56:63–87. doi: 10.1146/annurev.bi.56.070187.000431. [DOI] [PubMed] [Google Scholar]
- Horslen S. P., Clayton P. T., Harding B. N., Hall N. A., Keir G., Winchester B. Olivopontocerebellar atrophy of neonatal onset and disialotransferrin developmental deficiency syndrome. Arch Dis Child. 1991 Sep;66(9):1027–1032. doi: 10.1136/adc.66.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeken J., Carchon H., Stibler H. The carbohydrate-deficient glycoprotein syndromes: pre-Golgi and Golgi disorders? Glycobiology. 1993 Oct;3(5):423–428. doi: 10.1093/glycob/3.5.423. [DOI] [PubMed] [Google Scholar]
- Jaeken J., Carchon H. The carbohydrate-deficient glycoprotein syndromes: an overview. J Inherit Metab Dis. 1993;16(5):813–820. doi: 10.1007/BF00714272. [DOI] [PubMed] [Google Scholar]
- Jaeken J., De Cock P., Stibler H., Van Geet C., Kint J., Ramaekers V., Carchon H. Carbohydrate-deficient glycoprotein syndrome type II. J Inherit Metab Dis. 1993;16(6):1041–1041. doi: 10.1007/BF00711522. [DOI] [PubMed] [Google Scholar]
- Jaeken J., Eggermont E., Stibler H. An apparent homozygous X-linked disorder with carbohydrate-deficient serum glycoproteins. Lancet. 1987 Dec 12;2(8572):1398–1398. doi: 10.1016/s0140-6736(87)91287-6. [DOI] [PubMed] [Google Scholar]
- Jaeken J., Stibler H., Hagberg B. The carbohydrate-deficient glycoprotein syndrome. A new inherited multisystemic disease with severe nervous system involvement. Acta Paediatr Scand Suppl. 1991;375:1–71. [PubMed] [Google Scholar]
- Jaeken J., van Eijk H. G., van der Heul C., Corbeel L., Eeckels R., Eggermont E. Sialic acid-deficient serum and cerebrospinal fluid transferrin in a newly recognized genetic syndrome. Clin Chim Acta. 1984 Dec 29;144(2-3):245–247. doi: 10.1016/0009-8981(84)90059-7. [DOI] [PubMed] [Google Scholar]
- Konrad M., Merz W. E. Regulation of N-glycosylation. Long term effect of cyclic AMP mediates enhanced synthesis of the dolichol pyrophosphate core oligosaccharide. J Biol Chem. 1994 Mar 25;269(12):8659–8666. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee Y. C. High-performance anion-exchange chromatography for carbohydrate analysis. Anal Biochem. 1990 Sep;189(2):151–162. doi: 10.1016/0003-2697(90)90099-u. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Zeng Y. Pleiotropic resistance to glycoprotein processing inhibitors in Chinese hamster ovary cells. The role of a novel mutation in the asparagine-linked glycosylation pathway. J Biol Chem. 1989 Jan 25;264(3):1584–1593. [PubMed] [Google Scholar]
- McDowell W., Schwarz R. T. Dissecting glycoprotein biosynthesis by the use of specific inhibitors. Biochimie. 1988 Nov;70(11):1535–1549. doi: 10.1016/0300-9084(88)90290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellis S. J., Baenziger J. U. Separation of neutral oligosaccharides by high-performance liquid chromatography. Anal Biochem. 1981 Jul 1;114(2):276–280. doi: 10.1016/0003-2697(81)90480-2. [DOI] [PubMed] [Google Scholar]
- Merkle R. K., Cummings R. D. Lectin affinity chromatography of glycopeptides. Methods Enzymol. 1987;138:232–259. doi: 10.1016/0076-6879(87)38020-6. [DOI] [PubMed] [Google Scholar]
- März L., Hatton M. W., Berry L. R., Regoeczi E. The structural heterogeneity of the carbohydrate moiety of desialylated human transferrin. Can J Biochem. 1982 Jun;60(6):624–630. doi: 10.1139/o82-077. [DOI] [PubMed] [Google Scholar]
- Ohzeki T., Motozumi H., Hanaki K., Ohtahara H., Urashima H., Tsukuda T., Kobayashi S., Shiraki K., Ohno K. Carbohydrate-deficient glycoprotein syndrome in a girl with hypogonadism due to inactive follicle stimulating hormone. Horm Metab Res. 1993 Dec;25(12):646–648. doi: 10.1055/s-2007-1002198. [DOI] [PubMed] [Google Scholar]
- Petersen M. B., Brostrøm K., Stibler H., Skovby F. Early manifestations of the carbohydrate-deficient glycoprotein syndrome. J Pediatr. 1993 Jan;122(1):66–70. doi: 10.1016/s0022-3476(05)83488-2. [DOI] [PubMed] [Google Scholar]
- Plummer T. H., Jr, Tarentino A. L. Purification of the oligosaccharide-cleaving enzymes of Flavobacterium meningosepticum. Glycobiology. 1991 Jun;1(3):257–263. doi: 10.1093/glycob/1.3.257. [DOI] [PubMed] [Google Scholar]
- Powell L. D., Hart G. W. Quantitation of picomole levels of N-acetyl- and N-glycolylneuraminic acids by a HPLC-adaptation of the thiobarbituric acid assay. Anal Biochem. 1986 Aug 15;157(1):179–185. doi: 10.1016/0003-2697(86)90211-3. [DOI] [PubMed] [Google Scholar]
- Ramaekers V. T., Stibler H., Kint J., Jaeken J. A new variant of the carbohydrate deficient glycoproteins syndrome. J Inherit Metab Dis. 1991;14(3):385–388. doi: 10.1007/BF01811710. [DOI] [PubMed] [Google Scholar]
- Ripka J., Shin S., Stanley P. Decreased tumorigenicity correlates with expression of altered cell surface carbohydrates in Lec9 CHO cells. Mol Cell Biol. 1986 Apr;6(4):1268–1275. doi: 10.1128/mcb.6.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald A. G., Krag S. S. Lec9 CHO glycosylation mutants are defective in the synthesis of dolichol. J Lipid Res. 1990 Mar;31(3):523–533. [PubMed] [Google Scholar]
- Rosenwald A. G., Stanley P., Krag S. S. Control of carbohydrate processing: increased beta-1,6 branching in N-linked carbohydrates of Lec9 CHO mutants appears to arise from a defect in oligosaccharide-dolichol biosynthesis. Mol Cell Biol. 1989 Mar;9(3):914–924. doi: 10.1128/mcb.9.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald A. G., Stanley P., McLachlan K. R., Krag S. S. Mutants in dolichol synthesis: conversion of polyprenol to dolichol appears to be a rate-limiting step in dolichol synthesis. Glycobiology. 1993 Oct;3(5):481–488. doi: 10.1093/glycob/3.5.481. [DOI] [PubMed] [Google Scholar]
- Rosner M. R., Hubbard S. C., Ivatt R. J., Robbins P. W. N-asparagine-linked oligosaccharides: biosynthesis of the lipid-linked oligosaccharides. Methods Enzymol. 1982;83:399–408. doi: 10.1016/0076-6879(82)83037-1. [DOI] [PubMed] [Google Scholar]
- Sairam M. R., Linggen J., Sairam J., Bhargavi G. N. Influence of carbohydrates on the antigenic structure of gonadotropins: distinction of agonists and antagonists. Biochem Cell Biol. 1990 May;68(5):889–893. doi: 10.1139/o90-131. [DOI] [PubMed] [Google Scholar]
- Stibler H., Allgulander C., Borg S., Kjellin K. G. Abnormal microheterogeneity of transferrin in serum and cerebrospinal fluid in alcoholism. Acta Med Scand. 1978;204(1-2):49–56. doi: 10.1111/j.0954-6820.1978.tb08397.x. [DOI] [PubMed] [Google Scholar]
- Stibler H., Blennow G., Kristiansson B., Lindehammer H., Hagberg B. Carbohydrate-deficient glycoprotein syndrome: clinical expression in adults with a new metabolic disease. J Neurol Neurosurg Psychiatry. 1994 May;57(5):552–556. doi: 10.1136/jnnp.57.5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibler H., Jaeken J. Carbohydrate deficient serum transferrin in a new systemic hereditary syndrome. Arch Dis Child. 1990 Jan;65(1):107–111. doi: 10.1136/adc.65.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibler H., Westerberg B., Hanefeld F., Hagberg B. Carbohydrate-deficient glycoprotein (CDG) syndrome--a new variant, type III. Neuropediatrics. 1993 Feb;24(1):51–52. doi: 10.1055/s-2008-1071513. [DOI] [PubMed] [Google Scholar]
- Stoll J., Krag S. S. A mutant of Chinese hamster ovary cells with a reduction in levels of dolichyl phosphate available for glycosylation. J Biol Chem. 1988 Aug 5;263(22):10766–10773. [PubMed] [Google Scholar]
- Stoll J., Robbins A. R., Krag S. S. Mutant of Chinese hamster ovary cells with altered mannose 6-phosphate receptor activity is unable to synthesize mannosylphosphoryldolichol. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2296–2300. doi: 10.1073/pnas.79.7.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Radioactive tracer techniques in the sequencing of glycoprotein oligosaccharides. FASEB J. 1991 Feb;5(2):226–235. doi: 10.1096/fasebj.5.2.2004668. [DOI] [PubMed] [Google Scholar]
- Wada Y., Gu J., Okamoto N., Inui K. Diagnosis of carbohydrate-deficient glycoprotein syndrome by matrix-assisted laser desorption time-of-flight mass spectrometry. Biol Mass Spectrom. 1994 Feb;23(2):108–109. doi: 10.1002/bms.1200230211. [DOI] [PubMed] [Google Scholar]
- Wada Y., Nishikawa A., Okamoto N., Inui K., Tsukamoto H., Okada S., Taniguchi N. Structure of serum transferrin in carbohydrate-deficient glycoprotein syndrome. Biochem Biophys Res Commun. 1992 Dec 15;189(2):832–836. doi: 10.1016/0006-291x(92)92278-6. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Ideo H., Ohkura T., Fukushima K., Yuasa I., Ohno K., Takeshita K. Sugar chains of serum transferrin from patients with carbohydrate deficient glycoprotein syndrome. Evidence of asparagine-N-linked oligosaccharide transfer deficiency. J Biol Chem. 1993 Mar 15;268(8):5783–5789. [PubMed] [Google Scholar]
- Yamashita K., Ohkura T., Ideo H., Ohno K., Kanai M. Electrospray ionization-mass spectrometric analysis of serum transferrin isoforms in patients with carbohydrate-deficient glycoprotein syndrome. J Biochem. 1993 Dec;114(6):766–769. doi: 10.1093/oxfordjournals.jbchem.a124253. [DOI] [PubMed] [Google Scholar]
- Yasugi E., Nakasuji M., Dohi T., Oshima M. Major defect of carbohydrate-deficient-glycoprotein syndrome is not found in the synthesis of dolichyl phosphate or N-acetylglucosaminyl-pyrophosphoryl-dolichol. Biochem Biophys Res Commun. 1994 Apr 29;200(2):816–820. doi: 10.1006/bbrc.1994.1524. [DOI] [PubMed] [Google Scholar]



