Abstract
Background
Perioperative opioid administration results in postoperative nausea and vomiting (PONV) and acute opioid tolerance that manifests in increased postoperative pain. Esmolol is an ultra short acting cardioselective β1-adrenergic receptor antagonist, and it has been successfully used for perioperative sympatholysis and it reduces the opioid requirement during total intravenous anesthesia. We tested the hypothesis that perioperative esmolol administration results in decreased PONV and postoperative pain.
Methods
Sixty patients undergoing laparoscopic appendectomy were randomly assigned to two groups (Group E and Group C). The Group E patients were administered 5-10 µg/kg/min esmolol with remifentanil that was titrated to the autonomic response. The Group C patients received normal saline that was of the same volume as the esmolol in Group E, and the remifentanil was also titrated to the vital sign. Before intubation and extubation, the Group E patients were administered 1.0 mg/kg esmolol, and the Group C patients were administered normal saline of the same volume. The incidence and severity of PONV, the pain score, the rescue antiemetics and the rescue analgesics were assessed 30 min, 6 h and 24 h after surgery. The mean arterial pressure and heart rate under anesthesia were also recorded.
Results
PONV and postoperative pain were significantly increased in Group C. These patients needed more antiemetics and analgesics in the first 24 postoperative hours. The mean arterial pressure and heart rate were significantly higher in Group C at the time of intubation and extubation.
Conclusions
Perioperative esmolol administration contributes to the significant decrease in PONV and postoperative pain, and so this facilitates earlier discharge.
Keywords: Esmolol, PONV, Postoperative pain
Introduction
The development of anesthetics has facilitated quick anesthetic induction, the use of awake anesthesia and safe recovery. The advancement of tools and skills has allowed laparoscopic appendectomies to be performed in a short time and usually within 30 minutes. Because laparoscopic appendectomies require only a small incision and a low level of organ manipulation, they cause less pain and enteroplegia compared to laparatomies, and they allow for earlier discharge from the hospital [1]. Therefore, with the patient under general anesthesia, laparoscopic appendectomy allows faster and safer recovery.
Total intravenous anesthesia using propofol and remifentanil is commonly preferred because of its better rates of recovery of consciousness and the lower rate of PONV and pain after ceasing the administration of anesthetics [2]. PONV and pain following laparoscopy are the main causes for lengthening the period of hospitalization [3,4].
Esmolol is a β-adrenergic receptor antagonist that selectively affects the cardiovascular system. Its effect is rapid and it has a short half life. It is used as an anesthetic supplement to reduce the hemodynamic responses to surgical stimulation. This β-adrenergic receptor antagonist has a sympathetic nerve blocking effect on PONV and pain [5].
This study attempted to see the effects of a perioperative continuous infusion of esmolol on the PONV and pain in laparoscopic appendectomies with the patient under total intravenous anesthesia using propofol and remifentanil.
Materials and Methods
The study was performed on 60 patients between the ages 18-45, their ASA class was I or II and they were undergoing laparoscopic appendectomy. They were randomly placed into 2 groups: Group E (esmolol group, n = 30) and Group C (Group C, n = 30). The following patients were excluded from the study: the patients with side-effects or hypersensitivity towards the tested drugs, those with cardiovascular diseases, metabolic diseases, renal diseases, liver diseases, neurological disorders or pregnancy, those women who were breast-feeding and those who had taken antiemetics within the last 48 hours before the surgery. With the approval of the hospital's ethics committee, we visited patients before the surgery and explained the purpose of this research and we obtained their written consent. Thirty minutes before the surgery, glycopyrrolate 0.2 mg was intramuscularly administered to all the patients as premedication. Before anesthetic induction, the ECG, the automated noninvasive blood pressure device, the pulse-oximeter, the bispectral index (BIS) sensor and a thermometer were attached to the patients for monitoring. Before endotracheal intubation, Group E was intravenously administered with esmolol 1.0 mg/kg, and Group C was intravenously administered the same amount of normal saline. For anesthetic induction, propofol 2 mg/kg and rocuronium 0.8 mg/kg were intravenously administered before tracheal intubation. With a perioperative continuous infusion of remifentanil 0.2-0.5 µg/kg/min and propofol 75-85 µg/kg/min, the mean arterial blood pressure and heart rate were maintained within the preoperative levels. After anesthetic induction, Group E was continuously infused with esmolol 10 µg/kg/min and Group C was continuously infused with the same amount of normal saline. When perioperative hypotension (the mean arterial pressure below 60 mmHg) or bradycardia (the heartbeat rate below 40 bpm) occurred, ephedrine 10 mg or atropine 0.5 mg was intermittently administered in both groups.
Two surgeons who were experienced with laparoscopic appendectomy performed all the surgeries. Intraperitoneal CO2 insufflation was formed, and the peritoneal pressure was maintained at 12 mmHg during the surgery. After the intraperitoneal CO2 insufflation, the patients were placed in the Trendelenburg position and their left sides were turned down so that there was better access to the appendix.
To prevent surgical awareness and maintain an appropriate anesthetic depth during anesthesia in both groups, the remifentanil concentration was adjusted so that the BIS index reached 40-60. Mechanical ventilation was set so that the inhaled oxygen concentration reached 50% mixed with air. The end tidal CO2 was maintained at the normal level. For additional nerve muscle relaxation, rocuronium 0.1 mg/kg was intravenously administered, and warming blankets were used perioperatively to maintain the normal body temperature.
After skin-suturing, the intravenous infusion of propofol and remifentanil was stopped. For post-operative reversal of muscle relaxation, glycopyrrolate 0.008 mg/kg and pyridostigmine 0.03 mg/kg were mixed and intravenously infused. Right before extubation, Group E was infused with esmolol 1.0 mg/kg, and Group C was infused with natural saline of the same amount. After extubation, the continuous infusions of esmolol and natural saline were stopped. Drugs that could affect postoperative nausea and vomiting were not administered, and the amounts of remifentanil and esmolol administered up to that point were recorded.
For both groups, the patients' age, gender, weight, height, the length of operation, the length of time under anesthesia, the amount of perioperative fluid administration, smoker or nonsmoker, the presence of PONV and nausea, the amounts of perioperative infusion of esmolol, remifentanil and propofol and the time from the arrival into the recovery room to the time of discharge were all recorded.
In both groups, the presence and levels of pain, nausea and vomiting were recorded 30 minutes, 6 hours and 24 hours after the surgery. The recording was done by a person not involved with the anesthesia. There were 4 levels of nausea and vomiting (free, mild, moderate, severe) [6]. Free was defined as the absence of nausea and vomiting. Mild was when there was light nausea. Moderate was with 1-2 gag reflexes without actual vomiting and antiemetics were required. Severe was vomiting at least once or experiencing nausea at least twice and antiemetics were required at least once.
Metoclopramide 10 mg was administered by IV when the patient required antiemetics due to severe post-operative vomiting or continuous nausea. The level of pain was measured on a visual analogue scale (VAS) with grid points where 100 was the maximum and O indicated no pain. If the VAS score was 30+ or if the patient required analgesics, then diclofenac 90 mg was intramuscularly infused. The patients were discharged if they had no fever, bowel sounds could be heard and they could normally digest food.
In both groups, the mean arterial pressure (MAP) and heart rate (HR) were measured right before anesthetic induction, at the point of endotracheal intubation, 5 minutes after intubation, at the point of incision, 10 minutes after incision, at the point of extubation, 5 minutes after extubation and at the arrival point into the recovery room.
The recorded scores are shown as the mean ± standard deviation (SD) based on the percent of the number of patients (%). The patients' characteristics were compared with the VAS scores, and the MAP was compared with the HR using independent t-tests. PONV comparisons were done using the chi-square test. A P value of 0.05 or less was considered significant.
Results
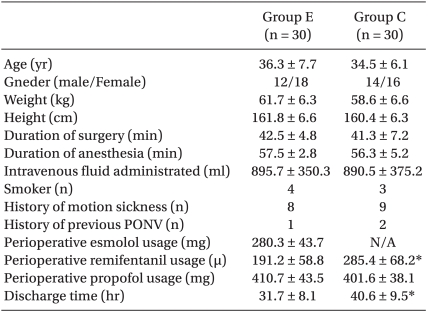
There were no significant differences found in the age, weight, height, the length of surgery, the length of anesthesia, the administered amount of fluid or the perioperatively amount of administered propofol. There were also no significant differences in the number of women who were more vulnerable to PONV, the patients with the history of PONV, the patients with the history of motion sickness and the nonsmokers (Table 1).
Table 1.
Patients' Characteristics
The data is presented as means ± SDs or the number of patients (n). There are no significant differences between the groups. Group E: esmolol group, Group C: control group. *P < 0.05 compared with Group E.
The amount of remifentanil perioperatively used in Group E was 191.2 ± 58.8 mg and it was 285.4 ± 68.2 mg in Group C. So Group E used a smaller amount of remifentanil (P = 0.035). The length of hospitalization from the point of post-surgery to the point of discharge was shorter in Group E (31.7 ± 8.1 h) than that in Group C (40.6 ± 9.5 h) (P = 0.027), (Table 1).
The rate of not experiencing PONV 30 minutes after the surgery was 77% in Group E (N = 23) and 43% in Group C (N = 13) (P = 0.025). Both 6hrs and 24 hrs after the surgery, the occurrence rate of PONV was lower in Group E than that in Group C, but no statistically significant difference was found (Table 2).
Table 2.
The Incidence and Severity of PONV and Metocloprimde Usage
The data is expressed as the numbers (percent) of the patient. Group E: esmolol group, Group C: control group. *P < 0.05 compared with Group E.
Post-operative antiemetics were used in 6 patients of Group E (20%) and in 11 patients of Group C (37%) and the difference was significant (P=0.04), (Table 2).
The VAS score 30 minutes post-surgery showed that the postoperative pain in Group E (45.1 ± 2.4 mm) was lower compared to that of Group C (60.2 ± 4.2 mm) (P = 0.014). Even 6 hr and 24 hr after the surgery, the VAS score was lower in Group E than that in Group C, but there was no statistically significant difference (Table 3).
Table 3.
VAS Scores and Diclofenac Usage
The data is presented as means ± SDs. Group E: esmolol group, Group C: control group. *P < 0.05 compared with Group E.
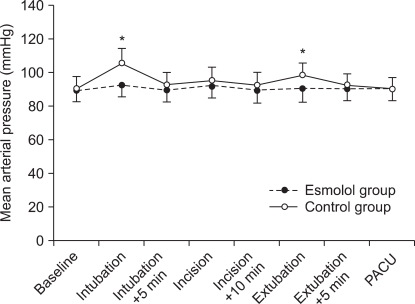
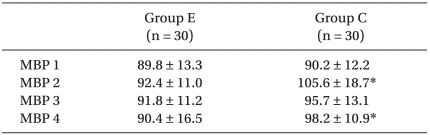
The change in MAP at the time of intubation in Group E (92.4 ± 11.0 mmHg) was lower than that in Group C (105.6 ± 18.7 mmHg)(P = 0.014). At the time of incision, there was no significant difference between the groups. At the time of extubation, the MAP in Group E (90.4 ± 16.5 mmHg) was lower than that in Group C (98.2 ± 10.9 mmHg)(P = 0.047), (Fig. 1, Table 4). There were no other significant differences.
Fig. 1.
Mean arterial pressure changes during anesthesia. *P < 0.05, significant inter-group difference. PACU: post anesthesia care unit.
Table 4.
The Changes of Mean Arterial Pressure (mmHg)
Data are mean ± SD. MBP 1: mean arterial pressure before anesthetic induction, MBP 2: mean arterial pressure of immediately after intubation, MBP 3: mean arterial pressure of immediately after incision, MBP 4: mean arterial of pressure immediately after extubation, Group E: esmolol group, Group C: control group. *P < 0.05 compared with Group E.
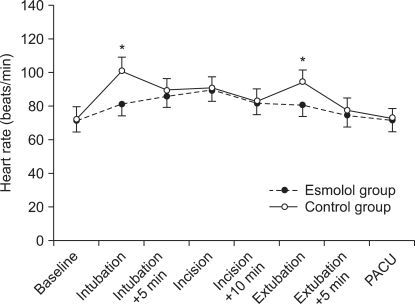
The perioperative HR change at the time of endotracheal intubation was lower in Group E (81.3 ± 12.4 bpm) than that in Group C (101.2 ± 14.8 bpm)(P = 0.024) (Fig. 2). At the time of incision, there was no significant difference between the two groups. But at the time of extubation, the HR was lower in Group E (80.8 ± 11.6 bpm) than that in Group C (94.5 ± 11.7 bpm) (P = 0.038), (Fig. 2, Table 5). There were no other significant differences during the surgery.
Fig. 2.
Heart rate changes during anesthesia. *P < 0.05, significant inter-group differences. PACU: post anesthesia care unit.
Table 5.
The Changes of Heart Rate (bpm)
Data are mean ± SD. HR 1: heart rate before anesthetic induction, HR 2: heart rate of immediately after intubation, HR 3: heart rate of immediately after incision, HR 4: heart rate of immediately after extubation, Group E: esmolol group, Group C: control group. *P < 0.05 compared with Group E.
Discussion
PONV and nausea more often occur in cases with the presence of perioperative hemodynamic instability, female patients, nonsmokers and patients with a past history of PONV or motion sickness. General anesthesia using inhalational anesthetics or nitrous oxide with the perioperative use of opioids can increase nausea and vomiting [3]. In the present study, there was no significant difference in such factors as gender, a past history of PONV, a history of motion sickness, smoking, the length of surgery and the amount of administered fluid. Therefore, the differences between Group E and Group C for PONV and pain are assumed to be due to the different use and dosage of drugs.
The perioperative use of esmolol as an anesthetic supplement has been recently reported to decrease the occurrence of PONV. Coloma et al. [7] stated that for outpatient gynecologic surgeries using desflurane and remifentanil, the use of esmolol in anesthesia decreases the occurrence rate of PONV. Ozturk et al. [8] reported that for laparoscopic cholecystectomy, the use of esmolol and alfentanil together reduces PONV. In the present study, Group E had fewer patients who were postoperatively administered antiemetics compared to that of Group C (Table 2). Although there is no definitely agreed upon theory, the perioperative use of β-adrenergic receptor antagonists reduces the required dosage of the perioperative opioids, which decreases the likelihood of opioids' side-effects such as nausea and vomiting [7,8,10]. To reduce the cardiovascular changes, the perioperative use of β-adrenergic receptor antagonists alone has no anesthetic effect, but when used as a supplement, they have an anesthetic sparing effect [7,9,10]. Coloma et al. [7] stated that if desflurane is used for general anesthesia in laparoscopic gynecologic surgeries, then esmolol can replace remifentanil. Zenichiro et al. [10] stated that the perioperative intravenous administration of the β-adrenergic receptor antagonist ladiolol reduces the minimal alveolar concentration (MAC) of sevoflurane. Johansen et al. [9] stated that when using propofol, nitrous oxide and morphine in anesthesia, esmolol reduces the required amount of anesthetics needed for a skin incision. When a great amount of opioid is used during surgery, depressed ventilation, excessive sedation, PONV, tingling, difficulty with voiding and ileus occur [11]. Therefore, the perioperative use of β-adrenergic receptor antagonists will have an opioid sparing effect, which can help prevent PONV [8]. In the present study, the administered dosage of remifentanil was significantly lower in Group E compared to that of Group C (Table 1), which was assumedly brought about the decreased PONV and the reduced use of antiemetics. It is currently not clearly known if the β-adrenergic receptor antagonist esmolol as a perioperative anesthesia supplement has an anesthetic sparing effect. Kurita et al. [12] stated that although the β-adrenergic receptor antagonist propranolol, which is lipid-soluble, has an anesthetic sparing effect in general anesthesia, landiolol and esmolol, which are more hydro-soluble, have lower penetrability into the blood-brain barrier and so it is unclear whether they have such an effect. Further studies on this are needed.
Another mechanism involved in the prevention of esmolol-related PONV could be the excessive perioperative cardiovascular changes [13]. So the use of esmolol can mitigate such perioperative hemodynamic changes and prevent PONV [14,15].
The dosage of remifentanil was set so that the hemodynamic changes in the groups would be in the 20% range of the baseline rate. But during endotracheal intubation and extubation, there were significant differences in the MAP and HR between Group E and Group C. The MAP and HR were maintainable in Group E because esmolol was used right before intubation and extubation.
Tan et al. [14] reported that the systolic blood pressure changes were stable in the patient group that was administered 1.0 mg/kg and nircadipine 30 µg/kg at the point of intubation as compared to that of the control group, which was administered natural saline. Dyson et al. [16] stated that the administration of esmolol 1.5 mg/kg before extubation will safely maintain the systolic blood pressure and HR. Kang et al. [17] stated that to keep the occurrence rate of bradycardia and systolic PHT within 5% due to ETI performed with thiopental sodium, vecuronium, and enflurane as anesthesia, the amount of required esmolol is around 1 mg/kg.
The experiment showed that the levels of PONV and pain and the use of analgesics were significantly reduced in Group E compared to that of Group C. Yavascaoglu et al. [18] used esmolol 0.5 mg/kg for anesthetic induction and they found that rocuronium as a muscle relaxant significantly reduces pain responses [18]. This was explained by Hageluken et al. [19], who stated that the β-adrenergic receptor antagonists have a direct effect in activating Gi-protein, which decreases the delivery of the spinal pain signals. Avram et al. [20] noted that the β-adrenergic receptor antagonists reduce the hepatic blood flow, which slows the metabolism of opioids and lengthens their anesthetic effect, and this reduces the need for the postoperative use of analgesics. Although Lee et al. [21] argued that the use of esmolol in laparoscopic gynecologic surgeries reduces the rate of PONV and pain (but not significantly), it is assumed that they did not simultaneously use esmolol with remifentanil, but rather they administered one or the other to a group. Further, only the presence/absence of PONV was recorded and the levels of PONV were not, which may have contributed to their difference compared with our present findings.
The mechanism to how perioperative infusion of esmolol reduces postoperative pain is explained by the fact that β-adrenergic receptor antagonists reduce the necessary perioperative dosage of opioids [7,9,10] and they decreases opioid tolerance. Opioids, and especially remifentanil or alfentanil, which are short acting opioids, they have fast elimination rates and they produce early tolerance. The high dosage of perioperative opioids or their continuous IV infusion can increase opioid tolerance and bring about severe postoperative pain [11,22,23].
Bruno et al. [22] compared the use of remifentanil with desflurane for anesthetic maintenance and they found that the group exposed to remifentanil experienced higher levels of pain. Also, a higher level of morphine was used as a postoperative palliative drug in the Group exposed to remifentanil but still, the level of PONV was worse than the Group exposed to desflurane. This may be due to rapid opioid tolerance. Although the tolerance rate is similar regardless of the opioid's potency, the time it takes to develop opioid tolerance is different. Alfentanil and remifentanil are short acting opioids with quickly developing tolerance [24].
Laparoscopic appendectomies have shorter recovery times and lengths of hospitalization compared to laparatomic appendectomies. They allow for faster recovery to normal daily routines and they have lower incidences of pain [25]. However, for better visibility, intraperitoneal CO2 insufflation is performed, which works as a vasodilator on the cerebral vessels, it increases the cerebral blood flow and cerebral blood pressure, and this increases PONV [4]. But when esmolol is used as a perioperative supplement, PONV and pain can be reduced and there is a faster recovery and return to daily activities.
In conclusion, using the perioperative β-adrenergic receptor antagonist esmolol as an anesthetic supplement can reduce the PONV and pain, but further research that will focus on this subject is still needed.
References
- 1.Seo KW, Choi YG, Choi JY, Yoon KY. Laparoscopic appendectomy is feasible for inexperienced surgeons in the early days of individual laparoscopic training courses. J Korean Surg Soc. 2009;76:23–27. [Google Scholar]
- 2.Jeong JH, Song SO, Kim HD. A comparison of the recovery characteristics of propofol and sevoflurane anesthesia under bispectral index system monitoring. Korean J Anesthesiol. 2004;46:528–534. [Google Scholar]
- 3.Lipp A, Kaliappan A. Focus on quality. Managing pain and ponv in day surgery. Curr Anaesth Crit Care. 2007;18:200–207. [Google Scholar]
- 4.Koivusalo AM, Kellokumpu I, Lindgren L. Gasless laparoscopic cholecystectomy: comparison of postoperative recovery with conventional technique. Br J Anaesth. 1996;77:576–580. doi: 10.1093/bja/77.5.576. [DOI] [PubMed] [Google Scholar]
- 5.Davidson EM, Szmuk P, Doursout MF, Chelly J. Antinociceptive and cardiovascular properties of esmolol following formalin injection in rats. Can J Anaesth. 2001;48:59–64. doi: 10.1007/BF03019816. [DOI] [PubMed] [Google Scholar]
- 6.Eberhart LH, Seeling W, Ulrich B, Morin AM, Georgieff M. Dimenhydrinate and metoclopramide alone or in combination for prophylaxis of PONV. Can J Anaesth. 2000;47:780–785. doi: 10.1007/BF03019481. [DOI] [PubMed] [Google Scholar]
- 7.Coloma M, Chiu JW, White PF, Armbruster SC. The use of esmolol as an alternative for fast-track outpatient gynecologic laparoscopic surgery. Anesth Analg. 2001;92:352–357. doi: 10.1097/00000539-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Ozturk T, Kaya H, Aran G, Aksun M, Savaci S. Postoperative beneficial effects of esmolol in treated hypertensive patients undergoing laparoscopic cholecystectomy. Br J Anaesth. 2007;100:211–214. doi: 10.1093/bja/aem333. [DOI] [PubMed] [Google Scholar]
- 9.Johansen JW, Flaishon R, Sebel PS. Esmolol reduces anesthetic requirement for skin incision during propofol/nitorous oxide/morphine anesthesia. Anesthesiology. 1997;86:364–371. doi: 10.1097/00000542-199702000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Wajima Z, Shiga T, Imanaga K, Inoue T, Sakamoto A. Intravenous landiolol, A Novel β-adrenergic blocker, reduces MAC of sevoflurane in human adults. Anesthesiology. 2006;105:A629. [Google Scholar]
- 11.White PF. The role of non-opioid analgesic techniques in the management of pain after ambulatory surgery. Anesth Analg. 2002;94:577–585. doi: 10.1097/00000539-200203000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Kurita T, Takata K, Morita K, Sato S. Lipophilic β-adrenoceptor antagonist propranolol increases the hypnotic and anti-nociceptive effects of isoflurane in a swine model. Br J Anaesth. 2008;100:841–845. doi: 10.1093/bja/aen089. [DOI] [PubMed] [Google Scholar]
- 13.Rothenberg DM, Parnass SM, Litwack K, McCarty RJ, Newman LM. Efficacy of ephedrine in the prevention of postoperative nausea and vomiting. Anesth Analg. 1991;72:58–61. doi: 10.1213/00000539-199101000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Tan PH, Yang LC, Shih HC, Lin CR, Lan KC, Chen CS. Combined use of esmolol and nicardipine to blunt the haemodynamic changes following laryngoscopy and tracheal intubation. Anaesthesia. 2002;57:1207–1212. doi: 10.1046/j.1365-2044.2002.02624_4.x. [DOI] [PubMed] [Google Scholar]
- 15.Unal Y, Ozsoylar O, Sariguney D, Arslan M, Yardim RS. The efficacy of esmolol to blunt the haemodynamic response to endotracheal extubation in lumbar disc surgery. Res J Med Sci. 2008;2:99–104. [Google Scholar]
- 16.Dyson A, Isaac PA, Pennant JH, Giesecke AH, Lipton JM. Esmolol attenuates cardiovascular responses to extubation. Anesth Analg. 1990;71:675–678. doi: 10.1213/00000539-199012000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Kang KC, Im JB, Joo JS. Appropriate dose of esmolol for protection of tachycardia and hypertension by endotracheal intubation. Korean J Anesthesiol. 1998;34:526–530. [Google Scholar]
- 18.Yavascaoglu B, Kaya FN, Ozcan B. Esmolol pretreatment reduces the frequency and severity of pain on injection of rocuronium. J Clin Anesth. 2007;19:413–417. doi: 10.1016/j.jclinane.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Hageluken A, Naurnberg B, Harhammer R, Schunack W, Seifert R. Lipophilic beta-adrenoceptor antagonists are effective direct activators of G-proteins. Biochem Pharmacol. 1994;47:1789–1795. doi: 10.1016/0006-2952(94)90307-7. [DOI] [PubMed] [Google Scholar]
- 20.Avram MJ, Krejcie TC, Henthorn TK, Niemann CU. Beta-adrenergic blockade affects initial drug distribution due to decreased cardiac output and altered blood flow distribution. J Pharmacol Exp Ther. 2004;311:617–624. doi: 10.1124/jpet.104.070094. [DOI] [PubMed] [Google Scholar]
- 21.Lee HY, Kwon WJ, Lee JU. The effects of esmolol, esmolol and nicardipine or remifentanil on mean blood pressure, heart rate and recovery in gynecologic laparoscopic surgery. Korean J Anesthesiol. 2008;55:709–715. [Google Scholar]
- 22.Guignard B, Bossard A, Coste C, Sessler D, Lebrault C, Alfonsi P, et al. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93:409–417. doi: 10.1097/00000542-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Vinik H, Kissin I. Rapid development of tolerance to analgesia during remifentanil infusion in humans. Anesth Analg. 1998;86:1307–1311. doi: 10.1097/00000539-199806000-00033. [DOI] [PubMed] [Google Scholar]
- 24.Kissin I, Brown PT, Brandley EL. Magnitude of acute tolerance to opioid is not related to their potency. Anesthesiology. 1991;75:813–816. doi: 10.1097/00000542-199111000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Lee SY, Park YG, Jang IT. The comparitive analysis between laparoscopic and open appendectomy. J Korean Surg Soc. 2004;67:65–69. [Google Scholar]