Abstract
We have developed a biodegradable composite scaffold for bone tissue engineering applications with a pore size and interconnecting macroporosity similar to those of human trabecular bone. The scaffold is fabricated by a process of particle leaching and phase inversion from poly(lactideco-glycolide) (PLGA) and two calcium phosphate (CaP) phases both of which are resorbable by osteoclasts; the first a particulate within the polymer structure and the second a thin ubiquitous coating. The 3–5 μm thick osteoconductive surface CaP abrogates the putative foreign body giant cell response to the underlying polymer, while the internal CaP phase provides dimensional stability in an otherwise highly compliant structure. The scaffold may be used as a biomaterial alone, as a carrier for cells or a three-phase drug delivery device. Due to the highly interconnected macroporosity ranging from 81% to 91%, with macropores of 0.8∼1.8 mm, and an ability to wick up blood, the scaffold acts as both a clot-retention device and an osteoconductive support for host bone growth. As a cell delivery vehicle, the scaffold can be first seeded with human mesenchymal cells which can then contribute to bone formation in orthotopic implantation sites, as we show in immune-compromised animal hosts. We have also employed this scaffold in both lithomorph and particulate forms in human patients to maintain alveolar bone height following tooth extraction, and augment alveolar bone height through standard sinus lift approaches. We provide a clinical case report of both of these applications; and we show that the scaffold served to regenerate sufficient bone tissue in the wound site to provide a sound foundation for dental implant placement. At the time of writing, such implants have been in occlusal function for periods of up to 3 years in sites regenerated through the use of the scaffold.
Key words: bone regeneration, scaffold, composite, biodegradable, clot retention, osteoconduction, cell delivery, extraction socket, sinus lift, clinical
Introduction
We have developed a biodegradable composite scaffold for bone tissue engineering applications with a pore size and interconnecting macroporosity similar to those of human trabecular bone, the development of which we have extensively reported elsewhere. 1–3 The continuing need for bone scaffolding materials is driven not only by the surgical requirement to either augment or alternatively to avoid reliance upon, traditional autograft procedures which are common practice in orthopedic and craniomaxillofacial surgery; but also to address the shortcomings of existing materials that may show limited degradation in vivo,4 an architecture which does not reflect that of the natural tissue,5 or a porosity and interconnected macroporosity that limits host tissue invasion.6 Furthermore, materials that are manufactured at temperatures above that which denatures proteins, nominally considered to be 60°C, cannot be processed to include biologically active molecules, such as growth factors since the processing temperature will denature the bio-effector.7,8 In addition to the use of biomaterials to replace bone grafts, the advent of tissue engineering strategies to increase their effectiveness by the incorporation of osteogenic-capable cells and/or growth factors adds further versatility to an appropriate carrier material. Given the appositional nature of bone formation, the scaffold material remains fundamental in bone tissue engineering strategies. Our strategy to develop a biomaterial for such an application has involved the fabrication of a totally biodegradable,9 osteoconductive, macroporous composite scaffold with a resultant architecture that is similar to trabecular bone, particularly with respect to the high level of interconnecting macroporosity, and with an ability to wick up blood. We believe the latter is important since the scaffold acts as both a clot-retention device and an osteoconductive support for host bone growth.
Here we provide a brief overview of the scaffold construction process; the biological rationales for the design modifications, including the need to enhance the compressive modulus of the polymeric component, the abrogation of the foreign body giant cell response to the latter and the rendering of an osteoconductive surface through the addition of a 3–5 μm thick surface CaP coating. We also show that the scaffold may be employed to deliver human osteogenic-capable cells into orthotopic sites in T-cell depleted rnu/rnu rats and that such cells contribute to bone formation in the recipient animals. Finally, we provide two case reports where the scaffold has been employed, in humans in lithomorph and particulate forms in dental extraction sockets and sinus lift procedures respectively to either maintain or increase, alveolar bone height prior to implant placement and the subsequent functional loading of such implants.
Our work demonstrates that it is possible to design, fabricate and clinically employ a completely biodegradable, osteoconductive, 3-phase composite material to augment or regenerate bone.
Scaffold Processing
The scaffold was made from poly(DL-lactide-co-glycolide) 75/25 with an inherent viscosity (IV) of 1.33 dL/g (Birmingham Polymers Inc., Birmingham, AL, USA); tetracalcium phosphate (TTCP) from Taihei chemical Industrial Co., (Osaka, Japan), and dicalcium phosphate anhydrous (DCPA) and dimethyl sulfoxide (DMSO) from Sigma Chemical Co., (St. Louis, Mo, USA). Sugar particles were purchased from Tate & Lyle North America Inc., (Toronto, Canada). The particles were sieved at varying sizes: 0.59∼0.85 mm, 0.85∼1.18 mm, 0.85∼2.0 mm and 1.18∼2.0 mm. These particles were used as the porogen.2
Calcium phosphate cement particles were prepared by mixing equimolar TTCP and DCPA with deionized distilled water (ddH2O) at 100% relative humidity for 24 hr. The reactant was ground and sieved through a series of custom-made sieves in pore size ranging from 5 to 45 μm. The average size of the CaP particle was 27.5 μm.2
The preparation procedure for the scaffold was modified from that described previously.10 Briefly, PLGA was dissolved in DMSO (10∼15% w/w) and then the PLGA solution was mixed with CaP particles in the CaP/PLGA ratio of 3:1 (w/w), and poured into a mold filled with sugar crystals, allowing the solution to diffuse throughout the mass of porogen crystals. After 2∼3 minutes the mold was transferred to a fridge at −18°C for 1 hr to set the mixture. The PLGA precipitated and the sugar crystals were leached out of the mixture in ddH2O at room temperature (20°C) for 3 days, to produce a 10 cm × 10 cm × 1.5 cm scaffold block.2
In order to deposit a thin surface layer of CaP onto and throughout the macroporous composite scaffolds, dry PLGA/ CaP cylinders or particulate formed by grinding such lithomorphs, were prewetted in 70% ethanol and immersed in so-called “simulated body fluid” using a composition and method adapted from that of Kokubo.11 Following coating, sample scaffolds were stored dry in a desiccator or packaged and gamma-sterilized for clinical use following industry standards.
Biological rationales driving design criteria. The original biological rationale for the scaffold design was to mimic, in a biodegradable polymeric material, the three dimensional architecture of natural human trabecular bone, since it is the latter that is employed in the majority of bone grafting procedures. Indeed, it should be emphasized that during such bone grafting, which involves the transport of small trabecular bone fragments from the harvesting site to the recipient site in the body, the bone fragments, which are bathed in blood and bone marrow, have no composite strength and cannot be used in load-bearing applications without the need for exogenous mechanical support. Thus it was not our intention to produce a synthetic analogue that could be used in load bearing applications, without such additional mechanical support. Specifically, the function of a trabecular bone graft is to move bone proteins and cells from one site to the other, and thus we argued that the 3-D architecture of the synthetic analogue should be suitable to permit ubiquitous osteogenic cell seeding prior to implantation. We have shown that the macropore size of a scaffold is critically important in permitting ubiquitous cell seeding, and that pores of the 200 micron range can be bridged by cells, while pores in the 450 micron range cannot be bridged by cells.12 This is critically important in the design of the interconnecting macroporosity of a scaffold since without sufficiently large interconnecting pores, bone will grow only on the outer margins of the scaffold.6
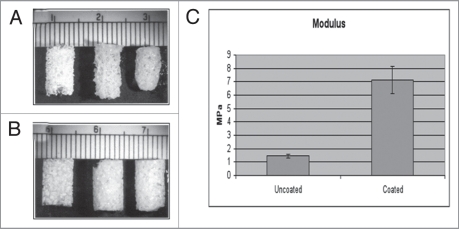
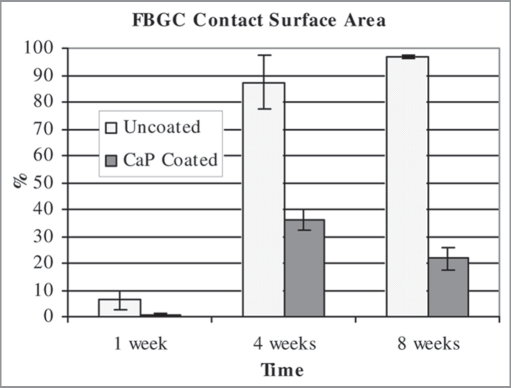
The compositional and mechanical characterization of our scaffold, that meets the above criteria, has been exhaustively reported elsewhere.2 In brief, the scaffold exhibits a highly interconnected macroporous structure with distinct strut structures similar to trabecular bone (Fig. 1A). The macropores are in the 0.8∼1.8 mm range with interconnecting pores exceeding 200 μm in size. The compressive strength and modulus of the scaffold, which were tested wet/37°C, were increased with the addition of an osteoclast-resorbable calcium phosphate particulate to the polymeric phase (Fig. 1B). Without this internal CaP phase, the base PLGA structure shrinks when incubated in culture medium, and even more in the presence of cells such that the scaffold structure is compromised. Thus the internal CaP phase obviates this problem, although it should be emphasized that our rationale was to increase structural strength to withstand shrinkage due to cell contractile forces, rather than to render the composite load-bearing under locomotor forces. However, the polymer generates a foreign body giant cell response upon implantation. This is an advantage in the degradation of bioabsorbable sutures, but a contraindication for use in bone. Thus, we coated the 2 phase scaffold with a thin 3–5 micron ubiquitous coating of a second osteoclast-resorbable calcium phosphate phase, and were able to demonstrate that this surface treatment not only further increased the mechanical properties of the scaffold (Fig. 1C), but also successfully abrogated the foreign body giant cell response (Fig. 2). We have called this 3-phase scaffold OsteoScafTM and refer to it as such in the clinical case reports below.
Figure 1.
(A) shows the PLGA scaffolds made of PLGA IV 1.13, PLGA concentration 12.5% and (left) dry control, (middle) wet control, (right) scaffold incubated with fibroblasts for 3 weeks. The PLGA scaffold was contracted by fibroblasts after incubation for 3 weeks. (B) shows the same polymer with a calcium phosphate particulate inclusion phase [CaP/PLGA ratio 2:1]: (left) dry control, (middle) wet control, (right) scaffold incubated with fibroblasts for 3 weeks. The CaP/PLGA composite scaffold retained its original shape in the presence of cultured cells. )A + B from reference Guan & Davies.2) (C) Compressive Modulus of uncoated and coated scaffold. Mean ± SD. Data courtesy of Limin Guan.
Figure 2.
Graph illustrating the percentage scaffold surface contacted by foreign body giant cells (FBGC) over time. The calcium phosphate coating is clearly shown to significantly decrease the number of FBGC at all time points. Data is mean ± standard deviation. n = 3 for each time point. From Lickorish et al.19
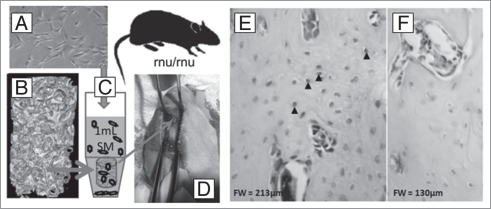
Human mesenchymal cells seeded on scaffold contribute to bone formation in nude rat. We have recently described a population of mesenchymal cells derived from the perivascular tissue of the umbilical cord,13 demonstrated that such cells show self renewal and multilineage differentiation potential at the single cell clonal level and that they can contribute to the healing of both bone and cartilage in a marrow ablation model in an immunocompromised mouse.14 We have employed the same cells, seeded them on the scaffolds described herein, and grown them ex vivo for several days before implanting the cell/scaffold constructs in femoral osteotomies in nude rats. Briefly, the cells were expanded in culture to third passage and transferred, at 1 × 10E6 cells in 1,000 μl phosphate buffered saline, into a 1.5 ml microcentrifuge tube containing a scaffold, and centrifuged at 30G and 4°C for five one-minute intervals with mixing between intervals to re-suspend the cell population. The cell/scaffold constructs were cultured for 5 days prior to transplantation. Figure 3 illustrates this approach which has been described in more detail elsewhere,15 and has specifically demonstrated that the scaffold can be employed as a delivery vehicle for osteogenic cells, and contribute to bone healing in osteotomies. In other work (not shown) we have also been able to demonstrate that this three-phase scaffold can be used as a delivery vehicle for biologically active molecules. Since the scaffold is processed completely at or below, ambient temperature, each of the three phases present a putative delivery opportunity with the calcium phosphate coating acting as a barrier to the burst release of drug from either the polymeric or CaP particulate phases.
Figure 3.
Human umbilical cord perivascular cells (HUCPVCs) (A) were added to scaffold (B) and seeded by mixing in a small conical tube (C). The cellseeded scaffold was then implanted in a femoral osteotomy created in the distal femur of a nude rat (D). On biopsy of the implant site 30 days later, and labeling the preparation with a HuN (an anti-human nuclear antibody), some of the osteocytes within the reparative bone matrix were seen to be HuN positive (E), while the negative control (F) showed no labeling.15
Human Case Studies
In the following case reports, each patient was referred to the Dental Implantology Specialty training program at the Faculty of Dentistry, Universidade Estadual Paulista (UNESP), in Araçtuba (SP), Brazil. In both cases, OsteoScaf™ was employed as a biomaterial with no addition of either cells or other compounds prior to implantation.
Case Report 1:
A sixty-seven year-old female patient complained of pain in the area of the upper right premolars. After clinical and radiographic examination, the treatment plan consisted of extraction of both upper right premolars, immediate grafting of the sockets with OsteoScaf™ for preservation of the alveolar bone architecture and further rehabilitation with dental implants.
After a mucoperiosteal flap was retracted, a longitudinal fracture along the root of the second premolar became evident (Fig. 4A). Bone loss on the buccal aspect of both premolar sockets could also be observed. The teeth were extracted with minimum trauma to the alveolar bone and the sockets were immediately grafted with two cylinders of OsteoScaf™ (3 × 10 mm) (Fig. 4B). The postoperative period was uneventful and clinical and radiographic postoperative follow-ups were conducted monthly for up to 120 days, when a biopsy sample was taken from the grafted area (not shown). Clinically, remnants of the scaffold were found embedded in the alveolar bone on the buccal aspect of the first premolar.
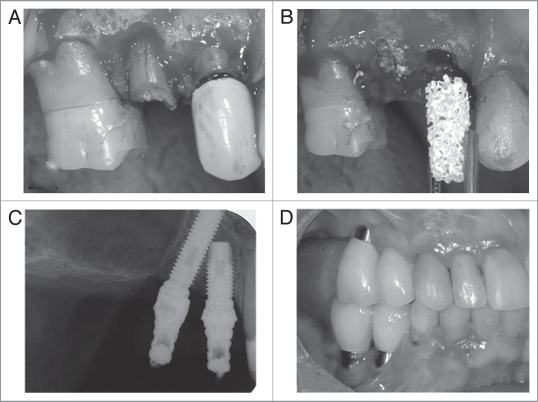
Figure 4.
(A) Surgical site showing longitudinal fracture along the root of the second premolar and bone loss on the buccal aspect of all sockets. (B) Both upper right premolars were extracted with minimal trauma to the alveolar bone and the sockets were immediately grafted with two cylinders of OsteoScaf™ (3 × 10 mm). (C) Radiograph after 14 months of OsteoScaf™ grafting. The patient was fully rehabilitated with prosthetic crowns installed over titanium dental implants. (D) Clinical follow-up 2 years after the treatment was completed.
Fourteen months after grafting with OsteoScaf™, the patient was fully rehabilitated with prosthetic crowns installed over titanium dental implants (Sistema Conexão de Prótese Ltda, SP, Brazil; 14: 4 × 10 mm; 15: 3.75 × 18 mm). The tilted implant insertion technique was adopted following Krekmanov et al.2 to avoid a sinus lift procedure. Figure 4C shows a follow-up radiograph immediately after the prostheses were installed. The bone margins remain at the level of the cervical portion of the implants. Figure 4D shows a clinical follow-up 2 years after the treatment was completed.
Case Report 2:
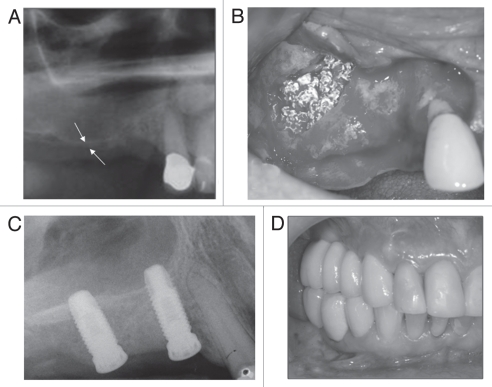
A radiographic examination of this 68 year-old female patient showed a severely resorbed alveolar ridge (2 mm in the 15 area [white arrows]) with extensive pneumatization of the right maxillary sinus (Fig. 5A). This clinical scenario contraindicated treatment with dental implants and therefore a sinus lift procedure, with grafting of the sinus with particulate OsteoScaf™, was considered the treatment of choice.
Figure 5.
(A) Radiograph showing a severely resorbed alveolar ridge (2 mm in the 15 area [white arrows]) with extensive pneumatization of the right maxillary sinus. (B) Photograph, taken at the time of surgery, showing the OsteoScaf™ immediately after the sinus lift and grafting procedure. The sinus was filled in with particles of OsteoScaf™ (2–8 mm in size), which were loosely packed to maintain the material's interconnected porosity. (C) A radiograph taken immediately after dental implant placement (180 days after OsteoScaf™ grafting) shows the considerable gain in bone height, which was sufficient to allow for primary stabilization of the implants. (D) Clinical follow-up of almost 2 years after completion of the treatment.
A mucoperiosteal flap was raised in the region planned for the sinus lift and a “window” was created in the bone of the anterolateral wall of the sinus for access to its interior. The sinus membrane was carefully detached from the floor of the sinus, without rupture and repositioned superiorly at a new desired height. The sinus was then filled in with particles of OsteoScaf™ (2–8 mm in size), which were loosely packed to avoid compromising the interconnected porosity of the material (Fig. 5B).
Again, the post-operative period was uneventful and no signs of infection or inflammatory response were observed due to the presence of the scaffold. Clinical and radiographic follow-ups were conducted for up to 180 days, when two dental implants were inserted in the grafted area. A radiograph taken immediately after dental implant placement shows the considerable gain in bone height (8 mm in the 15 area), which was sufficient to allow for primary stabilization of the implants (Fig. 5C). The patient was rehabilitated with a fixed prosthetic device over the implants (Sistema Conexão de Prótese Ltda, SP, Brazil; 14: 3.75 × 11.5 mm; 15: 4 × 10 mm). Figure 5D shows a clinical follow-up of almost 2 years after completion of the treatment.
Concluding Remarks
Through an iterative process we sought to design a biodegradable scaffold suitable for the regeneration of bone by mimicking the three dimensional architecture of human trabecular bone, adding an internal calcium phosphate phase to increase the mechanical strength to avoid shrinkage by cell and tissue ingrowth, and adding a calcium phosphate coating to render the surface osteoconductive and abrogate the foreign body giant cell response to the underlying polymer. However, when clinically employed as a biomaterial without the addition of cells, we believe it is the ability of the scaffold to wick-up blood, and retain the blood clot throughout the scaffold interstices, defined by both the porosity and interconnected macroporosity, which are the major driving forces for the patterns of healing observed. We have reported elsewhere on the phenomenon of osteoconduction,2,3 which we have defined as the recruitment and migration of osteogenic cells. By acting as a clot-retention device, in both lithomorph and particulate forms, in the socket and sinus examples illustrated, the scaffold compartmentalizes and retains the position of, the blood clot which provides the transitory three dimensional biological matrix through which the host osteogenic cells can migrate. Subsequent degradation of the scaffold leaves trabecular bone suitable to support implant placement and functional loading.
Acknowledgements
The authors acknowledge the generous help of Limin Guan who fabricated all the scaffolds for our studies, and the financial support from BoneTec Corporation, the Ontario Research and Development Challenge Fund (ORDCF grant to J.E.D) and the Canadian Institutes for Health Research (industry-partnered CIHR scholarship awarded to V.C.M).
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/12392
References
- 1.Holy CE, Shoichet MS, Davies JE. Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: investigating initial cell-seeding density and culture period. J Biomed Mater Res. 2000;51:376–382. doi: 10.1002/1097-4636(20000905)51:3<376::aid-jbm11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Guan L, Davies JE. Preparation and characterization of a highly macroporous biodegradable composite tissue engineering scaffold. J Biomed Mater Res A. 2004;71:480–487. doi: 10.1002/jbm.a.30173. [DOI] [PubMed] [Google Scholar]
- 3.Lickorish D, Guan L, Davies JE. A three-phase, fully resorbable, polyester/calcium phosphate scaffold for bone tissue engineering: evolution of scaffold design. Biomaterials. 2007;28:1495–1502. doi: 10.1016/j.biomaterials.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Piattelli M, Favero GA, Scarano A, Orsini G, Piattelli A. Bone reactions to anorganic bovine bone (Bio-Oss) used in sinus augmentation procedures: a histological long-term report of 20 cases in humans. Int J Oral Maxillofac Implants. 1999;14:835–840. [PubMed] [Google Scholar]
- 5.Walsh WR, Vizesi F, Michael D, Auld J, Langdown A, Oliver R, et al. Beta-TCP bone graft substitutes in a bilateral rabbit tibial defect model. Biomaterials. 2008;29:266–271. doi: 10.1016/j.biomaterials.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 6.Ishaug-Riley SL, Crane GM, Gurlek A, Miller MJ, Yasko AW, Yaszemski MJ, et al. Ectopic bone formation by marrow stromal osteoblast transplantation using poly (DL-lactic-co-glycolic acid) foams implanted into the rat mesentery. J Biomed Mater Res. 1997;36:1–8. doi: 10.1002/(sici)1097-4636(199707)36:1<1::aid-jbm1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz Z, Weesner T, van Dijk S, Cochran DL, Mellonig JT, Lohman CH, et al. Ability of deproteinized cancellous bovine bone to induce new bone formation. J Periodontol. 2000;71:1258–1269. doi: 10.1902/jop.2000.71.8.1258. [DOI] [PubMed] [Google Scholar]
- 8.Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Relat Res. 1981;157:259–278. [PubMed] [Google Scholar]
- 9.Holy CE, Dang SM, Davies JE, Shoichet M. In vitro degradation of a novel poly(lactide-co-glycolide) 75/25 foam. Biomaterials. 1999;20:1177–1185. doi: 10.1016/s0142-9612(98)00256-7. [DOI] [PubMed] [Google Scholar]
- 10.Holy CE. Bone tissue engineering on biodegradable polymers. Canada: Department of Chemical Engineering and Applied Chemistry, University of Toronto,; 1999. (Ph.D thesis) [Google Scholar]
- 11.Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J Biomed Mater Res. 1990;24:721–734. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- 12.Davies JE, Baksh D. Bone tissue engineering on biodegradable scaffolds. In: Ikada Y, Shimizu Y, editors. Tissue Engineering for Therapeutic Use. 1st edition. Vol. 4. New York: Elsevier Science BV; 2000. pp. 15–24. [Google Scholar]
- 13.Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220–229. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- 14.Sarugaser R, Hanoun L, Keating A, Stanford WL, Davies JE. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS One. 2009;4:6498. doi: 10.1371/journal.pone.0006498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matta R. Xenotransplantation of human umbilical cord perivascular cells in a femoral defect (Masters thesis) Canada: Institute of Biomaterials and Biomedical Engineering, University of Toronto,; 2009. [Google Scholar]
- 16.Krekmanov L, Kahn M, Rangert B, Lindström H. Tilting of posterior mandibular and maxillary implants for improved prosthesis support. Int J Oral Maxillofac Implants. 2000;15:405–414. [PubMed] [Google Scholar]
- 17.Davies JE, Hosseini MM. Histodynamics of endosseous wound healing. In: Davies JE, editor. Bone Engineering. 1st edition. Toronto: em squared Inc; 2000. pp. 1–14. [Google Scholar]
- 18.Davies JE. Understanding peri-implant endosseous healing. J Dent Educ. 2003;67:932–949. [available in colour at http://www.ecf.utoronto.ca/~bonehead/] [PubMed] [Google Scholar]
- 19.Lickorish D, Chan J, Song J, Davies JE. An in-vivo model to interrogate the transition from acute to chronic inflammation. Eur Cell Mater. 2004;8:12–19. doi: 10.22203/ecm.v008a02. Discussion 20. [DOI] [PubMed] [Google Scholar]