Abstract
Skeletal muscle can self-repair, but is unable to restore significant tissue loss, as consequence of trauma, congenital defects, tumor ablation or denervation. Intramuscular injection of autologous or allogenic derived myogenic cells (namely satellite cells and myoblasts) did not lead to efficient regeneration because of poor cell retention and survival, as well as immunorejection. In the last decade, tissue engineering looked at overcoming these problems by investigating alternative treatment options, i.e., the suspension of myogenic precursors in temporary matrix, formed by biodegradable and biocompatible materials. This approach allows to engineer custom architectured preconditioned implants, and locally deliver paracrine factors.
This article reviews current and potential strategies for the repair of damaged muscle and suggests some innovative approaches for the translation to the clinical setting.
Key words: tissue engineering, muscle, stem cell, satellite cell, biomaterial, niche, reconstruction
Introduction
Tissue engineering is a novel approach that attempts to mimic organogenesis.1 The creation of new skeletal muscle through tissue engineering represents an alternative for the replacement of tissue after severe damage: traumatic injury, dependent on aggressive tumor ablation or prolongued denervation, is a common clinical situation that often results in significant loss of muscle tissue requiring subsequent surgical reconstruction. Until now the treatment performed consisted in the transfer of muscle tissue from local or distant sites, a common practice associated with significant donor sites morbidity causing functional loss and volume deficiency.2 In a completely different context, tissue engineering approches could also be useful in muscle disfunctions, including skeletal myopathies such as Duchenne muscular dystrophy (DMD) or spinal muscular atrophy where local delivery of progenitors and stem cells is needed.3
However, in order to obtain an efficient skeletal muscle regeneration, there are two main components which must be taken into account: the cells with their regenerative potential and the way of delivery, i.e., the polymer in which cells are embedded. Moreover, whenever the aim is to create a tissue able to undergo regular turnover, a close look at the stem cell niche of such tissue is of paramount importance.
Stem Cells
Stem cells are fundamental in organ formation during development and for tissue regeneration in the postnatal life. A stem cell is a cell able to self-renew continuously and to give rise to differentiation into various cell types through asymmetric division.3 Stem cells are present in every developmental stage and are distinguishable on the basis of the developmental period in which they are present in embryonic, fetal and adult. Differences are related to their differentiation potential: embryonic stem (ES) cells can be derived from the inner blastocyst mass and can produce adult tissue from all three germ layers (ectoderm, mesoderm and endoderm); fetal cells retain a pluripotent differentiation potential, but more limited than the embryonic; in the postatal life (i.e., adult stem cells) however, there is a loss of plasticity, that passes through multipotency (capacity to form cells derived from the same germ layer) to unipotency (capacity to form only one specific type of adult cell).4
ES cells retain the highest differentiative potential. While therapeutically their contribution may be important, there are still many controversies that have not been adressed. Not only human ES cells are subjected to the ethical issue of the use of human embryos but there is consistent evidence that ES cells cause the formation of teratomas and share genetic programs with cancer stem cells.5 Moreover, it has been quite difficult to obtain skeletal muscle differentiation from ES cells, and little progress has been made toward the isolation of skeletal muscle progenitors from ES cells.6 This is mainly due to the paucity of paraxial mesoderm formation during embryoid bodies (EB) in vitro differentiation.6 Induced expression of Pax3 during EB differentiation enhances both paraxial mesoderm formation and the myogenic potential of cells within this population but the transplantation of Pax3-induced cells gave rise to teratomas. Isolating cells from EB through fluorescence-activated cell sorting (FACS) for platelet-derived growth factor-α (PDGFα) receptor, marker of paraxial mesoderm, and for the absence of Flk-1, marker of lateral plate mesoderm, it is possible to derive a population of cells with substantial muscle regeneration potential, that was demonstrated after intramuscular and systemic transplantation in dystrophic mice, and leaded to enhanced contractile function without the formation of teratomas.7
Alternatively it may be possible to avoid teratoma formation using cells from fetal tissues. Amniotic fluid stem cells, immunoselected for the surface antigen CD117 and clonally expanded, are able to differentiate in myogenic cells after conditioned medium incubation with the demetilating agent 5-aza-2′-deoxycytidine, leading to MyoD, MRF4 and desmin expression.8
However, the largest amount of data has been generated using adult stem cells. They can be divided in two cathegories: skeletal muscle specific or non-specific. The canonical myogenic progenitor is the muscle satellite cell (SC), characterized by its specific location on muscle fibers under the basal lamina.9 SCs can be isolated through single muscle fiber selection, and can be expanded from a culture of isolated fibers or after mechanical dissociation from their parental myofibers.10,11 They retain a high myogenic potential in vitro and more interestingly in vivo during the first few passages but they are unable to be expanded for longer in culture.12 It has been demonstrated that if SCs are obtained from single fiber explants, expanded and injected intramuscularly, they display poor proliferation, migration and regeneration potential. 13 The isolation technique has evolved to the mechanical dissociation of SCs from muscle fibers, through disgregation or the treatment, after enzymatic digestion of muscles, with a cocktail of collagenase and dispase on the selected single fibers.11,12,14 Therefore, SCs can be collected directly from a muscle biopsy and delivered in vivo avoiding the negative influence of in vitro culture. Freshly isolated SCs showed a higher regenerative potential, with implemented proliferation and migration.11,12,15 While anatomically they represent a well defined group of progenitors, it is still a matter of debate if SCs represent a heterogeneous cell population or are instead uniform. This debate could influence the use of SC for therapy and the isolation of the most effective SC subpopulation could be a valid approach to ameliorate the process of regeneration.11
Within the muscle there are at least two other cell types, muscle associated but not somite-derived, that present a high myogenic potential. The mesoangioblasts, vessel-associated stem cells, express early endothelial markers, such as Flk-1, CD34, stem cell antigen-1 and VE (vascular-endothelial)-cadherin, but not late markers, like Von Willebrand factor.16 They can be expanded for several passages, are not tumorigenic and, even if they do not express the transcription factors Myf5 and MyoD, they can be easily induced towards myogenesis upon co-culture with myoblasts.17 The advantage of the use of mesoangioblasts in cell therapy is that they present the surface protein vascular cell-adhesion molecule (VCAM-1), that mediates the interactions with vascular endothelium, allowing their systemic injection. The expression of α4-integrin and exposure of cells to stromal cell-derived factor-1 (SDF-1) or tumor necrosis factor-α (TNFα) improve up to fivefold the migration of wild-type mesoangioblasts to the dystrophic muscles and a consequent production of new fibers that express the normal copy of the mutated gene.18 Similarly, pericytes have also shown myogenic potential. They are, as the mesoangioblasts, vessel-associated progenitors, they do not express endothelial markers but they do express NG2 proteoglycan and alkaline phosphatase (ALP). Unlike the canonical myogenic precursors (SCs), pericyte-derived cells express myogenic markers only in differentiated myotubes, which they form spontaneously with high efficiency. Moreover, they generate numerous fibers expressing dystrophin when transplanted in dystrophic mice.19 More recently, other muscle stem cell populations have been characterized through selection with FACS, and tested for myogenic properties and contribution to the SC niche. SMPs (skeletal myogenic precursors) have been isolated for β1-integrin (adhesion protein) and CXCR4 (SDF-1 receptor). When engrafted in dystrophic mice, they could contribute up to the 94% of fibers, restoring dystrophin expression and significantly improving hystology and muscle function. Moreover, when transplanted, SMPs could enter the SC compartment, renewing the endogenous SC pool and participating in subsequent rounds of injury repair.14 Muscle stem cell (MuSCs) were also isolated according to a specific phenotypic pattern, specifically for the expression of α7-integrin (adhesion protein) and CD34, marker of haematopoietic cells but also of quiescent SCs, rapidly lost after activation and MyoD expression. They were clonally injected in muscles of NOD/SCID mice damaged with notexin, displaying a great potential both in forming new fibers and in reconstituting the SC niche constituting the in vivo proof of principle of differentiation and self-renewal potential.20 Interestingly, muscle side-population (SP) cells, i.e., cells characterized by the presence of the ABCG2 transporter, other than Pax7 and Syndecan-4, (markers of quiescent SCs), showed notable ability in reconstituting muscle fibers (contributing to 30% of total myonuclei) and in particular the SC compartment (with around 75% of SCs), in a model of muscle damage induced through barium chloride.21 This subpopulation gave rise to the best engraftment in the SC niche, underlining the important role of Syndecan-4, other than the stem cell properties of SP cells. Recently a novel population of myogenic cells, present in the interstitium between muscle fibers and not expressing, unlike in SCs, Pax7 at any stage has been discovered. Positive for cell stress mediator PW1, these cells, defined PW1-interstitial cells (PICs), are myogenic in vitro and efficiently contribute to muscle regeneration in vivo, including SCs and PICs. These cells represent a new and anatomically identifiable population of muscle progenitors, distinct from the SC lineage.22
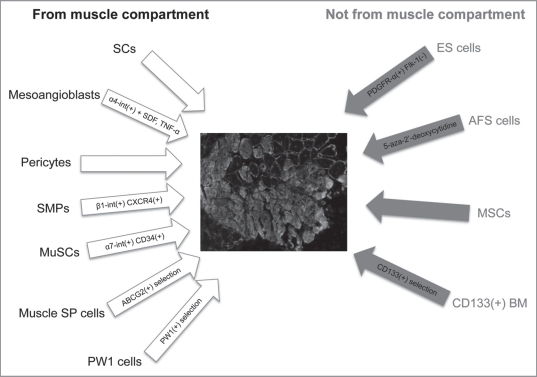
Considering adult stem cells, a common source of multipotency is constituted by mesenchymal stem cells (MSCs), which can be easily isolated from various adult tissues. Despite the low number (1 MSC per 10,000–100,000 bone marrow cells), bone marrow can be considered the most common place for their isolation.23 They retain the ability to differentiate toward different tissues, like bone, cartilage and fat, despite the fact that it remains a matter of debate whether MSCs are able to contribute efficiently to the functional regeneration of the skeletal muscle tissue and its niche.24 There are still evidences that bone marrow MSCs retain a great migration potential toward the areas of induced muscle degeneration (after direct injection), and are able to undergo complete myogenic differentiation, with regeneration of damaged fibers. In order to achieve an efficient myogenic differentiation their transduction with transcription factors such as MyoD, able to initiate myogenic differentiation, has been envisaged.25 CD133+ cells have also been investigated.26 They can be differentiated both in vivo and in vitro into myogenic lineages and may share a role for the treatment of muscular diseases together with other progenitors derived from the bone marrow (Fig. 1).27
Figure 1.
The scheme shows the stem cell types, derived from muscle and non-muscle compartments, able to contribute to muscle regeneration (in the center, represented as green fluorescent protein-positive fibers, counterstained with laminin, the contribution of GFP + SCs when injected after injury, unpublished data). From the muscle compartment (left): satellite cells (SCs); mesoangioblasts, overexpressing β4-integrin and after administration of SDF-1 and TNFα; pericytes; skeletal muscle precursors (SMPs), selected for α1-integrin and CXCR4; muscle stem cells (MuSCs), selected for α7-integrin and CD34; side-population (SP) cells, selected for the ABCG2 transporter; PW1-cells, selected for PW1. From the non-muscle compartment (right): embryonic stem (ES) cells, positively selected for PDGFRα and negatively for Flk-1; amniotic fluid stem (AFS) cells, treated with 5-aza-2′-deoxycytidine; mesenchymal stem cells (MSCs); CD133(+) BM, mesenchymal cells from bone marrow selected for CD133.
Biomaterials
In skeletal muscle tissue engineering, biomaterials play an essential role to support a correct myogenic process, since in vivo myogenic stem cells reside and differentiate within a three-dimensional environment. A variety of biomaterials (alginate, collagen, hyaluronan, hydroxyapatite and polyethilen-glycol) are currently being explored as three-dimensional scaffolds to study the effects of stem cell proliferation, migration, self-renewal and differentiation (Table 1).28–31 The peculiar properties of such biomaterials are biocompatibility, essential for blocking the immune-response in the host muscle, and biodegradability, that gradually allows the substitution of the scaffold by the newly formed muscle tissue.32
Table 1.
Biomaterials in muscle tissue engineering
| Material | + | − |
| Collagen | Differentiation | Migration |
| Matrigel | Proliferation | Immunogenicity |
| Sylgard | Matrix formation | Adhesion |
| Fibrin gel | Survival | Differentiation |
| PGA (polyglycolic acid) | Vascularization | Immunogenicity |
| Acellular matrix | Integration | Proliferation |
| Alginate | Adhesion | Degradation |
| Poly ɛ-caprolactone | Adhesion | Differentiation |
| Hyaluronan | Immunogenicity | Migration |
In this table the most common biomaterials used in muscle tissue engineering are reported, with indicated advantages(+) and disadvantages(−).
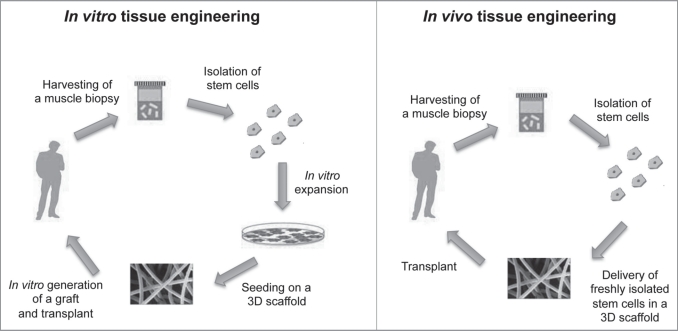
Therapeutic treatments for acquired and inherited skeletal myopathies and loss of functional muscle require either the implantation of differentiated muscle tissue constructs or the injection of muscle-precursor cells into sites of disfunction or tissue deficiency for subsequent formation of new muscle tissue.2,31 The injection of differentiated myoblasts has been the first step toward a cell therapy strategy in muscle pathologies; myoblasts incorporated and differentiated, improving muscle architecture, but large numbers of cells and sites to be injected were required.33,34 In order to improve their engraftment, myoblasts were then transfected in vitro as vehicles for the delivery of other recombinant proteins such as angiogenic factors and growth factors as insulin-like growth factor 1 (IGF-1), erythropoietin and vascular endothelial growth factor (VEGF).35–38 The myoblast-targeted gene therapy with the potential for local production and release of needed therapeutic proteins holds promise for the treatment of several myopathies as well as other diseases lacking important functional proteins.39–41 Alternatively, skeletal muscle tissue could be created in vitro with the development of bioartificial muscle, mediated by differentiation and maturation of SCs harvested from adult skeletal muscle, as an alternative source for treating muscular disorders.2,42 Therefore, we can consider two approaches to engineer skeletal muscle tissue: one approach uses in vitro-designed and pre-fabricated artificial muscle tissue in order to reimplant the neo-tissue after differentiation has taken place (in vitro tissue engineering); the second approach is based on the application of isolated SCs, previously expanded or not, with an appropriate transport matrix, which allows differentiation into myotubes in vivo to occur (in vivo tissue engineering, Fig. 2).43
Figure 2.
The schemes explain the differences between the approaches of in vitro and in vivo tissue engineering. In vitro tissue engineering (left). A muscle biopsy is collected from an individual, stem cells are isolated and then expanded through cell culture techniques; they are then seeded on a 3D scaffold and a graft is generated and then transplanted. In vivo tissue engineering (right): a muscle biopsy is collected from an individual, then stem cells are isolated and immediately delivered on a 3D scaffold, that is promptly transplanted.
To obtain efficient regeneration, scaffolds may contain various natural components of the ECM (extracellular matrix). Chondroitin sulfate is an important structural component of cartilage ECM, that provides mechanical support for cells and mediates intracellular communication.44 ECM components improve also the biomaterials capability to interface with natural tissue, particularly in case of damage or injury allowing the response to different signals coming from cells nearby the implanted scaffold.45,46 ECM degradation is controlled at multiple levels, and its degradation results in damage common on many diseases. Matrix metalloproteinases (MMPs) are proteolytic enzymes that play a major role in the degradation and remodeling of the ECM. Moreover, the presence of the interaction RGD (arginine-glycineaspartic acid) peptide, present in fibronectin, plays a important role in cell differentiation, as well as maintaining the overall fitness of cells encapsulated in hydrogels.47–49 This offers exciting progress in the field of tissue engineering, where it is becoming possible to engineer scaffolds that mimic the natural ECM, in addition to responding to the dynamic changes of cellular differentiation. The signaling however is highly complex and while building materials that focus on mimicking individual components of the ECM function will guide the field of tissue engineering it is difficult to predict how long it will take to engineer sophisticated materials able to mimick the in vivo performance of natural scaffolds.50
The Artificial Niche
When taking into account the generation of a new tissue, it is important to consider the way it may regenerate during the normal tissue turnover. Therefore, the final aim when combining stem cells and biopolymers, eventually loaded with factors, is to create an artificial niche, which allows the natural processes of differentiation and self-renewal. Stem cells refer to the local microenvironment that supports the mainteinance of stem cell identity and regulates the function of stem cells. The niche has been very well characterized in haematopoietic stem cells, intestinal crypt stem cells, hair follicle stem cells, neural stem cells and Drosophila germline stem cells.51–53 Stem cell niche has the property to allow the asymmetric generation and the commitment without disrupting the homeostasis of stem cells within the niche. SCs are localized along the surface of muscle fibers, under the basal lamina, so their niche has been physically characterized.10 More difficult has been the characterization of muscle SC niche signals, that can be divided in mechanical, electrical and chemical.54–56 The basal lamina itself is a major component of the ECM and consists mainly of laminin, collagen and proteoglycans. The anchoring to the basal lamina is vital for the mainteinance of a functional SCs niche.51 Another important component of SC niche is the microvasculature, that nourishes SCs, together with the endothelial cells associated with.57 Moreover, signals coming from macrophages, fibroblasts and muscle-resident stem cells are relayed to the SCs through the basal lamina. In conclusion, a combination of signals from the host muscle fiber, circulation system and ECM govern quiescence, activation and proliferation of SCs (Table 2). In order to mimick the natural niche, it is essential to build artificial three-dimensional microenvironments. In many cases, as it happens for the SCs, the microenvironment is a “polarized” structure, i.e., between two different macroenvironments, the basal lamina and the sorrounding basament membrane. The ideal model of niche should allow the recapitulation of this type of complex architecture and its manipulation at desired time during the muscle engineering, in order to assess how such polarity dictates when a cell is quiescent and when activated. Hydrogel engineering, in combination with photochemistry, allows the creation of complex three-dimensional microenvironments, with inducible variation of density, and consequently stiffness and cell adhesion properties.58 In case of in vivo transplantation of the artificial niche, two models have been proposed: one in which the biomaterial is used as a carrier for introducing stem cells into damaged, diseased or aged tissue, and one in which biomaterials are used to augment endogenous stem-cell function. In the first model, biomaterials are designed to act as carriers for the local delivery of stem cells, support cells or molecular niche cues. Moreover the material can provide protection and enhancement of viability of delivered cells; increase the number and stimulate the function of endogenous stem cells and deliver diffusible cytokines, in order to promote the mobilization of endogenous cells, involved in repair, such as endothelial progenitors in the formation of blood vessels; display regulatory proteins to enhance survival and to stimulate tissue-specific differentiation for large-scale tissue regeneration.59 In the second model, biomaterials can be used also for the local and specific delivery of bioactive niche components, that can be inhibitory or stimulatory molecules, with the ability to increase stem cell number or function when delivered into the niche. The most challenging goal is to create multicomponent, injectable biomaterials designed to act as de novo niches in vivo. Heavily damaged, necrotic tissue may have lost microenvironments suitable for the engraftment of stem cells, as happen in aged or dystrophic muscle but even after a significative trauma.61 In this case the artificial niche needs to be endowed with all the homing signals, constituted by molecules that mediate cell attraction and and cell-cell or cell-matrix adhesive interactions. When engrafted in the niche, stem cells need to receive the proper signals that mediate commitment and self-renewal processes. The biomaterial needs also to guarantee both access to vascular and neural cells, for the correct revascularization and reinnervation and protection for the cells from proteases that are developed by the host tissue after damage or in pathology. A useful technique for such approach is the use of fibrin gel with SCs, seeded in monolayer, in order guarantee adequate connection to the vascular system, for efficient transport of oxygen, carbon dioxide, nutrients and waste products.61
Table 2.
Signals in muscle satellite cell niche
| Signal | Source | Receptor | Function |
| HGF | ECM | c-Met | Activation |
| bFGF | ECM/SC | FGFR | Proliferation |
| IGF-1 | Circulation/ECM | IGFR-1 | Proliferation |
| Myostatin | Circulation/SC | ACVR-2 | Self-renewal |
| Wnt | ECM | Frizzled | Cell fate |
| BDNF | SC | P75NTR | No differentiation |
| Calcitonin | Circulation | CTR | Quiescence |
| SDF-1 | Myofiber | CXCR4 | Migration |
| EGF | ECM | ErbBR | Activation |
| TWEAK | Macrophage | Fn14 | Proliferation |
| NO | Myofiber | No receptor | Quiescence |
| Delta-1 | SC | Notch | Self-renewal |
| VLA4 | Myofiber | VCAM | Fusion |
| Laminin | Basal lamina | Integrin | Quiescence |
| M-cadherin | Myofiber | M-cadherin | Fusion |
In this table the factors that play a role in muscle SCs quiescence, activation and differentiation are reported, with indicated the source, the receptor that they interact with and their function.
Future Perspectives
Muscle tissue engineering has excitingly moved towards consistent results in reconstruction and therapy of muscle pathologies, and may overcome all the limitations derived from the canonical techniques of cell transplantation. Moreover, the use of biomaterials will help on finding novel biochemical and biophysical regulators of stem cell fate. Recent technologies allow the development of new biomaterials in which it is easier to create gradients, to modify photochemical properties or to upload cytokines. It remains difficult to identify markers that specifically and robustly distinguish stem cells from their progeny; multiple positive and negative markers are required, and in particular the investigation needs to be focused on finding stage specific markers that allow the isolation of cells at a specific stage in order to “tailor” the process of regeneration for different conditions of wasting or disease.
Abbreviations
- DMD
Duchenne muscular dystrophy
- ES
embryonic stem cell
- EB
embryoid body
- FACS
luorescence-activated cell sorting
- PDGFα
platelet-derived growth factor-α
- SC
satellite cell
- VCAM-1
vascular cell-adhesion molecule-1
- SDF-1
stromal cell-derived factor-1
- TNFα
tumor necrosis factor-α
- ALP
alkaline phosphatase
- SMP
skeletal myogenic precursors
- MuSC
muscle stem cell
- PIC
PW1-interstitial cell
- MSC
mesenchymal stem cell
- IGF-1
insulin-like growth factor-1
- VEGF
vascular endothelial growth factor
- ECM
extracellular matrix
- MMP
matrix metalloproteinase
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/12419
References
- 1.Mooney DJ, Mikos AG. Growing new organs. Sci Am. 1999;280:60–65. doi: 10.1038/scientificamerican0499-60. [DOI] [PubMed] [Google Scholar]
- 2.DiEdwardo CA, Petrosko P, Acarturk TO, Di Milla PA, LaFramboise WA, Johnson PC. Muscle tissue engineering. Clin Plast Surg. 1999;26:647–656. [PubMed] [Google Scholar]
- 3.Law PK, Goodwin TG, Fang Q, Deering MB, Duggirala V, Larkin C, et al. Cell transplantation as an experimental treatment for Duchenne muscular dystrophy. Cell Transplant. 1993;2:485–505. doi: 10.1177/096368979300200607. [DOI] [PubMed] [Google Scholar]
- 4.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 5.Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY. Module map of stem cell genes guides creatin of epithelial cancer stem cells. Cell Stem Cell. 2008;284:143–147. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- 7.Darabi R, Gehlbach K, Bachoo RM, Kamath S, Osawa M, Kamm KE, et al. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat Med. 2008;14:134–143. doi: 10.1038/nm1705. [DOI] [PubMed] [Google Scholar]
- 8.De Coppi P, Bartsch G, Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 9.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenblatt JD, Lunt AI, Parry DJ, Partridge TA. Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol Anim. 1995;31:773–779. doi: 10.1007/BF02634119. [DOI] [PubMed] [Google Scholar]
- 11.Rossi CA, Pozzobon M, Ditadi A, Archacka K, Gastaldello A, Sanna M, et al. Clonal characterization of rat muscle satellite cells: proliferation, metabolism and differentiation define an intrinsic heterogeneity. PLoS ONE. 2010;5:8523. doi: 10.1371/journal.pone.0008523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, et al. Stem cell function, self-renewal, and behavioural heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Beauchamp JR, Morgan JE, Pagel CN, Partridge TA. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 1999;144:1113–1122. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerletti M, Jurga S, Wiczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, et al. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 16.Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, et al. The meso-angioblasts: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates int most mesodermal tissues. Development. 2002;129:2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 17.Cossu G, Bianco P. Mesoangioblasts—vascular progenitors for extravascular mesodermal tissues. Curr Opin Genet Dev. 2003;13:537–542. doi: 10.1016/j.gde.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Galvez BG, Sampaolesi M, Brunelli S, Covarello D, Gavina M, Rossi B, et al. Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J Cell Biol. 2006;174:231–243. doi: 10.1083/jcb.200512085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satelliite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 20.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:503–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka KK, Hall JK, Troy AA, Cornelison DDW, Majka SM, Olwin BB. Syn22. decan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009;4:217–225. doi: 10.1016/j.stem.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell KJ, Pannerec A, Cadot B, Parlakian A, Besson V, Gomes ER, et al. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12:257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- 23.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:333–344. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 24.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goudenege S, Pisani DF, Wdiezonski B, Di Santo JP, Bagnis C, Dani C, et al. Enhancement of myogenic and muscle repair capacities of human adipose-derived stem cells with forced expression of MyoD. Mol Ther. 2009;17:1064–1072. doi: 10.1038/mt.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torrente Y, Belicchi M, Marchesi C, Dantona G, Cogiamanian F, Pisati F, et al. Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell Transplant. 2007;16:563–577. doi: 10.3727/000000007783465064. [DOI] [PubMed] [Google Scholar]
- 27.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, et al. Muscle regeneration by bone marrow-derived myogenic precursors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 28.Kavalkovich KW, Boynton RE, Murphy JM, Barry F. Chondrogenic differntiation of human mesenchymal stem cells within an alginate layer culture system. In Vitro Cell Dev Biol Anim. 2002;38:457–466. doi: 10.1290/1071-2690(2002)038<0457:cdohms>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Radice M, Brun P, Cortivo R, Scapinelli R, Battaliard C, Abatangelo G. Hyaluronan-based byopolimers as delivery vehicles for bone-marrow-derived mesenchymal progenitors. J Biomed Mater Res. 2000;50:101–109. doi: 10.1002/(sici)1097-4636(200005)50:2<101::aid-jbm2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 30.Nuttelman CR, Tripodi MC, Anseth KS. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. J Biomed Mater Res A. 2004;68:773–782. doi: 10.1002/jbm.a.20112. [DOI] [PubMed] [Google Scholar]
- 31.Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9:679–688. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- 32.Rossi CA, Elvassore N, Flaibani M, Pozzobon M, Blaauw B, Reggiani C, et al. Freshly isolated mouse satellite cells embedded in hyaluronan-based Hydrogel allow hystological and functional recovery of partially ablated tibialis anterioris muscle. ISSCR conference 2009.
- 33.Miller RG, Sharma KR, Pavlath GK, Gussoni E, Mynthier M, Lanctot AM, et al. Myoblast implantation in Duchenne muscular dystrophy: the San Francisco study. Muscle Nerve. 1997;20:469–478. doi: 10.1002/(sici)1097-4598(199704)20:4<469::aid-mus10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 34.Rando TA, Blau HM. Methods for myoblast transplantation. Methods Cell Biol. 1997;52:261–272. doi: 10.1016/s0091-679x(08)60382-9. [DOI] [PubMed] [Google Scholar]
- 35.Rando TA, Pavlath GK, Blau HM. The fate of myoblasts following transplantation into mature muscle. Exp Cell Res. 1995;220:383–389. doi: 10.1006/excr.1995.1329. [DOI] [PubMed] [Google Scholar]
- 36.Vandenburgh HH. Functional assessment and tissue design of skeletal muscle. Ann NY Acad Sci. 1981;961:201–202. doi: 10.1111/j.1749-6632.2002.tb03083.x. [DOI] [PubMed] [Google Scholar]
- 37.Prelle K, Wobus AM, Krebs O, Blum WF, Wolf E. Overexpression of insulin-like growth factor-II in mouse embryonic stem cells promotes myogenic differentiation. Biochem Biophys Res Commun. 2000;277:631–638. doi: 10.1006/bbrc.2000.3737. [DOI] [PubMed] [Google Scholar]
- 38.Powell C, Shansky J, Del Tatto M, Forman DE, Hennessey J, Sullivan K, et al. Tissue-engineered human bioartificial muscles expressing a foreign recombinant protein for gene therapy. Hum Gene Ther. 1999;10:565–577. doi: 10.1089/10430349950018643. [DOI] [PubMed] [Google Scholar]
- 39.Barr E, Leiden JM. Systemic delivery of recombinant proteins by genetically modified myoblasts. Science. 1991;254:1507–1509. doi: 10.1126/science.1962212. [DOI] [PubMed] [Google Scholar]
- 40.Deasy BM, Huard J. Gene therapy and tissue engineering based on muscle-derived stem cells. Curr Opin Mol Ther. 2002;4:382–389. [PubMed] [Google Scholar]
- 41.El Oakley RM, Brand NJ, Burton PB, McMullen MC, Adams GB, Poznansky MC, et al. Efficiency of a high-titer retroviral vector for gene transfer into skeletal myoblasts. J Thorac Cardiovasc Surg. 1998;115:1–8. doi: 10.1016/s0022-5223(98)70436-2. [DOI] [PubMed] [Google Scholar]
- 42.Law PK, Goodwin TG, Fang Q, Quinley T, Vastagh G, Hall T, et al. Human gene therapy with myoblast transfer. Transplant Proc. 1997;29:2234–2237. doi: 10.1016/s0041-1345(97)00312-6. [DOI] [PubMed] [Google Scholar]
- 43.Acarturk TO, Peel MM, Petrosko P, LaFramboise W, Johnson PC, DiMilla PA. Control of attachment, morphology and proliferation of skeletal myoblasts on silanized glass. J Biomed Mater Res. 1999;44:355–370. doi: 10.1002/(sici)1097-4636(19990315)44:4<355::aid-jbm1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 44.Bach AD, Beier JP, Stern-Staeter J, Horch RE. Skeletal muscle tissue engineering. J Cell Mol Med. 2004;8:413–422. doi: 10.1111/j.1582-4934.2004.tb00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 46.Nehrer S, Breinan HA, Ramappa A, Young G, Shortkroff S, Louie LK, et al. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials. 1997;18:769–776. doi: 10.1016/s0142-9612(97)00001-x. [DOI] [PubMed] [Google Scholar]
- 47.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 48.Pratt AB, Weber FE, Schmoekel HG, Muller R, Hubbell JA. Synthetic extracellular matrices for in situ tissue engineering. Biotechnol Bioeng. 2004;86:27–36. doi: 10.1002/bit.10897. [DOI] [PubMed] [Google Scholar]
- 49.Hwang NS, Varghese S, Zhang Z, Elisseeff J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng. 2006;12:2695–2706. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- 50.Salinas CN, Anseth KS. The influence of the RGD peptide motif and its contextual pesentation in PEG gels on human mesenchymal stem cell viability. J Tissue Eng Regen Med. 2008;2:296–304. doi: 10.1002/term.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salinas CN, Cole BB, Kasko AM, Anseth KS. Chondrogenic differentiation potential of human mesechymal stem cells photoencapsulated within poly(ethyleneglycol)-arginine-glycine-aspartic acid-serine thiol-methacrylate mixed-mode networks. Tissue Eng. 2007;13:1025–1034. doi: 10.1089/ten.2006.0126. [DOI] [PubMed] [Google Scholar]
- 52.Deans TL, Elisseeff JH. Stem cells in musculoskeletal engineered tissue. Curr Opin Biotechnol. 2009;20:537–544. doi: 10.1016/j.copbio.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 53.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbours: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 54.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 55.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 56.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 57.Molgo J, Colasantei C, Adams DS, Jaimovich E. IP3 receptors and Ca2+ signals in adult skeletal muscle satellite cell in situ. Biol Res. 2004;37:635–639. doi: 10.4067/s0716-97602004000400019. [DOI] [PubMed] [Google Scholar]
- 58.Tatsumi R, Liu X, Pulido A, Morales M, Sakata T, Dial S, et al. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am J Physiol Cell Physiol. 2006;290:1487–1494. doi: 10.1152/ajpcell.00513.2005. [DOI] [PubMed] [Google Scholar]
- 59.Hahn MS, Miller JS, West JL. Three-dimensional biochemical and biomechanical patterning of hydrogels for guiding cell behaviour. Adv Mater. 2006;18:2679–2684. [Google Scholar]
- 60.Wosnick JH, Shoichet MS. Three-dimensional chemical patterning of transparent hydrogels. Chem Mater. 2008;20:55–60. [Google Scholar]
- 61.Kloxin AM, Kasko AM, Salinas CM, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koning M, Harmsen MC, van Luyn MJ, Werker PM. Current opportunities and challenges in skeletal muscle tissue engineering. J Tissue Eng Regen Med. 2009;3:407–415. doi: 10.1002/term.190. [DOI] [PubMed] [Google Scholar]