Abstract
An injectable, biodegradable and glucose-responsive hydrogel derived from natural polysaccharide derivatives was synthesized to deliver adipogenic factor of insulin in vitro for adipose tissue engineering. The biodegradable hydrogel based N-succinyl-chitosan (SCS) and aldehyde hyaluronic acid (AHA) with covalently conjugated glucose oxidase and catalase. The gelation is attributed to the Schiff-base reaction between amino and aldehyde groups of SCS and AHA, respectively. The morphologies and compressive modulus of the freeze-dried hydrogels demonstrated that the incorporated insulin and enzymes results in the formation of a tighter network structure in composite hydrogels. The immobilized enzymes triggered conversion of glucose reduces the pH value of the microenvironment, and results in hydrolysis and increasing swelling of the network basing on Schiff-base cross-linking. The pH inside the hydrogel, kept in PBS solution at pH 7.4 and 37°C, linearly dropped from 7.40 to 7.17 during 4 h of initial period, then slowly increased to 7.36 after 24 h. Correspondingly, the swelling ratio increased from 20.8 to 28.6 at 37°C in PBS with 500 mg/dL glucose. In PBS buffer with 500 mg/dL glucose, about 10.8% of insulin was released from the hydrogel after 8 h of incubation while upon observation. The results demonstrated that the adipogenic factor of insulin would be released from this biodegradable hydrogel device into the local microenvironment in a controlled fashion by the swelling of hydrogel network. These preliminary studies indicate that the biodegradable and glucose-responsive hydrogel may have potential uses in adipose tissue engineering applications.
Key words: injectable hydrogels, drug delivery, insulin, adipogenic factor, tissue engineering
Introduction
Injectable, biodegradable hydrogels could be utilized as drug delivery systems and cell scaffolds for tissue engineering, which allow easy and homogenous drug or cell distribution within any defect size or shape.1,2 Recently, injectable hydrogels carrying adipose-derived stem cells (ASCs) have been highlighted with promising potential for adipose tissue engineering.3–6 For practical adipose tissue engineering applications, however, the challenge is to deliver the necessary adipogenic factors such as insulin, insulin-like growth factor-1 (IGF-1) and dexamethasone to induce adipogenesis.7–10
Unlike conventional drug delivery, insulin delivery systems require a glucose-sensing ability to specifically interacts with glucose and senses its levels.11–13 Steady release of insulin in a normal glucose environment is not desirable because it could cause hypoglycemia in the patient when blood glucose level is decreased. In particular, glucose-responsive hydrogels possess special properties in response to external stimuli, which renders these gels advanced compared to typical systems for self-regulating insulin delivery.14–16 In order to achieve insulin release, hydrogel swelling is required when glucose concentration increases.
An extensively studied strategy of glucose-responsive system is to immobilize enzymes of glucose oxidase (GOD) and catalase (CTL) into pH-sensitive hydrogels derived from synthetic copolymers to sense the change in the blood glucose concentration. 17–19 Several groups have developed pH-sensitive and non-biodegradable polymeric hydrogels based on GOD entrapment or immobilization within the gel, such as N,N-dimethylacrylamide (DMAAm), poly(DEAEM-g-EG), poly(MAAc-g-EG), poly(MPBA-co-AAm), NIPAAm-MAA-EGDMA, NIPAAm-MAA, poly(HEMA-co-DMAEMA) and poly(DEAEM-HEMA-g-EG).14,18,20–25 Despite extensive studies of pharmaceutical usage, these devices are unsuitable for serving as cell scaffolds due to their poor injectability and non-biodegradability. Insulin is virtually the only effective drug for the treatment of diabetes as well as known as a key adipogenic factor for adipose regeneration.3–6 To the best of our knowledge, the use of biodegradable and glucose-responsive hydrogel as an injectable cell scaffold for adipogenic factor delivery and adipose tissue engineering has received limited attention.
Herein, we report on the use of a new class of biodegradable and glucose-responsive hydrogels derived from natural polysaccharide derivatives to deliver insulin in vitro, potentially resulting in functional adipose tissue. The glucose-responsive hydrogel based N-succinyl-chitosan (SCS) and aldehyde hyaluronic acid (AHA) with entrapped insulin and enzymes. The gelation is attributed to the Schiff-base reaction between amino and aldehyde groups of SCS and AHA, respectively. This composite hydrogel derived from such natural polysaccharide derivatives is an ideally injectable scaffold system as it resembles the extracellular matrices of tissues comprised of various glycosaminoglycans (GAGs) and can create a more biomimetic microenvironment with improved biocompatibility.
Results and Discussion
Synthesis of hydrogels
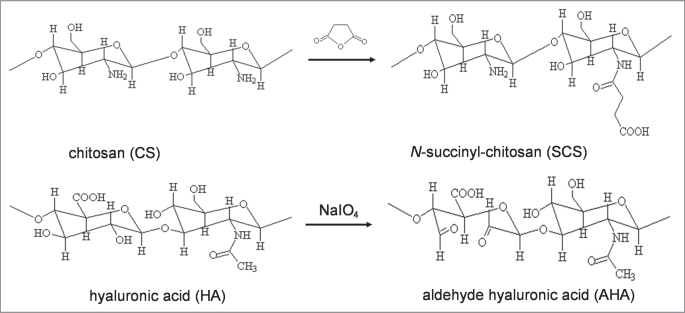
SCS was obtained by introduction of succinyl groups into the chitosan N-terminal of the glucosamine units (Fig. 1), which is water soluble at various pHs.26 Aldehyde groups were introduced to hyaluronic acid by reaction with sodium periodate, which oxidizes the vicinal hydroxyl groups to dialdehydes (Fig. 1), thereby opening the sugar ring to form dialdehyde derivatives.27 Biodegradable and pH-sensitive hydrogels basied on Schiff-base bond cross-linking were generated by mixing SCS (2 wt% in PBS, pH 7.4) and AHA (2 wt% in PBS, pH 7.4) solutions. Insulin, the key adipogenic factor with negative charges, was trapped during the preparation of composite hydrogel. Glucose oxidase (GOD) and catalase (CTL) were simultaneously incorporated in the hydrogel network during Schiff-base bond cross-linking between amino and aldehyde groups of biomolecules (Fig. 2).
Figure 1.
Synthetic route of N-succinyl-chitosan (SCS) and aldehyde hyaluronic acid (AHA).
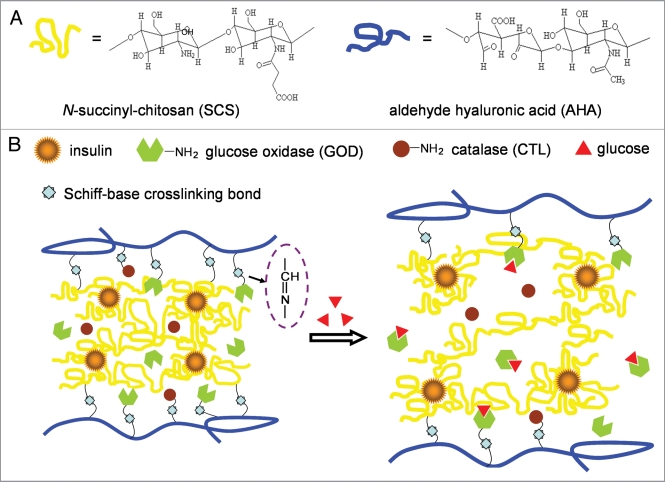
Figure 2.
Schematic illustration showing the strategy of biocompatible and glucose-responsive polysaccharide hydrogel for self-regulating insulin delivery. SCS and enzymes are convalently linked with AHA through Schiff-base reaction synchronously. Insulin is loaded by ionic reaction and released form gel by swelling of the hydrogel network.
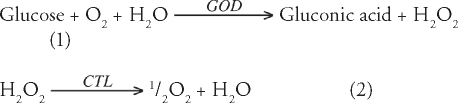
In the presence of an environment of glucose, the immobilized GOD converts glucose to gluconic acid (pKa 3.4) and hydrogen peroxide (H2O2) in presence of oxygen.14 The CTL converts hydrogen peroxide (H2O2) produced from the reaction between GOD and glucose to oxygen and water, which remedies the oxygen depletion problem in the gel under a physiological condition. In addition, GOD is also stabilized by CTL by reducing the concentration of H2O2 which adversely influences GOD activity. The stoichiometry of the reactions are:21–23
 |
Formation of acid results in temporarily lowering of the pH within the hydrogel, which triggers swelling in pH-sensitive hydrogel and consequently facilitating the release of insulin by diffusion-mediated process.24
Gelation of hydrogels
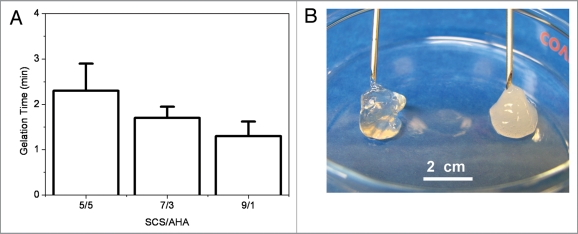
The influence of volume ratios of SCS/AHA (5:5, 7:3 and 9:1) on gelation rate of composite hydrogels was studied (Fig. 3A). The results proved that the ratio of SCS/AHA played an important role in the formation of hydrogel. When insulin and enzymes concentrations were respectively mixed with 3 and 2 mg/mL (5 units CAT per unit GOD), all gelation occurred within 3 min with three different volume ratios of SCS/AHA. With increasing ratios of SCS in the composite hydrogels from 5/5 to 9/1, the gelation time slightly decreased, but no significant difference was found (p > 0.05). The slightly positive charges of protonated amino groups in SCS could form the electrostatic linkages with the negative charges in insulin. The formulations of 5/5 and 7/3 has less amino groups per unit weight after electrostatic crosslinking and requires additional time to form Schiff-base crosslink bonds between SCS and AHA molecules, which resulted in a longer gelation time. Insulin with low molecular weight has better penetrability, which tended to tightly combine with partial SCS molecules with electrostatic bonds, which lead to a more opaque and compact appearance than the pure polysaccharide gel without insulin (SCS/AHA 9:1, Fig. 3B).
Figure 3.
(A) Gelation time of insulin-SCS-AHA/enzymes hydrogels as a function of volume ratio of SCS/AHA at 4°C. (B) Polysaccharide SCS-AHA (Left) and insulin-SCS-AHA/enzymes (Right) hydrogel prepared by mixing SCS and AHA solutions (SCS/AHA 9:1). Insulin and enzymes concentration were fixed with 3 mg/mL and 2 mg/mL, respectively. Enzymes were at the ratio of 5 units CAT per unit GOD, and values reported are an average n = 3, ±standard deviation.
Microstructure and compressive modulus
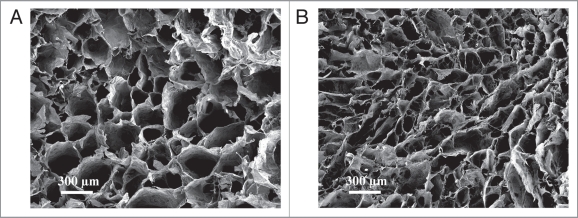
SEM images characterized the microstructure of SCS-AHA (SCS/AHA 9:1) and insulin-SCS-AHA/enzymes (SCS/AHA 9:1) hydrogels after freeze-drying (Fig. 4A and B). According to cross-section morphologies, both of the hydrogels displayed a continuous and porous structure by virtue of the freeze-drying step, with the pores being the result of ice crystal formation, resembling other natural macromolecular hydrogel system structures.26 The pore diameter of the hydrogel with insulin and enzymes is in the range of ∼50–200 µm (Fig. 4B), which is smaller than the ∼100–400 µm of pure polysaccharide gel (Fig. 4A). The morphologies of the freeze-dried hydrogels demonstrated that the incorporated insulin and enzymes results in the formation of a tighter network structure in composite hydrogels due to an additionally electrostatic crosslinking.
Figure 4.
SEM images of the lyophilized SCS-AHA (A) and insulin-SCS-AHA/enzymes (B) composite hydrogels. Enzymes are at the ratio of 5 units CAT per unit GOD.
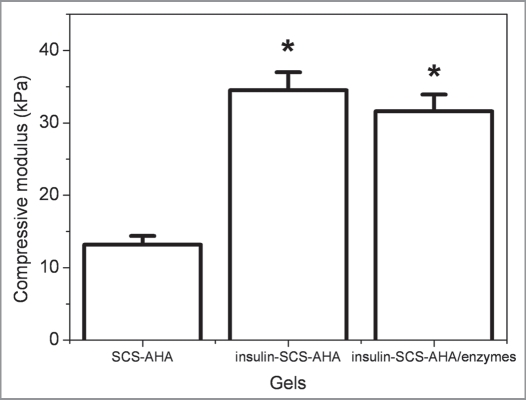
Compressive modulus of the hydrogels was determinedby a dynamic mechanical analysis method (Fig. 5). With incorporation of insulin/enzymes contents, the compressive modulus of the composite hydrogels was improved correspondingly. The insulin-SCS-AHA and insulin-SCS-AHA/enzymes hydrogels had a significantly larger compressive modulus than the SCS-AHA hydrogel (p < 0.05), which were 34.5 and 31.6 kPa, respectively, whereas no difference was found between them.
Figure 5.
Compressive modulus of insulin-SCS-AHA/enzymes (SCS/AHA 9:1) composite hydrogels at room temperature. Insulin and enzymes concentration were fixed with 3 mg/mL and 2 mg/mL, respectively. Enzymes are at the ratio of 5 units CAT per unit GOD, and values reported are an average n = 3, ±standard deviation.
Degradation of hydrogels
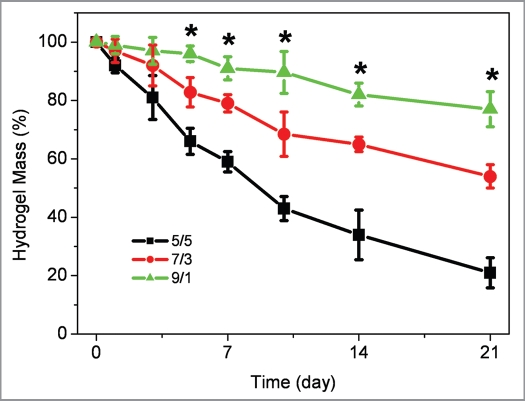
Biodegradable polymeric systems, especially biodegradable hydrogels, have been attractive candidates for a variety of tissue engineering applications.28–30 The degradation properties of composite hydrogels were monitored as a function of incubation time in PBS at 37°C. As shown in Figure 6, the ratio of SCS/AHA has a significant influence on the weight loss behavior of insulin-SCS-AHA/enzymes composite hydrogels. The insulin-SCS-AHA/enzymes hydrogels with a higher ratio of SCS demonstrated a slower weight loss than hydrogels with less SCS composition. Compared with 5/5 and 7/3, a 9/1 gel ratio formed a more sufficient Schiff-base bonds between SCS and AHA. As a result, the 9/1 hydrogels lost its weight steadily up to 21 days and showed a significantly slower weight losing rate than the others after 5 days incubation. At day 21, the weight remaining ratio of 5/5, 7/3 and 9/1 hydrogel were 21%, 54% and 77%, respectively. This result suggested that the ratio of 9/1 SCS/AHA is an appropriate candidate for insulin loading and release.
Figure 6.
Weight loss of insulin-SCS-AHA/enzymes hydrogel with different volume ratio of SCS/AHA in PBS at 37°C. Enzymes are at the ratio of 5 units CAT per unit GOD, and values reported are an average n = 3, ±standard deviation.
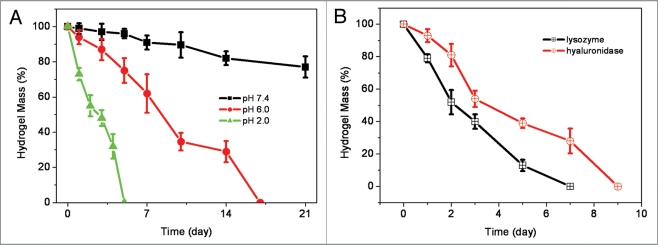
Although hydrolysis rate of SCS/AHA composite hydrogel is slow in PBS with neutral pH,26 this polysaccharide hydrogel exhibits responsive to pH due to the instability of Schiff-base structure at acidic aqueous.31,32 At pH of 6.0 and 2.0, the insulin-SCS-AHA/enzymes (SCS/AHA 9:1) gel showed significantly faster weight loss behavior compared to neutral pH (Fig. 7A). Since chitosan and hyaluronic acid are enzymatically degradable polysaccharides, the enzymatic resistance of composite insulin-SCS-AHA/enzymes (SCS/AHA 9:1) hydrogels was also investigated in lysozyme (1 mg/mL) and hyaluronidase (100 U/mL) as a function of incubation time (Fig. 7B). Both of lysozyme and hyaluronidase resulted in a significant mass loss, and the hydrogels were degraded after 7 and 9 days, respectively.
Figure 7.
(A) Weight loss of insulin-SCS-AHA/enzymes (SCS/AHA 9:1) hydrogel incubated in PBS with different pHs at 37°C. (B) Degradation of insulin-SCS-AHA/enzymes hydrogels (SCS/AHA 9:1) in PBS with 1 mg/mL lysozyme or 100 U/mL hyaluronidase at 37°C with respect to weight loss. Enzymes are at the ratio of 5 units CAT per unit GOD, and values reported are an average n = 3, ±standard deviation.
Swelling kinetics of hydrogels
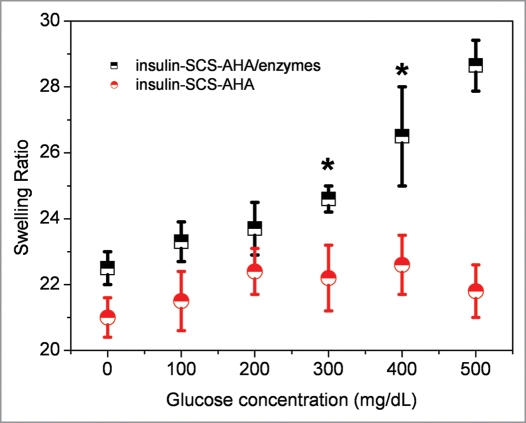
Swelling kinetics of the hydrogels were evaluated in response to step changes in glucose concentration, using the glucose-sensitive insulin-SCS-AHA/enzymes hydrogel described above. Figure 8 indicates the effects of immobilized enzymes on the glucose dependent swelling of the gel. The swelling kinetics experiments took place in PBS, pH 7.4, after 24 hours incubation with several glucose concentrations, showed higher swelling ratios at higher glucose concentrations than at lower glucose concentrations. The swelling ratio of the gel immobilized enzymes linearly increased from 22.5 to 28.6, whereas no significant swelling ratio changed on the gel without enzymes. The swelling mechanism depends on diffusion of glucose into the hydrogel matrix followed by the glucose conversion to gluconic acid and Schiff-base structure hydrolysis. Based on these results, the system showed a highly glucose-responsive property with immobilized enzymes at a ratio of 5 units catalase per unit glucose oxidase.
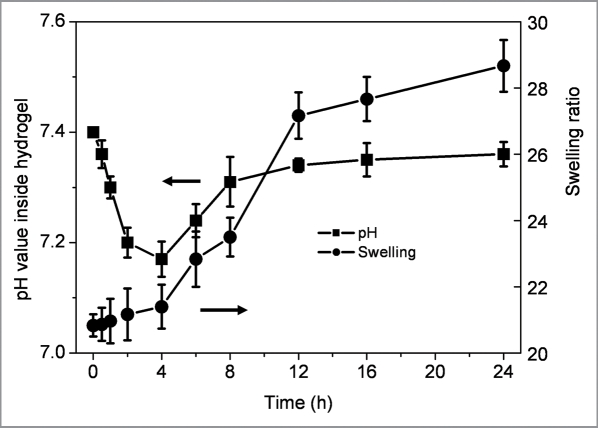
Figure 9 shows the glucose dependent internal pH and swelling of the enzymes immobilized polysaccharide hydrogel. The pH inside the hydrogel, kept in PBS solution at pH 7.4 and 37°C, linearly dropped from 7.40 to 7.17 during 4 h of initial period, then slowly increased to 7.36 after 24 h. The swelling ratio of the hydrogel increased from 20.8 to 28.6. The swelling value of gels rapidly increased with the decrease in pH after 4 hours may due to the hydrolysis reaction of partial Schiff-base bonds. Interestingly, the swelling level coincided with the pH change inside the hydrogel with a noticeable hysteresis for the tested time period. With pH decreasing in the aqueous environment, insulin retained its hydrophilicity in the hydrogel at a lower pH, resulting in the network remaining in a sol state, synchronously increasing the swelling of hydrogel, which would be more favorable for glucose response.23,24
Figure 9.
Internal pH and swelling ratio kinetics of insulin-SCS-AHA/enzymes (SCS/AHA 9:1) hydrogels at 37°C in PBS with 500 mg/dL glucose. Enzymes are at the ratio of 5 units CAT per unit GOD, and values reported are an average n = 5, ±standard deviation.
Controlled insulin release
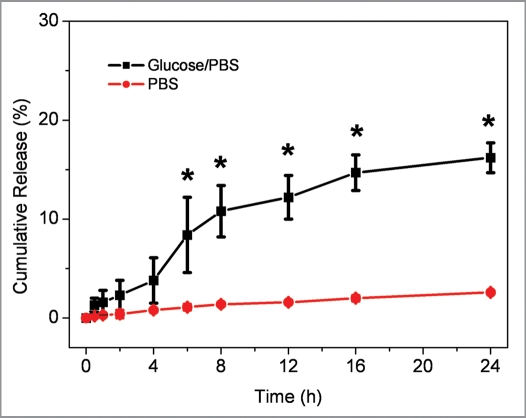
To evaluate the ability of the glucose-responsive system to effectively deliver insulin, insulin cumulative release behavior from the insulin-SCS-AHA/enzymes hydrogels in vitro was investigated at 37°C in PBS (pH 7.4) with a glucose concentration of 500 mg/dL (Fig. 10). In PBS buffer with glucose, about 10.8% of insulin was seen to be released from the hydrogel after 8 h of incubation. The release rate gradually decreased and finally the rate went close to zero due to lose of the enzyme bioactivities. While in PBS buffer without glucose, the results showed that there was a significantly low release rate of protein detected from the hydrogels. Therefore, the CAT/GOD immobilized insulin-polysaccharide Schiff-base crosslinking hydrogels can tune the release of insulin with response to external glucose.
Figure 10.
Cumulative release profiles of insulin from hydrogels at 37°C in PBS with/without 500 mg/dL glucose, respectively. Insulin and enzymes concentration were fixed with 3 mg/mL and 2 mg/mL, respectively. Enzymes were immobilized at a ratio of 5 units CTL per unit GOD. Values reported are an average n = 5, ±standard deviation.
In conclusion, we have designed a biodegradable and glucose-responsive polysaccharide hydrogel system immobilized with enzymes via Schiff-base cross-linking reaction. This hydrogel will be useful for adipogenic factor delivery for adipose tissue engineering. The immobilized enzymes triggered conversion of glucose reduces the pH value of the microenvironment, and results in hydrolysis and increasing swelling of the network. As a result, the adipogenic factor would be released from this biodegradable hydrogel device into the local microenvironment in a controlled fashion by the swelling of hydrogel network. These preliminary studies indicate that the biodegradable and glucose-responsive hydrogel maintain its bioactivity and may have potential uses in adipose tissue engineering applications. Further studies including adipose-derived stem cells encapsulation and differentiation are underway to promote adipogenesis in vitro and in vivo.
Materials and Methods
Materials
Chitosan (deacetylation degree: 85%, Mη : 4 × 105), hyaluronic acid sodium (molecular weight, ∼1.6 × 106), succinic anhydride, sodium periodate, ethylene glycol, t-butyl carbazate, ninhydrin, human insulin, GOD (type VII from Aspergillus niger) and CAT (from Aspergillus niger) were purchased from Sigma-Aldrich, USA. Insulin Human Elisa Kit was purchased from Invitrogen, Eugene, Oregon, USA. All chemicals and reagents were used as received.
Synthesis of S-CS
0.5 g of chitosan was dissolved in 40 mL, 5% (v/v) lactic acid solution and then 160 mL methanol was added to dilute the solution. 1.5 g of succinic anhydride was added to this solution with stirring at room temperature. After 24 h, the succinyl modified chitosan was precipitated by adjusting the solution pH to 6∼7. The precipitate was filtered, re-dissolved in H2O, and dialyzed for 3 days. The purified product was freezedried and stored at 4°C. The determined substitution degree of SCS was 38% by the ninhydrin assay.33
Synthesis of A-HA
For AHA preparation, 1.0 g hyaluronic acid sodium was dissolved in 100 mL nanopure H2O at a concentration of 10 mg/ml. An aqueous solution of sodium periodate (0.5 M, 5 ml) was added dropwise, and the reaction was stirred for 2 h at room temperature in the dark. 1 mL Ethylene glycol was then added to inactivate any unreacted periodate. The reaction was stirred for 1 h at ambient temperature and the solution was purified by exhaustive dialysis against H2O for 3 days, and the dry product was obtained by freeze-drying. Determination of the actual aldehyde content of AHA revealed an extent of oxidation of 45% by t-butyl carbazate assay.34
Hydrogel preparation
SCS and AHA solutions were prepared by dissolving 200 mg polysaccharides in 10 mL PBS (pH 7.4) separately at a concentration of 2 wt % and cooled to 4°C, respectively. 33 mg human insulin solution were dissolved in 1 mL PBS containing 3 vol % of HCl in an ice bath. GOD and CAT were added to cold SCS solution under stirring. For preparation of insulin-loaded gels, 50 µL cold insulin solution was add into 450 µL SCS solution containing enzymes, and mixed with 50 µL AHA, which resulted in formation of opaque gels. The final concentration of enzymes ware fixed with 2 mg/mL, which were at the ratio of 5 units CAT per unit GOD. The gelation time of composite hydrogels was monitored at 4°C. The gels were heated to 37°C for 5 min and resulted in composite gels.
Morphologies
Morphologies of insulin-SCS-AHA/enzymes composite hydrogels were characterized by scanning electron microscopy (SEM) after gelation. The hydrogels were lyophilized at −50°C for 24 h (Freezone 4.5, Labconco, USA) and then gold-coated using a Cressington 108 Auto (Cressington, Watford UK). The surface and cross-sectional morphologies were viewed using a JSM-6330F SEM (JEOL, Peabody, MA) operated at 10 kV accelerating.
Weight loss and degradation
Weight loss of initial hydrogels (W0, dried weight) was monitored as a function of incubation time in PBS at 37°C. At specified time intervals, hydrogels were removed from the PBS and lyophilized (−50°C) and weighed (Wt). The weight loss ratio was defined as 100% x (W0 − Wt)/W0. The weight remaining ratio was defined as 1–100% x (W0 - Wt)/W0. Degradation of lyophilized hydrogels was examined with respect to weight loss under aqueous conditions in the presence of 1 mg/mL lysozyme and 100 U/mL hyaluronidase, respectively.
Compressive modulus
Mixtures of solutions described above were injected into a 12-well culture plate for 5 min to obtain columned hydrogels (∼22 mm diameter, ∼7 mm height). Compressive modulus of elasticity was measured in the elastic region of hydrogel using a dynamic mechanical analyzer (ELF3200, Endura TEC) in unconfined compression up to 20% strain at room temperature.
Swelling properties
The known weights of hydrogels were immersed in PBS and kept at 37°C. For swelling experiment, the initially swollen hydrogel (∼0.55 mL) was immersed in 5 mL PBS with varying glucose concentrations (100, 200, 300, 400 and 500 mg/dL) and kept at 37°C. At predetermined intervals, the hydrogels were removed and immediately weighed with a microbalance after the excess of water lying on the surfaces was absorbed with a filter paper. The swelling ratio (SR) was calculated using the following equation of SR = (Ws − Wd)/Wd, where Ws and Wd are the weights of the hydrogels at the swelling state and at the dry state, respectively. For pH inside gel experiment, ∼1.1 mL of gel was suspended in 10 mL of PBS with glucose at 37°C. The internal pH of the hydrogel was measured by using a pH meter (Accumet AB15, Fisher Scientific, USA) at specified time intervals.
Insulin release
0.55 mL of gels were suspended in 5 mL of PBS with glucose at 37°C, provided a reservoir into which insulin could be released from the gel complex and subsequently measured. At predetermined intervals, a 2.5 mL sample of release medium was extracted from the sample vials and replenished with 2.5 mL fresh release medium to maintain a constant volume. Insulin released into the release medium in vitro was determined at different time point, the supernatants were analyzed with Insulin Human Elisa Kit assay to measure the amount of released insulin.
Statistical analysis
The experimental data from all the studies were analyzed using Analysis of Variance (ANOVA). Statistical significance was set to p value ≤0.05. Results are presented as mean ± standard deviation.
Figure 8.
Swelling ratio of insulin-SCS-AHA/enzymes (SCS/AHA 9:1) hydrogels in PBS at 37°C as a function of glucose concentration after 24 h incubation. Enzymes are at the ratio of 5 units CAT per unit GOD, and values reported are an average n = 5, ±standard deviation.
Acknowledgements
We thankfully acknowledge Professor Steven Abramowitch for assistance with the mechanical testing, NIH R01CA114246-01A1 (J.P.R.), DOD W81XWH-08-2-0032 (J.P.R. and K.G.M.) and Center for Biologic Imaging.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/12037
References
- 1.Kretlow JD, Klouda L, Mikos AG. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:263–273. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Drury JL, Mooney DJ. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 3.Beahm EK, Walton RL, Patrick CW. Progress in adipose tissue construct development. Clin Plast Surg. 2003;30:547–558. doi: 10.1016/s0094-1298(03)00072-5. [DOI] [PubMed] [Google Scholar]
- 4.Shenaq SM, Yuksel E. New research in breast reconstruction: Adipose tissue engineering. Clin Plast Surg. 2002;29:111–125. doi: 10.1016/s0094-1298(03)00089-0. [DOI] [PubMed] [Google Scholar]
- 5.Alhadlaq A, Tang M, Mao JJ. Engineered adipose tissue from human mesenchymal stem cells maintains predefined shape and dimension: implications in soft tissue augmentation and reconstruction. Tissue Eng. 2005;11:556–566. doi: 10.1089/ten.2005.11.556. [DOI] [PubMed] [Google Scholar]
- 6.Tan H, Ramirez CM, Miljkovic N, Li H, Rubin JP, Marra KG. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials. 2009;30:6844–6853. doi: 10.1016/j.biomaterials.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol. 2005;40:229–242. doi: 10.1080/10409230591008189. [DOI] [PubMed] [Google Scholar]
- 8.Schugart EC, Umek RM. Dexamethasone signaling is required to establish the postmitotic state of adipocyte development. Cell Growth Differ. 1997;8:1091–1098. [PubMed] [Google Scholar]
- 9.Tchoukalova YD, Hausman DB, Dean RG, Hausman GJ. Enhancing effect of troglitazone on porcine adipocyte differentiation in primary culture: a comparison with dexamethasone. Obes Res. 2000;8:664–672. doi: 10.1038/oby.2000.85. [DOI] [PubMed] [Google Scholar]
- 10.Yuksel E, Weinfeld AB, Cleek R, Jensen J, Wamsley S, Waugh JM, et al. Augmentation of adipofascial flaps using the long-term local delivery of insulin and insulin-like growth factor-1. Plast Reconstr Surg. 2000;106:373–382. doi: 10.1097/00006534-200008000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Brange J, Volund A. Insulin analogs with improved pharmacokinetic profiles. Adv Drug Deliv Rev. 1999;35:307–335. doi: 10.1016/s0169-409x(98)00079-9. [DOI] [PubMed] [Google Scholar]
- 12.Hoare T, Pelton R. Charge-switching, amphoteric glucose-responsive microgels with physiological swelling activity. Biomacromolecules. 2008;9:733–740. doi: 10.1021/bm701203r. [DOI] [PubMed] [Google Scholar]
- 13.Ichikawa H, Peppas NA. Novel complexation hydrogels for oral peptide delivery: In vitro evaluation of their cytocompatibility and insulin-transport enhancing effects using Caco-2 cell monolayers. J Biomed Mater Res. 2003;67:609–617. doi: 10.1002/jbm.a.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravaine V, Ancla C, Catargi B. Chemically controlled closed-loop insulin delivery. J Control Release. 2008;132:2–11. doi: 10.1016/j.jconrel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Trewyn BG, Slowing II, Lin VSY. Mesoporous silica nanoparticle-based double drug delivery system for glucose-responsive controlled release of insulin and cyclic AMP. J Am Chem Soc. 2009;131:8398–8400. doi: 10.1021/ja901831u. [DOI] [PubMed] [Google Scholar]
- 16.Kataoka K, Miyazaki H, Bunya M, Okano T, Sakurai Y. Totally synthetic polymer gels responding to external glucose concentration: Their preparation and application to on-off regulation of insulin release. J Am Chem Soc. 1998;120:12694–12695. [Google Scholar]
- 17.Guiseppi-Elie A, Brahim SI, Narinesingh D. A chemically synthesized artificial pancreas: Release of insulin from glucose-responsive hydrogels. Adv Mater. 2002;14:743–746. [Google Scholar]
- 18.Brown LR, Edelman ER, Fischel-Ghodsian F, Langer R. Characterization of glucose-mediated insulin release from implantable polymers. J Pharm Sci. 1996;12:1341–1345. doi: 10.1021/js9600686. [DOI] [PubMed] [Google Scholar]
- 19.Brahim S, Narinesingh D, Guiseppi-Elie A. Bio-smart hydrogels: Co-joined molecular recognition and signal transduction in biosensor fabrication and drug delivery. Biosens Bioelectron. 2002;17:973–981. doi: 10.1016/s0956-5663(02)00089-1. [DOI] [PubMed] [Google Scholar]
- 20.Podual K, Doyle FJ, III, Peppas NA. Glucose-sensitivity of glucose oxidase-containing cationic copolymer hydrogels having poly(ethylene glycol) grafts. J Control Release. 2000;67:9–17. doi: 10.1016/s0168-3659(00)00195-4. [DOI] [PubMed] [Google Scholar]
- 21.Luo R, Li H, Lam KY. Modeling the effect of environmental solution pH on the mechanical characteristics of glucose-sensitive hydrogels. Biomaterials. 2009;30:690–700. doi: 10.1016/j.biomaterials.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Traitel T, Cohen Y, Kost J. Characterization of glucose-sensitive insulin release systems in simulated in vivo conditions. Biomaterials. 2000;21:1679–1687. doi: 10.1016/s0142-9612(00)00050-8. [DOI] [PubMed] [Google Scholar]
- 23.Uchiyama T, Kiritoshi Y, Watanabe J, Ishihara K. Degradation of phospholipid polymer hydrogel by hydrogen peroxide aiming at insulin release device. Biomaterials. 2003;24:5183–5190. doi: 10.1016/s0142-9612(03)00441-1. [DOI] [PubMed] [Google Scholar]
- 24.Kang SI, Bae YH. A sulfonamide based glucose-responsive hydrogel with covalently immobilized glucose oxidase and catalase. J Control Release. 2003;86:115–121. doi: 10.1016/s0168-3659(02)00409-1. [DOI] [PubMed] [Google Scholar]
- 25.Morishita M, Lowman AM, Takayama K, Nagai T, Peppas NA. Elucidation of the mechanism of incorporation of insulin in controlled release systems based on complexation polymers. J Control Release. 2002;81:25–32. doi: 10.1016/s0168-3659(02)00019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan H, Chu CR, Payne KA, Marra KG. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30:2499–2506. doi: 10.1016/j.biomaterials.2008.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia X, Colombo G, Padera R, Langer R, Kohane DS. Prolongation of sciatic nerve blockade by in situ crosslinked hyaluronic acid. Biomaterials. 2004;25:4797–4804. doi: 10.1016/j.biomaterials.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Leach JB, Schmidt CE. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials. 2005;26:125–135. doi: 10.1016/j.biomaterials.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Huang AH, Yeger-McKeever M, Stein A, Mauck RL. Tensile properties of engineered cartilage formed from chondrocyte- and MSC-laden hydrogels. Osteoarthr Cartilage. 2008;16:1074–1082. doi: 10.1016/j.joca.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coleman RM, Case ND, Guldberg RE. Hydrogel effects on bone marrow stromal cell response to chondrogenic growth factors. Biomaterials. 2007;28:2077–2086. doi: 10.1016/j.biomaterials.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Braun KP, Cody RB, Jones DR, Peterson CM. A structural assignment for a stable acetaldehyde-lysine adduct. J Biol Chem. 1995;270:11263–11266. doi: 10.1074/jbc.270.19.11263. [DOI] [PubMed] [Google Scholar]
- 32.Viguera AR, Villa MJ, Goñi FM. A water-soluble polylysine-retinaldehyde Schiff base. Stability in aqueous and nonaqueous environments. J Biol Chem. 1990;265:2527–2532. [PubMed] [Google Scholar]
- 33.Curotto E, Aros F. Quantitative determination of chitosan and the percentage of free amino groups. Anal Biochem. 1993;211:240–241. doi: 10.1006/abio.1993.1263. [DOI] [PubMed] [Google Scholar]
- 34.Bouhadir KH, Hausman DS, Mooney DJ. Synthesis of cross-linked poly (aldehyde guluronate) hydrogels. Polymer. 1999;40:3575–3584. [Google Scholar]