Abstract
The biological methyl donor S-adenosyl-l-methionine [(S,S)-AdoMet] can spontaneously break down under physiological conditions to form the inactive diastereomer (R,S)-AdoMet, which may interfere with cell function. Although several lower organisms metabolize (R,S)-AdoMet via homocysteine methyltransferases, it is unclear how mammals deal with it. In this paper, we show that the mouse liver extracts, containing the BHMT-2 homocysteine methyltransferase candidate for a similar activity, recognizes (S,S)-AdoMet but not (R,S)-AdoMet. We find no evidence for the enzymatic breakdown of (R,S)-AdoMet in these extracts. Thus, mammals may metabolize (R,S)-AdoMet using a different strategy than other organisms.
Introduction
The thermodynamic instability of biomolecules leads to the potential for the accumulation of damaged DNA, RNA, proteins, lipids, and small molecules in aging organisms.1–3 The success of aging organisms may depend on how well these damaged molecules are repaired, removed, or metabolized. We have focused our interest on how cells deal with the spontaneous breakdown of the metabolite S-adenosylmethionine (AdoMet), which is widely employed in biology as a methyl donor but can also function in a variety of other reactions, including the transfer of aminopropyl and aminobutyryl groups and the generation of deoxyadenosyl radicals.4
AdoMet can exist in two diastereoisomeric states with respect to its sulfonium ion.5,6 The S configuration (S,S)-AdoMet is the only form that is produced enzymatically, as well as the only form used in almost all biological methylation reactions5,7–10 Under physiological conditions, however, the sulfonium ion can spontaneously racemize to the R form, producing (R,S)-AdoMet.5 As of yet, (R,S)-AdoMet has no known physiological function and may inhibit cellular reactions.
Previously, we found two Saccharomyces cerevisiae enzymes that are capable of recognizing age-damaged (R,S)-AdoMet and using it to methylate homocysteine to form methionine.11 This reaction results in the elimination of the altered form of AdoMet and allows the regeneration of normal AdoMet by the actions of S-adenosylhomocysteine hydrolase, methionine synthase, and AdoMet synthetase. We found that yeast strains with deletions in the SAM4 and MHT1 genes encoding these enzymes accumulate (R,S)-AdoMet; such accumulation is not seen in strains containing one or both of these enzyme activities (C. Vinci and S. Clarke, unpublished). We have further shown that (R,S)-AdoMet-specific homocysteine methyltransferase activity is not specific to yeast, occurring in extracts of Arabidopsis thaliana, Drosophila melanogaster, and Caenorhabditis elegans.11 Similar activity, however, was thus far not detected in the Mus musculus,11 even though it appears that mammalian cells can avoid the accumulation of (R,S)-AdoMet.12,13
Currently, we are searching for a mechanism by which mammals can either metabolize or dispose of (R,S)AdoMet. We first looked at BHMT-2, which is a mammalian homolog of the yeast Sam4 and Mht1 proteins. Its main function is to metabolize S-methylmethionine in a homocysteine-dependent methyltransferase reaction; however, it can metabolize AdoMet as well, albeit to a much lesser degree.14 Thus, we were intrigued by the possibility that the AdoMet substrate used by BHMT-2 was in fact (R,S)-AdoMet, providing a metabolic pathway to avoid the accumulation. We test this hypothesis in this paper. We have found that the use of AdoMet by BHMT-2 appears to occur only for the normal S,S isomer, suggesting that alternate pathways may exist to metabolize (R,S)-AdoMet.
Results
We first wanted to clarify whether a mouse liver cytosolic fraction could utilize (R,S)-AdoMet when incubated for extended periods of time with larger amounts of extract protein than used previously.11 We were interested in the activity of BHMT-2 not only because it is a homolog of the yeast Mht1 and Sam4 homocysteine methyltransferases11 but also that it uses S-methyl-l-methionine (SMM) as the major methyl donor (as does Mht1) and also more poorly AdoMet.14,15 We were particularly interested in the possibility that part or all of the activity with AdoMet was due to the (R,S)-AdoMet content, similar to the situation with yeast Mht1.
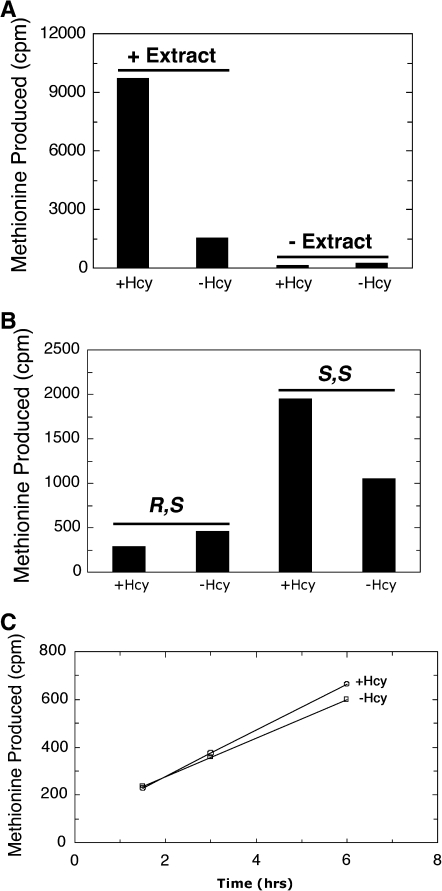
We first demonstrated the presence of BHMT-2 in our extracts using [methyl-14C]SMM as a substrate. After 60 min of incubation at 37°C, roughly one third of the radiolabeled SMM is metabolized, presumably to methionine, in a homocysteine dependent reaction (Fig. 1A). We found that the activity was linearly dependent upon both time and the amount of extract (data not shown). We then asked if (R,S)-AdoMet or (S,S)-AdoMet could react with homocysteine to form methionine (Fig. 1B). When mouse liver extracts were incubated for 4.5 h at 37°C, we found a small homocysteine-dependent activity with (S,S)-AdoMet, but no detectable activity with (R,S)-AdoMet. Although there may be a small amount of breakdown of (R,S)-AdoMet in these experiments, it is independent of homocysteine and, thus, not due to the activity of BHMT-2 or other homocysteine methyltransferases. This result suggests that the small amount of BHMT-2 activity observed previously with AdoMet may be specific to the S,S form (Fig. 1B).
FIG. 1.
Metabolism of (R,S)-AdoMet in mouse liver extracts. (A) Activity of BHMT2 in mouse liver extracts. BHMT2 activity with S-methyl-l-methionine (SMM) was measured as described previously19 with modifications. Extracts (50 μL of 7.5 mg/mL) prepared as described20 were incubated in a final volume of 200 μL with 30,000 cpm of [methyl-14C]SMM in 50 mM Tris-HCl, pH 7.5, 0.8 mM DL-homocysteine, and 140 mM 2-mercaptoethanol for 60 min at 37°C. The 14C-labeled methionine product was separated from 14C-SMM via Dowex AG1X4 anion-exchange columns and counted. Controls were performed without extract and/or homocysteine. (B) (R,S)- and (S,S)-AdoMet-utilizing activities in mouse liver extracts. For each assay, 140 μL of 7.5 mg/mL mouse liver extract was incubated with 4000 cpm of [methyl-3H](R,S)- or (S,S)-AdoMet prepared as described11 in 0.1 M sodium phosphate, pH 7.0, and 0.8 mM dl-homocysteine, final volume of 200 μL, for 4.5 h at 37°C. The [3H]methionine produced was separated from the [3H]AdoMet via Dowex 50WX8 cation-exchange columns as described11 and counted. Controls were done without extract and/or without homocysteine. (C) Assessing the breakdown of (R,S)-AdoMet in mouse liver extracts. Mouse liver extract (140 μL of 7.5 mg/mL) was incubated with 4000 cpm of [methyl-3H](R,S)-AdoMet for 1.5, 3, and 6 h at 37°C in 0.1 M sodium phosphate, pH 7.0, with and without 0.8 mM dl-homocysteine with a final volume of 200 μL. The 3H-labeled product produced was separated from the AdoMet and counted as in B. The rates of decay were calculated for the assays with and without homocysteine. Hcy, homocysteine.
To examine the breakdown of (R,S)-AdoMet in mouse extracts more closely, we conducted a time-course experiment in which (R,S)-AdoMet was incubated in mouse liver extract at 37°C for 1.5, 3, and 6 h with and without homocysteine (Fig. 1C). We did observe the slow formation of product that is not bound to the cation-exchange resin, but this formation is not dependent on homocysteine. The rate constant of loss of (R,S)-AdoMet was determined to be 1.2 × 10−5 s−1 and 1.0 × 10−5 s−1 for the reactions with and without homocysteine, respectively. Because the rates of spontaneous degradation of AdoMet to S-pentosylmethionine and methylthioadenosine are 3 × 10−6 s−1 and 4.6 × 10−6 s−1 respectively,12 it is probable that the products measured above are a mixture of these two spontaneous degradation products. These results suggest that (R,S)-AdoMet is not broken down enzymatically. Thus, it appears that mammals may have lost the efficient mechanism by which lower organisms can dispose of this damaged molecule.
Discussion
It is clear from the previous results that mouse liver12 and rat brain13 do not accumulate significant amounts of (R,S)-AdoMet. How this is accomplished is unclear; the results of this study make it unlikely that an (R,S)-AdoMet-dependent homocysteine methyltransferase is involved, as is the case in yeast, plants, and lower animals.
It is possible that the difference in metabolism of (R,S)-AdoMet in mammals and lower organisms results from the ability of the latter to use both (S,S)-AdoMet and (R,S)-AdoMet as a rich nutritional sources of sulfur, nitrogen, and carbon. This use may involve homocysteine methylation, leading to methionine and S-adenosylhomocysteine formation and the further metabolism of these compounds. In fact, in preliminary experiments, we have found that a sam4−/met17− mutant of the yeast Saccharomyces cerevisiae grows slightly faster in media supplemented with a 50/50 (R,S)/(S,S)-AdoMet mixture than with a 20/80 mixture. This result suggests that the Mht1 protein, which can use (R,S) but not (S,S)-AdoMet,11 allows (R,S)-AdoMet in the media to be a nutrient source. Similarly, sam4− yeast were found to grow better than sam4−/mht1− yeast.16 There may be a significant amount of AdoMet in the R,S form in the yeast natural enviroment; its utilization may give organisms that can use it a selective advantage.
What are the mechanisms for the removal of (R,S)-AdoMet in mammalian cells? It is possible that there are specific “radical SAM” enzymes that convert this molecule to methionine and the deoxyadenosyl radical.17 Alternatively, (R,S)-AdoMet may accumulate in older tissues and contribute to the loss of function with age. There is precedence for the accumulation in mammalian cells of substances such as lipofuscin that can be metabolized in lower organisms such as soil bacteria.18
Acknowledgments
We thank Dr. Timothy Garrow for providing radiolabeled methylmethionine and for his helpful advice through this work. We also thank Dr. Jonathan Lowenson for preparation of the mouse liver extracts. This work was supported by grants from the National Institutes of Health (AG 032303) and the Ellison Medical Foundation. S.G.C. received support from the UCLA Older Americans Independence Center, National Institutes of Health/National Institute on Aging (NIH/NIA) grant P30-AG028748.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Baynes JW. From life to death—the struggle between chemistry and biology during aging: the Maillard reaction as an amplifier of genomic damage. Biogerontology. 2000;1:235–246. doi: 10.1023/a:1010034213093. [DOI] [PubMed] [Google Scholar]
- 2.Hipkiss AR. On the “struggle between chemistry and biology during aging”—implications for DNA repair, apoptosis and proteolysis, and a novel route of intervention. Biogerontology. 2001;2:173–178. doi: 10.1023/a:1011599321168. [DOI] [PubMed] [Google Scholar]
- 3.Clarke S. Aging as war between chemical and biochemical processes: Protein methylation and the recognition of age-damaged proteins for repair. Ageing Res Rev. 2003;2:263–285. doi: 10.1016/s1568-1637(03)00011-4. [DOI] [PubMed] [Google Scholar]
- 4.Fontecave M. Atta M. Mulliez E. S-Adenosylmethionine: Nothing goes to waste. Trends Biochem Sci. 2004;5:243–249. doi: 10.1016/j.tibs.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 5.De La Haba G. Jamieson GA. Mudd SH. Richards HH. S-Adenosylmethionine: The relation of configuration at the sulfonium center to enzymatic reactivity. J Am Chem Soc. 1959;81:3975–3980. [Google Scholar]
- 6.Cornforth JW. Reichard SA. Talalay P. Carrell HL. Glusker JP. Determination of the absolute configuration at the sulfonium center of S-adenosylmethionine. Correlation with the absolute configuration of the diastereomeric S-carboxymethyl-(S)-methionine salts. J Am Chem Soc. 1977;99:7292–7300. doi: 10.1021/ja00464a032. [DOI] [PubMed] [Google Scholar]
- 7.Cannon LM. Butler FN. Wan W. Zhou ZS. A stereospecific colorimetric assay for (S,S)-adenosylmethionine quantification based on thiopurine methyltransferase-catalyzed thiol methylation. Anal Biochem. 2002;308:358–363. doi: 10.1016/s0003-2697(02)00267-1. [DOI] [PubMed] [Google Scholar]
- 8.Bentley R. Role of sulfur chirality in the chemical processes of biology. Chem Soc Rev. 2005;34:609–624. doi: 10.1039/b418284g. [DOI] [PubMed] [Google Scholar]
- 9.Zappia V. Zydek-Cwick CR. Schlenk F. The specificity of S-adenosyl-L-methionine sulfonium stereoisomers in some enzyme systems. Biochim Biophys Acta. 1969;178:185–187. doi: 10.1016/0005-2744(69)90147-8. [DOI] [PubMed] [Google Scholar]
- 10.Borchardt RT. Wu YS. Potential inhibitors of S-adenosylmethionine-dependent methyltransferases. 5. Role of the asymmetric sulfonium pole in the enzymatic binding of S-adenosyl-L-methionine. J Med Chem. 1976;19:1099–1103. doi: 10.1021/jm00231a004. [DOI] [PubMed] [Google Scholar]
- 11.Vinci CR. Clarke SG. Recognition of age-damaged (R,S)-adenosyl-L-methionine by two methyltransferases in the yeast Saccharomyces cerevisiae. J Biol Chem. 2007;282:8604–8612. doi: 10.1074/jbc.M610029200. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman JL. Chromatographic analysis of the chiral and covalent instability of S-adenosyl-L-methionine. Biochemistry. 1986;25:4444–4449. doi: 10.1021/bi00363a041. [DOI] [PubMed] [Google Scholar]
- 13.Beaudouin C. Haurat G. Laffitte JA. Renaud B. The presence of (+)-S-adenosyl-L-methionine in the rat brain and its lack of effect on phenylethanolamine N-methyltransferase activity. J Neurochem. 1993;61:928–935. doi: 10.1111/j.1471-4159.1993.tb03604.x. [DOI] [PubMed] [Google Scholar]
- 14.Garrow TA. Szegedi SS. Castro C. Assigning enzymatic function to betaine-homocysteine S-methyltransfearse-2 (BHMT2) as an S-methylmethionine (SMM)-specific homocysteine (Hcy) methyltransferase. FASEB J. 2006;20:A606. (abstract). [Google Scholar]
- 15.Szegedi SS. Castro CC. Koutmos M. Garrow TA. Betaine-homocysteine S-methyltransferase-2 is an S-methylmethionine-homocysteine methyltransferase. J Biol Chem. 2008;283:8939–8945. doi: 10.1074/jbc.M710449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas D. Becker A. Surdin-Kerjan Y. Reverse methionine biosynthesis from S-adenosylmethionine in eukaryotic cells. J Biol Chem. 2000;275:40718–40724. doi: 10.1074/jbc.M005967200. [DOI] [PubMed] [Google Scholar]
- 17.Layer G. Moser J. Heinz DW. Jahn D. Schubert WD. Crystal structure of coproporphyrinogen III oxidase reveals cofactor geometry of radical SAM enzymes. EMBO J. 2003;22:6214–6224. doi: 10.1093/emboj/cdg598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Grey ADNJ. Rae M. Ending Aging. 1st. St. Martin's Press; 2007. Upgrading the biological Incinerators; pp. 101–133. [Google Scholar]
- 19.Garrow TA. Purification, kinetic properties, and cDNA cloning of mammalian betaine-homocysteine methyltransferase. J Biol Chem. 1996;271:22831–22838. doi: 10.1074/jbc.271.37.22831. [DOI] [PubMed] [Google Scholar]
- 20.Miranda TB. Lowenson JD. Clarke S. A new type of protein methylation activated by tyrphostin A25 and vanadate. FEBS Lett. 2004;577:181–186. doi: 10.1016/j.febslet.2004.09.080. [DOI] [PubMed] [Google Scholar]