Abstract
Parkinson disease (PD) is a neurodegenerative disorder that provides a useful model for testing cell replacement strategies to rejuvenate the affected dopaminergic neural systems, which have been destroyed by aging and the disease. We first showed that grafts of fetal dopaminergic neurons can reverse parkinsonian motor deficits induced by the toxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), validating the feasibility of cellular repair in a primate nervous system. Subsequent clinical trials in Parkinson patients showed encouraging results, including long-term improvement of neurological signs and reduction of medications in some patients. However, many experienced little therapeutic benefit, and some recipients experienced dyskinesias, suggesting a lack of regulated control of the grafts. We have since attempted to improve cell replacements by placing grafts in their correct anatomical location in the substantia nigra and using strategies such as co-grafting fetal striatal tissue or growth factors into the physiologic striatal targets. Moreover, the use of fetal cells depends on a variable supply of donor material, making it difficult to standardize cell quality and quantity. Therefore, we have also explored possibilities of using human neural stem cells (hNSCs) to ameliorate parkinsonism in nonhuman primates with encouraging results. hNSCs implanted into the striatum showed a remarkable migratory ability and were found in the substantia nigra, where a small number appeared to differentiate into dopamine neurons. The majority became growth factor–producing glia that could provide beneficial effects on host dopamine neurons. Studies to determine the optimum stage of differentiation from embryonic stem cells and to derive useful cells from somatic cell sources are in progress.
Introduction
Parkinson disease (PD) is a progressive neurodegenerative disorder that results from a loss of dopamine-producing neurons in a small region of the rostral mesencephalon termed the substantia nigra. These dopaminergic neurons project to distant sites in the subcortical grey matter known as the neostriatum, which is a critical area for the control of voluntary movement. Epidemiological studies have demonstrated that aging is the strongest risk factor for developing PD, with approximately 30% of persons with a mean age of 75 years1,2 and more than 50% of persons older than 85 years exhibiting parkinsonian signs.1 The typical signs of PD, namely bradykinesia, rigidity, motor freezing, resting tremor, difficulty in initiating movement, postural instability, incoordination, and difficulty with speech and swallowing are common in older persons with and without idiopathic PD.1–3 Corresponding to these changes, there is loss of the nigrostriatal dopamine system integrity with age,4–6 with a linear decline in the number of pigmented (i.e., dopamine) neurons in the substantia nigra,5,7–9 with greater losses in patients with the disease. Although the mechanisms underlying the death of dopamine neurons are not fully known, several have been suggested, including mitochondrial DNA deletions10–12 and complex 1 mutations,13 subtelomeric methylation,14 decreased vascular endothelial growth factor (VEGF)15 and Nurr1 levels16, increased α-synuclein,17,18 and decreased estrogen levels,19,20 to provide some examples. All of these are associated with aging.
PD has been treated pharmacologically with the amino acid precursor of dopamine, L-3,4-dihydroxyphenylalanine (L-DOPA, Levodopa), which crosses the blood–brain barrier and is converted to dopamine in residual neurons of the substantia nigra to activate dopamine receptors in the neostriatum. The drug can be effective for many years, but eventually some patients become refractive to therapy due in part to the continuing loss of dopamine neurons and associated changes in receptors. After prolonged exposure to L-DOPA, dyskinesias and other side effects such as hallucinations often develop and the treatment has diminished efficacy.21–23 The loss of effectiveness of L-DOPA may be compounded by further effects of aging as well as some unfortunate effects of L-DOPA metabolism that may increase metabolic and oxidative stress and exacerbate neuronal loss.24
Efforts have been made to develop "cell replacements" for the missing and damaged dopamine neurons as an alternative that might fully restore function, consistent with strategies for engineered negligible senescence. Although pathological processes and aging might eventually have deleterious effects on replaced cells, they offer the prospect of full restoration of the functional losses and potential rejuvenation. The earliest reported effort to transplant adult neural tissue (in 1890) was completely unsuccessful.25 Slow progress began with the discovery in 1917 that embryonic tissue could survive grafting into the brain.26 Neural grafts of primary fetal dopamine neurons and immediate precursors have been tested as an experimental approach for therapy in PD since 1979, when the first encouraging results were reported in dopamine-depleted rodents.27,28 Our first work in nonhuman primates established an encouraging degree of efficacy in severely debilitated animals,29 proving that functional brain cell transplantation was not unique to rodents. We used the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to induce all of the classic signs of parkinsonism and create a model for study. Animals that received multiple grafts of carefully dissected fetal dopamine neurons demonstrated a remarkable reversal of motor signs. Survival of grafted dopaminergic neurons was robust and was characterized by the presence of extensive neuritic outgrowth into the host striatum and increased dopamine in the vicinity of the grafts.30 The survival of cryopreserved human fetal neural tissue transplanted into monkeys, with careful respect for the various ethical concerns, paved the way for human studies.31
Several clinical trials were conducted and some positive effects were seen in reducing the symptoms of the disease (for reviews, see refs. 32 and 33). Importantly, many subjects showed a reduction in L-DOPA requirements, reduced dyskinesias, and fewer on–off fluctuations during pharmacotherapy. However, other subjects failed to show substantial improvement, which has led to efforts to provide a more consistent number and type of cells implanted. This has been technically difficult to accomplish due to the variability and availability of human fetal donor tissue. Although there are many disadvantages to primary fetal tissue, including the association with elective abortions, several reports of long-lasting functional benefits after 9–16 years are encouraging.34–36 The presence of some changes associated with PD in the transplanted cells in 4 of the 9 patients suggests an ongoing pathological process that might be prevented by future interventions.
Stem cells offer a unique opportunity to implant cells of known characteristics and in numbers that can be determined based on the severity of the disorder. Neural stem cells also are known to be prolific producers of neuronal growth factors such as glial-derived neurotrophic factor (GDNF), nerve growth factor (NGF), and others that promote survival of neurons in the central nervous system (CNS).37 Accordingly, they offer another opportunity for cellular repair in addition to cell replacement. The present contribution reviews our recent work in this area and offers suggestions for future trials based on findings in a translational model that is highly relevant to human trials. We briefly describe our efforts to develop strategies for more physiologic circuit restoration of the nigrostriatal system using different combinations of fetal cells and gene therapy, as well as our studies using neural stem cells in nonhuman primates.
Almost all of the preclinical and clinical studies of transplantation for PD have placed the grafts directly into the putamen and/or the caudate, the presumed targets of the dopamine-producing cells of the substantia nigra (SN). This placement was due to the belief that the distances between the structures were too great for grafted cells to bridge, especially in the absence of the various factors that directed such outgrowth during fetal development. Grafts placed into the SN were shown to have functional effects and projections to the striatum at postnatal days 3 and 10, but not by day 20 in the neonatal rat,38 confirming the belief that placements in the SN would not be effective in adult animals or patients. Mendez and Hong39 and Mendez et al.40 have suggested that the double ventral mesencephalon (VM) grafts were producing unknown growth factors and that the presence of the VM grafts in the SN added to the functional benefits, a procedure that has also been taken into the clinic.41 It was still believed unlikely, however, that any improved results were due to outgrowth of cells from the SN, especially because dopamine precursors were also placed directly into the striatum.
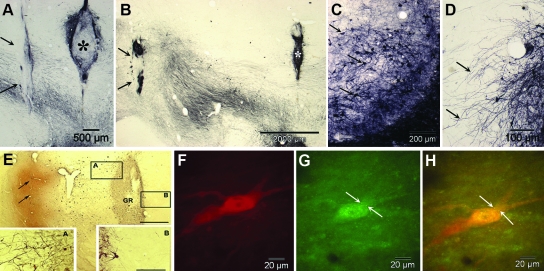
Our group has used fetal lateral ganglionic eminence (anlagen of the striatum) in a series of experiments to determine the potential attractant effect of placements at increasing distances from VM grafts into the SN. Beginning with graft placements in close proximity, it was apparent that the "striatal" grafts attracted outgrowth from the VM tissue. In a series of studies,42 we showed that neurites from grafted dopaminergic neurons extended 5–7 mm, almost the full distance to the caudal portion of the caudate in the St. Kitts green monkey43 (see Fig.1A–D).
FIG. 1.
(A and B) Co-grafts of fetal ventral mesencephalon (asterisks) and striatum (arrows) implanted at 2.5 mm (A) and 5.0 mm (B) apart show survival of dopaminergic neurons (arrows), seen to advantage in C, and outgrowth of neurites (arrows in D) preferentially to the striatal grafts. Similar extension of dopamine positive neurites resulting in dense patterns of terminal fibers was not seen as extensively when the grafts were separated by 7.5 mm (not shown). (E) Injection of adeno-associated virus/glial-derived neurotrophic factor (AAV2-GDNF) directly into the host striatum (arrows) resulted in enhanced survival and neuritic outgrowth from grafts of dopaminergic neurons (GR) with a prominent polarity of the neurites extending toward the region of the vector as seen in detail in rectangles A and B. (F–G) Images of a grafted dopaminergic neuron that shows granules (arrows) of Fluoro-Gold transported in a retrograde direction from the site of injection in the target region, i.e., the striatum, to the graft in the mesencephalon. F is stained for tyrosine hydroxylase alone, G is fluorescence of Fluoro-Gold alone, and G is a combined view of E and F. (A–D are adapted from Sladek et al. 200843; E is reprinted with permission from Elsworth et al. 2008,44 and F–H are unpublished images from Redmond et al. 200945).
In studies of GDNF, which is essential for the survival and outgrowth of developing midbrain dopamine neurons, we confirmed prior studies that GDNF elicits directional neuritic outgrowth of fetal VM neurons grafted into the striatum when GDNF overexpression was induced by an adeno-associated virus (AAV2) vector harboring the GDNF gene.44 Because GDNF is released into interstitial fluid, we wondered whether this effect might be sufficient to attract neurite outgrowth over the distance from the SN to the rostral caudate.
We then studied adult male monkeys (Chlorocebus sabaeus), with different combinations of VM tissue placed immediately dorsal to or within the SN and either striatal co-grafts or injections of the AAV2/GDNF vector into the target regions.45 In this experiment, 7–20 days prior to sacrifice, the retrograde tracer Fluoro-Gold (FG) was injected into the identical striatal targets on the ipsilateral and contralateral sides to the SN grafts in 2 animals. Several animals received striatal and nigral co-grafts at increasing distances along the trajectory of the ascending pathway to the striatum from the SN. After 6 months, the animals were killed and the brains were studied using immunohistochemical methods with unbiased stereological counting. FG-labeled tyrosine hydroxylase–positive (marker for dopamine neurons) cells were found in the host SNs as well as labeling small numbers of cells in the grafts, but not in control animals. There was no FG labeling of any of the co-grafts, suggesting that the attractant effect did not extend beyond the striatal co-grafts location substantially into the striatum where the FG injections were made. These results, we believe, support the interpretation that VM grafts placed in the region of the SN have the potential to extend neurites to their physiological targets in the striatum, if growth factors and other conditions are suitable (see Fig. 1E–H).
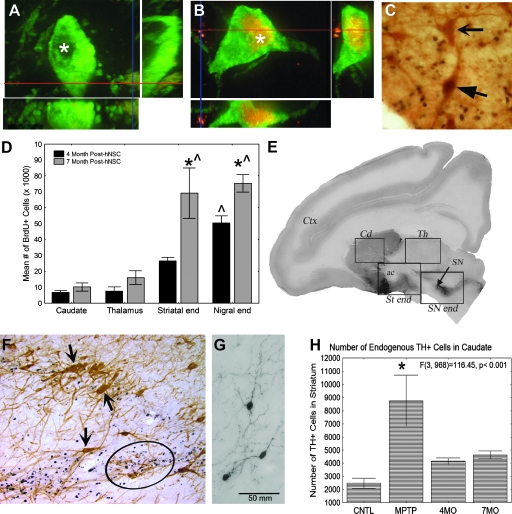
In parallel to these studies, we have also studied human stem cells, derived from a neuroectoderm-derived structure, the telencephalic ventricular zone of an early second-trimester human cadaver, as an alternative to primary fetal cells (human neural stem cells [hNSCs]). These cells have the potential to be maintained and expanded in culture and to differentiate into all of the cell types of the CNS. Implanting stem/progenitor cells constitutively capable of multiple actions, including differentiation into various cells and secreting cytokines, might allow them to develop in a parkinsonian brain to yield the most appropriate types, numbers, and locations of cells as determined by the host milieu.37 We studied 27 adult male monkeys (Chlorocebus sabaeus) in several cohorts with different outcome periods up to 8 months after injection into the nigrostriatal system of MPTP-injected and uninjected normal monkeys.46–48 Briefly, we found three categories of effects: (1) Differentiation of some NSCs into cells that show classic markers of dopamine neurons; (2) significant migration of hNSCs; and (3) normalizing effects of hNSCs on MPTP-induced abnormalities in the host, as illustrated in Fig. 2. In addition to these potentially normalizing effects, we found a substantial improvement in the parksonian behaviors induced by MPTP in 5 severely affected monkeys, compared with 3 controls. This improvement did not result in a complete recovery from parkinsonism, although it was highly significant statistically as well as functionally, allowing animals to move and feed themselves, which previously they had been unable to do. It is also not clear from these studies which of the mechanisms might be responsible—the homeostatic and normalizing effects observed in the host brain associated with the various types of hNSCs present or the small number of apparently dopamine cells that had differentiated from the hNSCs in the SN.
FIG. 2.
Over the course of three studies, spontaneous human neural stem cell (hNSC) differentiation (A–C), hNSC migration (D–E), and the mitigating effects of hNSC on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced effects were studied. MPTP-treated monkeys were implanted bilaterally with undifferentiated hNSCs in the caudate and unilaterally in the substantia nigra (SN). hNSCs were labeled with nuclear bromodeoxyuridine (BrdU) prior to implantation. To evaluate spontaneous differentiation, confocal microscopic analysis of TH and BrdU staining in the SN revealed host nigral neurons that were TH-ir (tyrosine hydrolase-immunoreactive) in the cytoplasm but negative for BrdU in the nucleus (*)(A). A subpopulation of TH-ir cells were BrdU-ir (* red nuclei)(B). Red and blue lines indicate corresponding points in the orthogonal planes, confirming localization of the label within the pictured cell after the summation of serial optical sections. (C) Some donor-derived BrdU-ir cells in this region were also immunoreactive for a secondary marker of dopamine neurons, dopamine transporter (DAT) (closed arrow). These cells were juxtaposed with host DAT-ir neurons, indicated by the lack of black BrdU staining in the nucleus (open arrow). Migration of hNSCs was indicated by the fact that very few BrdU-ir cells were found in the caudate nucleus, which was bilaterally implanted 4 or 7 months prior to analyses (D). There were significantly more BrdU-ir cells found along the nigrostriatal pathway (ST (striatal) end and SN end) than in the caudate nucleus, an area specifically implanted with hNSCs. As many cells were found in the thalamus, an unimplanted site, as were found in the caudate nucleus. (*) Significantly greater than the same region in the 4-month-old animals; (^) significantly different from the other brain areas. Significance level p < 0.05. Parasagittal section of a monkey brain, stained for TH, with boxes depicting the four areas in which counts of BrdU+ cells were made (E). The caudate nucleus was implanted bilaterally with BrdU prelabeled hNSCs, whereas the SN was implanted unilaterally only. Most BrdU-positive cells appeared in the areas between the caudate and the SN, along the nigrostriatal pathway (boxes St end and SN end). The thalamus also was included as a control area. Cx, Cerebral cortex; Cd, caudate; ac, anterior commissure; Th, thalamus; SN, substantia nigra. Many apparently undifferentiated BrdU-ir hNSCs (circle indicates some of these black cells) were found intermingled with host Th-ir nigral neurons (brown cells, arrows) in both the implanted and unimplanted sides of the SN (F). After MPTP lesioning, TH-ir neurons found in the caudate nucleus increase in number (G), a compensatory but abnormal change induced by MPTP. TH-ir neurons in the caudate nucleus are typically small bipolar cells with long varicose processes. In MPTP-exposed brains implanted with hNSCs, the number of TH-ir cells in the caudate nucleus decrease to near normal control parameters, even though the hNSCs migrated away from the caudate nucleus (H). (A, B, C, and H are modified and reproduced, with permission, from Redmond et al.46 D and E are modified and reproduced, with permission, from Bjugstad et al.47 F and G are previously unpublished.)
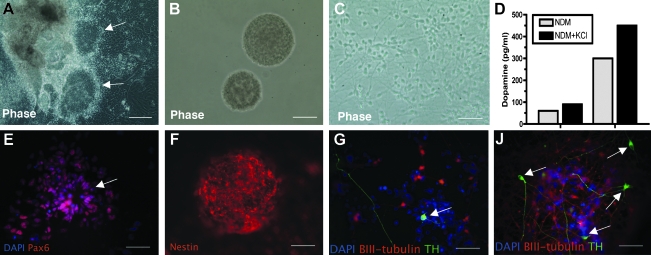
We and our collaborators are working now to derive therapeutic cells from embryonic stem cells differentiated to the "neural" stage or to a larger percentage of dopamine cells in culture. Several methods have been reported, with functional improvements in rodent models, but the results have been less successful than had been hoped.49–53 Several studies in primates nonetheless support the idea that improved methods can be found in the future to fully restore function to the level seen after the best fetal precursor grafts.50,54 We hope to identify an optimal level of differentiation, in which cells remain migratory and have sufficient supportive factors to sustain them during a more physiologic circuit restoration, such as we saw with AAV2/GDNF injections in the target regions with fetal precursor cells. We now have cultures with about 10% of TH+ cells, which also release dopamine into the medium (see Fig. 3). We are presently investigating such dopamine-enriched cultured cells in MPTP-treated monkeys. We hope soon to study parkinsonian monkeys injected with cells derived from adult fibroblasts (induced pluripotent somatic cells [iPS cells]) that have been differentiated into neural and dopaminergic cells.55
FIG. 3.
Dopaminergic neurons differentiated from human embryonic stem (hES) cells release dopamine into medium. Briefly, human embryonic stem cells (H1 from Wicell) (passages P44–P54) were cultured in an undifferentiated state on feeder-free and serum-free conditions. They were differentiated into neural precursor cells (NPCs) by culturing them as floating cell aggregates (embryonic bodies) for 3 weeks in a medium supplemented with recombinant human Noggin and basic fibroblast growth factor (bFGF). The NPCs exhibited columnar morphology, formed neural rosettes (arrows, A) and expressed Pax6, immature neuronal marker (E). The selected neural structures were then cultured in suspension for 1 week to generate neurospheres (B). The majority of cells in the spheres stained positively for the neural precursor marker nestin (F). The spheres were then differentiatied into dopaminergic neurons according to the method of Yang et al.53 (C). The number of TH (green)/βIII-tubulin (red)–positive neurons (arrowheads) increased from 1 week (G) to 4 weeks (H) of the differentiation protocol. To explore whether the hESC-derived TH+ neurons were truly dopaminergic, hESC-derived dopaminergic neurons were analyzed for the release of dopamine (D). Cultures containing dopamine-differentiated cells were incubated in neural differentiation medium (NDM, control conditions) or in the same medium supplemented with KCl (which causes activity-dependent dopamine release) for 30 min. The media were then collected and dopamine levels were assayed by reverse-phase high-performance liquid chromatography (HPLC). As the percentage of TH+ cells in each plate varied somewhat (20–50%), examples of the potassium-stimulated release into the media from the cells are shown (D). In control conditions, 60 and 300 pg·mL−1 dopamine were detected. The dopamine levels were elevated when the neurons were depolarized with potassium (72 and 450 pg·mL−1, respectively).
There is much yet to do before stem cell–derived and reprogrammed cells are ready for rational and controlled clinical trials, including additional studies of toxicity, inappropriate migration, cell overgrowth, and immune rejection. Improved reliability of efficacy and more physiologic circuit reconstruction using tropic and trophic factors are also important goals. Future studies of brain repair and rejuvenation will also benefit from new discoveries of the genetic programs for brain development and improved tools for reprogramming cells, which will make it possible in the future to repair and replace the broken circuits in neurodegenerative diseases.
Acknowledgments
We thank the staff at St. Kitts Biomedical Research Foundation for their invaluable contributions to these studies, especially Dr. Milton C. Whittaker, Dr. Ricaldo Pike, Ernell Nisbett, Clive Wilson I and II, O'Neal Whattley, Xavier Morton, Shervin Liddie, Steve Whittaker, and Samuel Phipps. This research was supported by National Institute of Neurological Disorders and Stroke (NINDS) grants PO1-NS044281 and UO1-NS046028, the Axion Research Foundation, and the Michael J. Fox Foundation for Parkinson's Research. Some of this material is based upon work supported by the State of Connecticut under the Connecticut Stem Cell Research Grants Program. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the State of Connecticut, the Department of Public Health of the State of Connecticut or Connecticut Innovations, Incorporated.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Bennett DA. Beckett LA. Murray AM. Shannon KM. Goetz CG. Pilgrim DM. Evans DA. Prevalence of parkinsonian signs and associated mortality in a community population of older people. The New England journal of medicine. 1996;334:71–76. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- 2.Rajput A. Movement disorders and aging. In: Watts R, editor; Koller W, editor. Movement Disorders: Neurologic Principles and Practice. New York: McGraw-Hill; 1997. pp. 673–686. [Google Scholar]
- 3.Nichols ME. Meador KJ. Loring DW. Poon LW. Clayton GM. Martin P. Age-related changes in the neurologic examination of healthy sexagenarians, octogenarians, and centenarians. J Geriatr Psychiatry Neurol. 1994;7:1–7. doi: 10.1177/089198879400700101. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson A. Winblad B. Influence of age and time interval between death and autopsy on dopamine and 3-methoxytyramine levels in human basal ganglia. J Neur Transmission. 1976;38:271–276. doi: 10.1007/BF01249444. [DOI] [PubMed] [Google Scholar]
- 5.McGeer PL. McGeer EG. Suzuki JS. Aging and extrapyramidal function. Arch Neurol. 1977;34:33–35. doi: 10.1001/archneur.1977.00500130053010. [DOI] [PubMed] [Google Scholar]
- 6.Adolfsson R. Gottfries CG. Roos BE. Winblad B. Post-mortem distribution of dopamine and homovanillic acid in human brain, variations related to age, and a review of the literature. J Neur Transmission. 1979;45:81–105. doi: 10.1007/BF01250085. [DOI] [PubMed] [Google Scholar]
- 7.Hiral S. Ageing of the substantia nigra. Adv Neurol Sci. 1968;7:12845–12849. [Google Scholar]
- 8.Mann DM. Yates PO. Possible role of neuromelanin in the pathogenesis of Parkinson's disease. Mech Ageing Dev. 1983;21:193–203. doi: 10.1016/0047-6374(83)90074-x. [DOI] [PubMed] [Google Scholar]
- 9.Fearnley JM. Lees AJ. Ageing and Parkinson's disease: Substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 10.Bender A. Krishnan KJ. Morris CM. Taylor GA. Reeve AK. Perry RH. Jaros E. Hersheson JS. Betts J. Klopstock T. Taylor RW. Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 11.Biskup S. Moore DJ. Detrimental deletions: Mitochondria, aging and Parkinson's disease. Bioessays. 2006;28:963–967. doi: 10.1002/bies.20471. [DOI] [PubMed] [Google Scholar]
- 12.Burton A. mtDNA deletions associated with ageing and PD. Lancet Neurol. 2006;5:477. doi: 10.1016/s1474-4422(06)70464-1. [DOI] [PubMed] [Google Scholar]
- 13.Smigrodzki R. Parks J. Parker WD. High frequency of mitochondrial complex I mutations in Parkinson's disease and aging. Neurobiol Aging. 2004;25:1273–1281. doi: 10.1016/j.neurobiolaging.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Maeda T. Guan JZ. Oyama J. Higuchi Y. Makino N. Aging-associated alteration of subtelomeric methylation in Parkinson's disease. J Gerontol. 2009;64:949–955. doi: 10.1093/gerona/glp070. [DOI] [PubMed] [Google Scholar]
- 15.Villar-Cheda B. Sousa-Ribeiro D. Rodriguez-Pallares J. Rodriguez-Perez AI. Guerra MJ. Labandeira-Garcia JL. Aging and sedentarism decrease vascularization and VEGF levels in the rat substantia nigra. Implications for Parkinson's disease. J Cereb Blood Flow Metab. 2009;29:230–234. doi: 10.1038/jcbfm.2008.127. [DOI] [PubMed] [Google Scholar]
- 16.Chu Y. Kompoliti K. Cochran EJ. Mufson EJ. Kordower JH. Age-related decreases in Nurr1 immunoreactivity in the human substantia nigra. J Comp Neurol. 2002;450:203–214. doi: 10.1002/cne.10261. [DOI] [PubMed] [Google Scholar]
- 17.Jellinger KA. Alpha-synuclein lesions in normal aging, Parkinson disease, and Alzheimer disease: evidence from the Baltimore Longitudinal Study of Aging (BLSA) J Neuropathol Exp Neurol. 2005;64:554. doi: 10.1093/jnen/64.6.554. [DOI] [PubMed] [Google Scholar]
- 18.Chu Y. Kordower JH. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson's disease? Neurobiol Dis. 2007;25:134–149. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Leranth C. Roth RH. Elsworth JD. Naftolin F. Horvath TL. Redmond DE., Jr. Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: Implications for Parkinson's disease and memory. J Neurosci. 2000;20:8604–8609. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ragonese P. D'Amelio M. Callari G. Salemi G. Morgante L. Savettieri G. Age at menopause predicts age at onset of Parkinson's disease. Mov Disord. 2006;21:2211–2214. doi: 10.1002/mds.21127. [DOI] [PubMed] [Google Scholar]
- 21.Lewitt PA. Levodopa for the treatment of Parkinson's disease. N Engl J Med. 2008;359:2468–2476. doi: 10.1056/NEJMct0800326. [DOI] [PubMed] [Google Scholar]
- 22.Hauser RA. Levodopa: Past, present, and future. Eur Neurol. 2009;62:1–8. doi: 10.1159/000215875. [DOI] [PubMed] [Google Scholar]
- 23.Lang AE. When and how should treatment be started in Parkinson disease? Neurology. 2009;72:S39–S43. doi: 10.1212/WNL.0b013e318198e177. [DOI] [PubMed] [Google Scholar]
- 24.Alexander T. Sortwell CE. Sladek CD. Roth RH. Steece-Collier K. Comparison of neurotoxicity following repeated administration of l-dopa, d-dopa and dopamine to embryonic mesencephalic dopamine neurons in cultures derived from Fisher 344 and Sprague-Dawley donors. Cell Transplantat. 1997;6:309–315. doi: 10.1177/096368979700600313. [DOI] [PubMed] [Google Scholar]
- 25.Thompson WG. Successful brain grafting. NY Med J. 1890;51:701–702. [Google Scholar]
- 26.Dunn EH. Primary and secondary findings in a series of attempts to transplant cerebral cortex in the albino rat. J Comp Neurol. 1917;27:565–582. [Google Scholar]
- 27.Björklund A. Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res. 1979;177:555–560. doi: 10.1016/0006-8993(79)90472-4. [DOI] [PubMed] [Google Scholar]
- 28.Perlow MJ. Freed WJ. Hoffer BJ. Seiger A. Olson L. Wyatt RJ. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 1979;204:643–653. doi: 10.1126/science.571147. [DOI] [PubMed] [Google Scholar]
- 29.Redmond DE., Jr. Sladek JR., Jr. Roth RH. Collier TJ. Elsworth JD. Deutch AY. Haber S. Fetal neuronal grafts in monkeys given methylphenyltetrahydropyridine. Lancet. 1986;1:1125–1127. doi: 10.1016/s0140-6736(86)91839-8. [DOI] [PubMed] [Google Scholar]
- 30.Elsworth JD. Taylor JR. Redmond DE., Jr. Collier TJ. Sladek JR. Roth RH. Biochemical assessment of reversal of MPTP-induced parkinsonism following intrastriatal transplants of fetal substantia nigra in primates. Restor Neurol Neurosci. 1989;1:59. [Google Scholar]
- 31.Redmond DE., Jr. Naftolin F. Collier TJ. Leranth C. Robbins RJ. Sladek CD. Roth RH. Sladek JR., Jr. Cryopreservation, culture, and transplantation of human fetal mesencephalic tissue into monkeys. Science. 1988;242:768–771. doi: 10.1126/science.2903552. [DOI] [PubMed] [Google Scholar]
- 32.Redmond DE., Jr. Cellular replacement therapy for Parkinson's disease—where are we today? Neuroscientist. 2002;8:457–488. doi: 10.1177/107385802237703. [DOI] [PubMed] [Google Scholar]
- 33.Bjorklund A. Dunnett SB. Fifty years of dopamine research. Trends Neurosci. 2007;30:185–187. doi: 10.1016/j.tins.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Kordower JH. Chu Y. Hauser RA. Freeman TB. Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 35.Liu X. Huang J. Chen T. Wang Y. Xin S. Li J. Pei G. Kang J. Yamanaka factors critically regulate the developmental signaling network in mouse embryonic stem cells. Cell Res. 2008;18:1177–1189. doi: 10.1038/cr.2008.309. [DOI] [PubMed] [Google Scholar]
- 36.Mendez I. Vinuela A. Astradsson A. Mukhida K. Hallett P. Robertson H. Tierney T. Holness R. Dagher A. Trojanowski JQ. Isacson O. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nat Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flax JD. Aurora S. Yang C. Simonin C. Wills AM. Billinghurst LL. Jendoubi M. Sidman RL. Wolfe JH. Kim SU. Snyder EY. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol. 1998;16:1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- 38.Bentlage C. Nikkhah G. Cunningham MG. Bjorklund A. Reformation of the nigrostriatal pathway by fetal dopaminergic micrografts into the substantia nigra is critically dependent on the age of the host. Exp Neurol. 1999;159:177–190. doi: 10.1006/exnr.1999.7110. [DOI] [PubMed] [Google Scholar]
- 39.Mendez I. Hong M. Reconstruction of the striato-nigro-striatal circuitry by simultaneous double dopaminergic grafts: A tracer study using fluorogold and horseradish peroxidase. Brain Res. 1997;778:194–205. doi: 10.1016/s0006-8993(97)01055-x. [DOI] [PubMed] [Google Scholar]
- 40.Mendez I. Baker KA. Hong M. Simultaneous intrastriatal and intranigral grafting (double grafts) in the rat model of Parkinson's disease. Brain Res Brain Res Rev. 2000;32:328–339. doi: 10.1016/s0165-0173(99)00091-0. [DOI] [PubMed] [Google Scholar]
- 41.Mendez I. Dagher A. Hong M. Gaudet P. Weerasinghe S. McAlister V. King D. Desrosiers J. Darvesh S. Acorn T. Robertson H. Simultaneous intrastriatal and intranigral fetal dopaminergic grafts in patients with Parkinson disease: A pilot study. Report of three cases. J Neurosurg. 2002;96:589–596. doi: 10.3171/jns.2002.96.3.0589. [DOI] [PubMed] [Google Scholar]
- 42.Collier T. Elsworth J. Taylor J. Sladek J., Jr. Roth R. Redmond D., Jr. Peripheral nerve-dopamine neuron co-grafts in MPTP-treated monkeys: Augmentation of tyrosine hydroxylase-positive fiber staining and dopamine content in host systems. Neuroscience. 1994;61:875–889. doi: 10.1016/0306-4522(94)90410-3. [DOI] [PubMed] [Google Scholar]
- 43.Sladek JR., Jr. Bjugstad KB. Collier TJ. Bundock EA. Blanchard BC. Elsworth JD. Roth RH. Redmond DE., Jr. Embryonic substantia nigra grafts show directional outgrowth to cografted striatal grafts and potential for pathway reconstruction in nonhuman primate. Cell Transplant. 2008;17:427–444. [PubMed] [Google Scholar]
- 44.Elsworth JD. Redmond DE., Jr. Leranth C. Bjugstad KB. Sladek JR., Jr. Collier TJ. Foti SB. Samulski RJ. Vives KP. Roth RH. AAV2-mediated gene transfer of GDNF to the striatum of MPTP monkeys enhances the survival and outgrowth of co-implanted fetal dopamine neurons. Exp Neurol. 2008;211:252–258. doi: 10.1016/j.expneurol.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Redmond DE., Jr. Elsworth JD. Roth RH. Leranth C. Collier TJ. Blanchard B. Bjugstad KB. Samulski RJ. Aebischer P. Sladek JR., Jr. Embryonic substantia nigra grafts in the mesencephalon send neurites to the host striatum in non-human primate after overexpression of GDNF. J Comp Neurol. 2009;515:31–40. doi: 10.1002/cne.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redmond DE., Jr. Bjugstad KB. Teng YD. Ourednik V. Ourednik J. Wakeman DR. Parsons XH. Gonzalez R. Blanchard BC. Kim SU. Gu Z. Lipton SA. Markakis EA. Roth RH. Elsworth JD. Sladek JR., Jr. Sidman RL. Snyder EY. Behavioral improvement in a primate Parkinson's model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci USA. 2007;104:12175–12180. doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bjugstad KB. Teng YD. Redmond DE., Jr. Elsworth JD. Roth RH. Cornelius SK. Snyder EY. Sladek JR., Jr. Human neural stem cells migrate along the nigrostriatal pathway in a primate model of Parkinson's disease. Exp Neurol. 2008;211:362–369. doi: 10.1016/j.expneurol.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wakeman DR. Hofmann MR. Redmond DE., Jr. Teng YD. Snyder EY. Long-term multilayer adherent network (MAN) expansion, maintenance, and characterization, chemical and genetic manipulation, and transplantation of human fetal forebrain neural stem cells. Curr Prot Stem Cell Biol. 2009. Chapter 2:Unit2D 3. [DOI] [PubMed]
- 49.Bjorklund LM. Sanchez-Pernaute R. Chung S. Andersson T. Chen IY. McNaught KS. Brownell AL. Jenkins BG. Wahlestedt C. Kim KS. Isacson O. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci USA. 2002;99:2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takagi Y. Takahashi J. Saiki H. Morizane A. Hayashi T. Kishi Y. Fukuda H. Okamoto Y. Koyanagi M. Ideguchi M. Hayashi H. Imazato T. Kawasaki H. Suemori H. Omachi S. Iida H. Itoh N. Nakatsuji N. Sasai Y. Hashimoto N. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005;115:102–109. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho MS. Lee YE. Kim JY. Chung S. Cho YH. Kim DS. Kang SM. Lee H. Kim MH. Kim JH. Leem JW. Oh SK. Choi YM. Hwang DY. Chang JW. Kim DW. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:3392–3397. doi: 10.1073/pnas.0712359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Keeffe FE. Scott SA. Tyers P. O'Keeffe GW. Dalley JW. Zufferey R. Caldwell MA. Induction of A9 dopaminergic neurons from neural stem cells improves motor function in an animal model of Parkinson's disease. Brain. 2008;131:630–641. doi: 10.1093/brain/awm340. [DOI] [PubMed] [Google Scholar]
- 53.Yang D. Zhang ZJ. Oldenburg M. Ayala M. Zhang SC. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells (Dayton, Ohio) 2008;26:55–63. doi: 10.1634/stemcells.2007-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferrari D. Sanchez-Pernaute R. Lee H. Studer L. Isacson O. Transplanted dopamine neurons derived from primate ES cells preferentially innervate DARPP-32 striatal progenitors within the graft. Eur J Neurosci. 2006;24:1885–1896. doi: 10.1111/j.1460-9568.2006.05093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wernig M. Zhao JP. Pruszak J. Hedlund E. Fu D. Soldner F. Broccoli V. Constantine-Paton M. Isacson O. Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci USA. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]