Abstract
This study examines the relationship between cognitive functioning and depressive symptoms across 3 years in a prospective study of 273 community-dwelling, Hispanic older adults in Miami. The analyses extend the literature by testing for a bidirectional or reciprocal relationship between depressive symptoms and cognitive functioning over time and by examining the relationship between these variables among Hispanics, an understudied population at risk of developing depressive symptoms and cognitive impairments. Structural equation modeling with a cross-lagged panel design showed that depressive symptoms were unrelated to subsequent cognitive functioning. However, cognitive functioning was related to subsequent depressive symptoms at every time point, such that poorer cognitive functioning was related to higher depressive symptoms. Findings suggest that cognitive declines may predict depressive symptoms in community-dwelling Hispanic older adults.
Keywords: Cognitive functioning, Depressive symptoms, Hispanics/Latinos
A number of studies among older adults have documented a cross-sectional association between symptoms of depression and poorer cognitive functioning (Ganguli, Du, Dodge, Ratcliff, & Chang, 2006; La Rue, Swan, & Carmelli, 1995; Lichtenberg, Ross, Millis, & Manning, 1995). This finding has been notably consistent across studies, despite the use of diverse samples and research methods. Indeed, in clinical studies, cognitively impaired individuals frequently evidence depressive symptoms, and depressed individuals often show symptoms that can interfere with cognitive performance, such as attention and concentration difficulties, psychomotor slowing, and motivational impairments or apathy (Baune, Suslow, Arolt, & Berger, 2006; Lyketsos et al., 2002). Among older adults, depression and depressive symptoms have been associated with poorer cognitive performance in various domains, including episodic memory (specifically, learning and delayed recall), information processing speed, executive functioning, and global cognitive functioning (Bierman, Comijs, Jonker, & Beekman, 2005; Butters et al., 2004; Ganguli et al.). Studies exploring possible causal or temporal relationships between depressive symptoms and cognitive functioning have yielded mixed findings.
There is some evidence that certain cognitive impairments and depressive symptoms in older adults may share a similar pathophysiology. For instance, “vascular cognitive impairment or dementia,” which is characterized by a vascular pathology in cortical and subcortical regions of the brain, may lead to the disruption of the frontostriatal pathways (cf., Alexopolous et al., 2002). This may result in both cognitive impairments (e.g., declines in executive functioning, attention, and information processing speed) and behavioral changes (e.g., depression, apathy, and anxiety; see O’Brien, 2006).
Some studies have found that depressive symptoms often precede cognitive deterioration, particularly in individuals who later develop Alzheimer’s disease or dementia (Gatz, Tyas, St. John, & Montgomery, 2005; Wilson et al., 2002). Depressive symptoms may represent an early indicator or prodrome of cognitive deterioration (Gatz et al.; Geerlings et al., 2000), or they may contribute to subsequent cognitive decline among older adults (Bassuk, Berkman, & Wypij, 1998; Ganguli et al., 2006; Wilson, Mendes de Leon, Bennett, Bienias, & Evans, 2004). The latter is important because interventions to manage depressive symptoms may be helpful in preventing or slowing cognitive decline. Nonetheless, these studies have yielded mixed results, with some finding that depressive symptoms have no impact on cognitive functioning (Dufouil, Fuhrer, Dartigues, & Alpérovitch, 1996; Ganguli et al.) and others finding that elevated or persistent depressive symptoms predict cognitive decline (Barnes, Alexopoulos, López, Williamson, & Yaffe, 2006; Paterniti, Verdier-Taillefer, Dufouil, & Alpérovitch, 2002) or increase the risk for dementia or Alzheimer’s disease (Gatz et al.; Lichtenberg et al., 1995; Ownby, Crocco, Acevedo, John, & Loewenstein, 2006).
In other research, depressive symptoms have predicted cognitive decline only among certain subgroups. Bassuk and colleagues (1998) reported that depressive symptoms predicted later cognitive losses among older adults showing initially moderate cognitive impairments, but not among those lacking cognitive impairments. In a similar vein, Geerlings and associates (2000) found that depression predicted cognitive decline but only among older adults with higher levels of education.
A less frequently examined hypothesis is that cognitive decline contributes to the development or exacerbation of depressive symptoms. This is a compelling research question: If it is true, then depressive symptoms can further affect the cognitively impaired person’s quality of life and independent functioning, and they can complicate the management of the cognitive impairments. Indeed, some studies have shown that depressive symptoms tend to follow cognitive impairments or occur concurrently, instead of occurring earlier (Dufouil et al., 1996; Ganguli et al., 2006). A possible explanation is that as older adults become aware of growing cognitive deficits, they experience psychological distress or depression (Bassuk et al., 1998; Bierman, Comijs, Jonker, & Beekman, 2007). Alternatively, individuals with lower cognitive functioning may be at increased risk for depression over time, because higher cognitive functioning has been associated with greater self-efficacy in several life domains that can influence quality of life, including regulating one’s mood, performing activities of daily living (e.g., exercising), and utilizing social support (Baltes & Lang, 1997; Shifren, Park, Bennett, & Morrell, 1999).
Comparisons of these study findings are complicated by design and methodological differences across different projects. Differences include the selection of clinical versus community samples, the duration of study follow-up periods, and different strategies for conceptualizing and measuring cognitive decline and depression. For instance, cognitive decline has been defined on the basis of selected cutoff points, as well as by the clinical diagnosis of mild cognitive impairment or dementia. Depression has been measured by clinical diagnosis, as well as through self-reported symptoms using different assessments. Further complicating conclusions is that even when a longitudinal or temporal relationship between depression and cognitive decline has been documented, the underlying biological or clinical mechanisms that explain this relationship remain largely unclear.
In this study, we examine the relationship between cognitive functioning and depressive symptoms across three years (i.e., four time points) in a community sample of Hispanic older adults living in Miami, Florida. On the basis of the existing literature, which shows effects in both directions, we hypothesized that there would be a bidirectional or reciprocal relationship between depressive symptoms and cognitive functioning across time, with poorer cognitive functioning predicting higher depressive symptoms and higher depressive symptoms predicting poorer cognitive functioning. This study extends the existing literature in three ways.
First, in our analyses we test for a reciprocal relationship between depressive symptoms and cognitive functioning. To our knowledge, these relationships have not been studied simultaneously by using the same data set. Second, whereas many previous studies have used categorical outcome variables based on cutoff scores or clinical diagnoses, in this study we examine continuous outcomes. Although helpful in interpreting results, categorizing outcomes may also mask or dilute actual changes that may be occurring across time. Third, we examine the relationship of cognitive functioning and depressive symptoms in an understudied population: Hispanic older adults. This group may be at high risk of depressive symptoms and cognitive impairments for a variety of reasons, including limited financial resources, low education, and residence in inner-city neighborhoods (Black et al., 1999; Espino, Lichtenstein, Palmer & Hazuda, 2001; Falcón & Tucker, 2000; Perrino, Brown, Mason, & Szapocznik, in press; Tang et al., 1998). Given the rapidly expanding population of Hispanic older adults in the United States, this research has important clinical and public health implications.
METHODS
Research Design
We collected data as part of a larger, population-based, prospective cohort study of Hispanic older adults in Miami, Florida, entitled “The Hispanic Elders’ Behavioral Health Study,” which focuses on the relationship between neighborhood environmental factors and older adults’ well-being, in particular the built (physical) environment and residents’ mental health outcomes. To obtain a population-based sample, we enumerated all 16,000 households in a single urban Miami community to identify all Hispanic adults who were 70 years of age or older. One Hispanic older adult was randomly selected from each block on which at least one older adult lived. Of all 3,322 older adults enumerated, 521 were randomly chosen and approached for participation, and 273 participants provided informed consent and completed the baseline assessment. Of the 521 older adults initially approached, 30 had died since enumeration, 87 had moved or could not be located or contacted, 95 refused participation, 10 had incorrect home addresses, 24 did not meet full eligibility criteria, and 2 moved to a different block from which a participant had already been sampled. The study was approved by the University of Miami’s Institutional Review Board.
Participants and Procedure
To participate, individuals had to (a) be 70 years of age or older, (b) have immigrated from a Spanish-speaking country, (c) reside in this specific Miami neighborhood, (d) reside in housing in which they could walk outside (we excluded nursing homes), (e) be of sufficient physical health to go outside; and (f) have a score of 17 or higher on the Mini-Mental State Examination, or MMSE (Folstein, Folstein, & McHugh, 1975), a test of global cognitive functioning. We lowered the standard MMSE cutoff point from 24 to 17, given concerns about the MMSE’s lower sensitivity and specificity in detecting cognitive impairments among those with lower educational levels, and possible bias with regard to language use and immigrant status (Black et al., 1999; Ostrosky-Solis, López-Arango, & Ardila, 2000).
Participants completed yearly, home-based assessments in Spanish at baseline and at 12-, 24-, and 36-month follow-ups. We compensated them $25, $30, $35, and $40 for assessment completion, respectively. Baseline assessments were completed between the year 2002 and 2004. At baseline, the mean age of the older adults was 78.5 (range = 70–100); 59% were female and 41% were male. Approximately 86% were born in Cuba or relocated to Cuba as children, and 10% were born in a Central or South American country. Participants had lived in the United States an average of 29 years. The sample was of low socioeconomic status, with 71% reporting an annual household income of less than $10,000, and most reporting working-class jobs prior to retirement (e.g., factory worker, housekeeper). The sample averaged 7.3 years of education (SD = 4.3; range = 0–20 years). Of the participants, 34% were married, 35% were widowed, 20% were separated or divorced, and 11% had never married.
Measures
Depressive symptoms
We used a Spanish translation of the 20-item Center for Epidemiologic Studies–Depression scale (CES-D; Radloff, 1977) to assess self-reported depressive symptoms in the preceding week. We computed a continuous total sum score, such that higher scores indicated more depressive symptoms. The CES-D has demonstrated good test–retest reliability and has been used successfully with Hispanic and Spanish-speaking older adults (Black et al., 1999). At baseline, Cronbach’s alpha for the CES-D in this sample was a α = 0.86.
Cognitive functioning
We used three aspects of cognitive functioning to create a latent cognitive functioning variable at each time point: tracking ability, short-term verbal learning and memory, and short-term object memory.
We assessed tracking ability by using the Color Trails Test, Part 2 (D’Elia, Satz, Uchiyama, & White, 1996), in which participants are asked to connect numbered and colored circles in the proper sequence, alternating between two colors. The Color Trails Test has been shown to be a reliable and valid measure of attention or executive functioning in geriatric populations (La Rue, Romero, Ortiz, Liang, & Lindeman, 1999). In this study, we assessed tracking ability according to the amount of time taken to complete Part 2.
We measured Short-term verbal learning and memory by using the second edition of the Spanish-language version of the California Verbal Learning Test (Delis, Kramer, Kaplan, & Ober, 2000). Participants are read a list of words and are then asked to verbally recall the words across several trials. In this study we used total learning across five successive presentations of the same word list, or the summed free recall for Trials 1–5 of List A, as an indicator of verbal learning and memory. This has been used as a global index of verbal learning ability (Lamar, Resnick, & Zonderman, 2003).
We assessed short-term object memory by using the Fuld Object Memory Evaluation (Fuld, 1977), as modified by Loewenstein and colleagues (2001). Participants are asked to recall 10 common household objects across a series of learning trials. Acceptable reliability and validity has been demonstrated in samples of community-dwelling, healthy older adults as well as patients with Alzheimer’s disease (Loewenstein et al.). In this study, we assessed short-term object memory by the summed recall across the three learning trials of the Fuld Object Memory Evaluation.
Control and descriptive variables
We assessed these variables at baseline, and included the following: gender, age, marital status, years of education, financial strain, and global cognitive functioning. We assessed financial strain by using a single item about how difficult it was to pay for basic needs, using a response scale ranging from 1 (easy) to 7 (very difficult). We assessed global cognitive functioning with the MMSE (Folstein et al., 1975), a commonly used screening measure with both Hispanic and non-Hispanic older adults; it is scored on a 0- to 30-point scale.
Analytic Model
Our preliminary analyses examined variable distributions, sample characteristics, and attrition. Our primary analyses used Amos 6.0 (SPSS, 2005) and focused on structural equation modeling. We used a cross-lagged panel design to test the relationships between depressive symptoms and cognitive functioning across time. Analyses considered age, gender, education, marital status, and financial strain at baseline as possible control variables. Post hoc analyses examined the impact of baseline mental status and missing data caused by deaths on the relationship between depressive symptoms and cognitive functioning. Among the strengths of using a cross-lagged panel analysis approach is that it allows the simultaneous analysis of the two dependent variables, thereby permitting the identification of possible bidirectional associations between depressive symptoms and cognitive functioning across time.
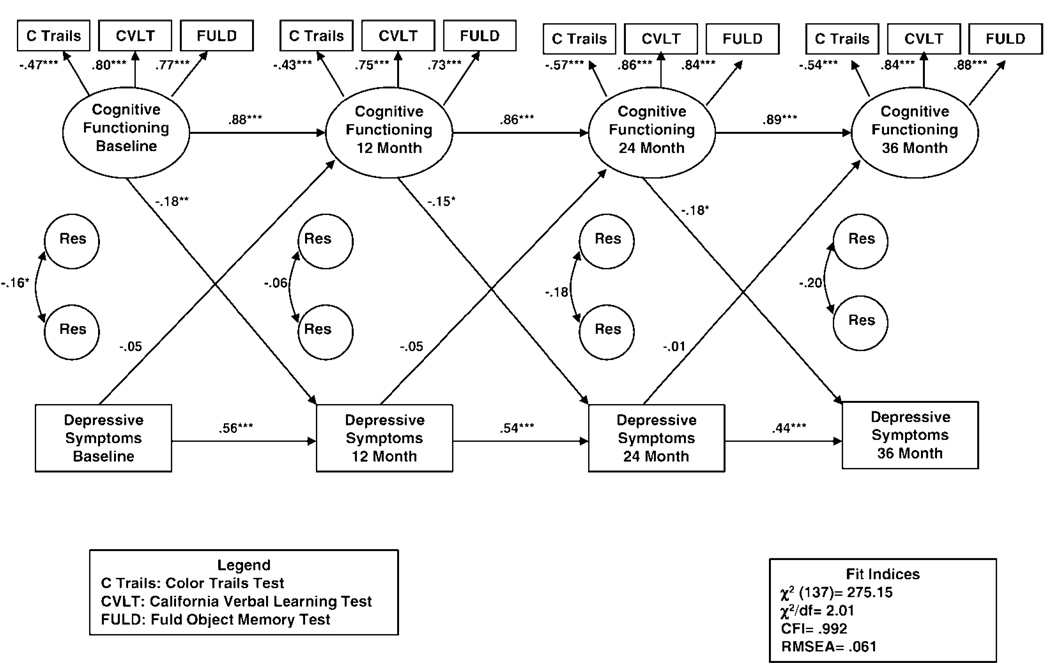
The structural equation modeling analyses involved developing an initial full model in which all paths were estimated, including all cross-lagged paths between depressive symptoms and cognitive functioning. We removed nonsignificant paths from the model with two exceptions. First, we always estimated the cross-lagged paths. Second, we initially drew paths from the control variables to the dependent variables only at baseline. If these paths were statistically significant, then in additional analyses we examined whether the control variable was significantly related to the dependent variable at later time points. For the purposes of consistency and clarity, if a control variable was significant at any of the postbaseline time points, then we included the control variable and outcome for all time points, regardless of whether they were significant. The full model is presented in Figure 1. To reduce clutter and highlight the cross-lagged pathways, which are the focus of this study, we specify the path coefficients for the control variables in Table 2 rather than Figure 1.
Figure 1.
Relationship between cognitive functioning and depressive symptoms across time: The full model. (CFI = Comparative Fit Index; RMSEA = root mean square error of approximation; Res = residual.)
Table 2.
Bivariate Correlations Among Baseline Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Age | — | |||||||||
| 2. | Gendera | .19** | — | ||||||||
| 3. | Financial strain | −.02 | .13* | — | |||||||
| 4. | Marital statusb | .15* | .22*** | .02 | — | ||||||
| 5. | Education | −.12* | −.09 | −.03 | .04 | — | |||||
| 6. | MMSE-B | −.30*** | −.21** | −.08 | −.03 | .34*** | — | ||||
| 7. | CES–D-B | .07 | .20** | .24*** | .18** | −.04 | −.19** | — | |||
| 8. | CVLT-B | −.25*** | −.12* | −.04 | −.02 | .23*** | .43*** | −.15* | — | ||
| 9. | Fuld-B | −.26*** | −.07 | .003 | −.07 | .08 | .37*** | −.13* | .62*** | — | |
| 10. | Trails-B | .25*** | .17** | .07 | .02 | −.27*** | −.46*** | .18** | −.36*** | −.37*** | — |
Notes: MMSE = Mini-Mental State Examination; CES–D = Center for Epidemiological Studies–Depression scale; CVLT = California Verbal Learning Test; Fuld = Fuld Object Memory Evaluation; Trails = Color Trails Test.
Gender dummy codes: male = 1; female = 2.
Marital status dummy codes: married or never married = 1; disrupted marriage (e.g., separated, divorced, widowed) = 2.
p < .05;
p < .01;
p < .001.
RESULTS
Attrition or Missing Data Across Time Points
Participants decreased from 273 at baseline to 194 at the 36-month follow-up. By the 36-month follow-up, 77 participants were lost to follow-up (36 died, 16 refused to participate, 9 moved out of greater Miami, and 16 could not be located). Two participants either refused or could not complete any of the analytic variables of interest (three cognitive measures and one depressive symptoms measure) at the 36-month interview. Fewer than 1% of the yearly evaluations were missed by survivors at each time point for reasons other than those given here (i.e., the participant could not be engaged for that year’s evaluation). Additional missing data for the depressive symptoms and cognitive functioning measures were as follows: (a) CES-D, less than 1%; (b) Fuld Object Memory Evaluation, less than 3%; (c) California Verbal Learning Test, less than 5%; and (d) Color Trails Test, less than 10%. We addressed the missing data by using a full information maximum likelihood algorithm (Arbuckle, 1996).
Depressive Symptoms and Global Cognitive Functioning in the Sample
At baseline, we found that slightly more than 35% of the participants (n = 96) met the criteria for clinically relevant levels of depressive symptoms (Perrino et al., in press), based on the commonly used CES-D cutoff score of 16 and above (see Cho et al., 1993). We found clinically relevant symptoms in 29% of the sample at the 12-month follow-up, 28% at 24 months, and 29% at 36 months. Similarly, we examined the distribution of global cognitive functioning at each time point by using the study’s MMSE cutoff point of 17, as well as the more conservative estimate of 24 that has been used in other studies. At baseline, all participants scored 17 or above on the MMSE (study entry criterion), whereas 33% scored below 24 on the MMSE. At 12 months, 3% of participants scored below 17 on the MMSE, whereas 23% scored below 24 on the MMSE. These percentages varied modestly over time, with 5% and 27% below 17 and 24, respectively, at 24 months, and 3% and 25% below 17 and 24, respectively, at 36 months.
Means and standard deviations of the three cognitive measures and depressive symptoms scale at each of the four assessments are presented in Table 1. On average, mean values for the four outcome measures were fairly stable over time. This was the case whether one examined the means based on all data at each time point (as presented in Table 1) or whether one examined the means based on only those individuals who remained in the sample through the 36-month follow-up. However, individual older adults did show meaningful changes in their own scores over time, with some showing increases and others showing decreases over the course of the study. When examining individual change over time, we found that the mean level of change between the initial baseline assessment and the 36-month assessment on the cognitive functioning and depressive scales was 0.69 to 0.78 SD, with some individuals increasing and others decreasing over time. In other words, on average, the typical older adult increased or decreased nearly 0.75 SD in cognitive functioning or depressive symptoms. However, because this change was positive for some and negative for others, the overall mean levels across all individuals stayed fairly flat.
Table 1.
Number, Means, and Standard Deviations of Cognitive Functioning and Depressive Symptoms
| Follow-Up | ||||
|---|---|---|---|---|
| Test | Baseline | 12 Month | 24 Month | 36 Month |
| Color Trails | 207.81 (96.00) | 196.80 (93.10) | 207.34 (92.67) | 191.81 (97.93) |
| n = 261 | n = 220 | n = 197 | n = 176 | |
| California Verbal Learning Test | 29.80 (10.92) | 30.62 (10.69) | 30.35 (11.39) | 31.98 (12.05) |
| n = 268 | n = 223 | n = 204 | n = 185 | |
| FULD Object Memory Evaluation | 20.91 (4.86) | 20.30 (4.77) | 21.09 (5.14) | 21.88 (4.92) |
| n = 272 | n = 231 | n = 210 | n = 188 | |
| Depressive Symptoms | 12.99 (10.64) | 11.27 (9.09) | 11.31 (9.63) | 11.37 (8.71) |
| n = 273 | n = 231 | n = 214 | n = 192 | |
.
Correlation Matrix
Table 2 presents the bivariate correlations between variables at baseline, which suggested that increasing age was associated with poorer cognitive functioning but that age was unrelated to depressive symptoms. Women tended to have poorer cognitive functioning scores and greater depressive symptoms than men did. Financial strain was related to more depressive symptoms. Higher levels of education tended to be related to better cognitive functioning but were unrelated to depressive symptoms. Higher depressive symptoms were consistently related to worse cognitive functioning.
Cognitive Functioning Measurement Model
We conducted analyses to assess for factor invariance in the cognitive functioning latent variable across time points. This is important because, when possible, it is desirable to constrain factor loadings across time, given that degrees of freedom are added and fewer parameters have to be estimated. For the present model, factor loadings for the cognitive functioning latent variables were invariant across the four time points (see Figure 1). Specifically, the chi-square difference test between the models with and without constrained factor loadings was not statistically significant, χ2(9) = 10.27, p = 0.33. Consequently, we constrained the factor loadings to be equal at each time point. In addition, we allowed error terms for each of the cognitive functioning indicators to correlate with the corresponding indicators at the other time points. Fit indices suggested a good fit between the measurement model and data, with Comparative Fit Index = 0.977, root mean square error of approximation = 0.054, and χ2/df = 1.781. As we expected, the chi-square test was statistically significant: χ2(39) = 69.468, p = .002.
Model Development
Figure 1 shows the full model, in which all cross-lagged paths were estimated. Model fit indices showed an adequate fit to the data, specifically χ2/df = 2.01, Comparative Fit Index = 0.922, and root mean square error of approximation = 0.061; model χ2(137) = 275.15, p < .001. Regression weights and p values for the control variables are shown in Table 3. We allowed the control variables to correlate with each other in the model. We considered marital status as a control variable, but it was not a significant predictor of either cognitive functioning or depressive symptoms and therefore we did not include it in the final model. Global cognitive functioning at baseline was also considered as a control variable, but we removed it from the model because of its overlap with the cognitive functioning latent variables. However, to assess the potentially important impact of baseline cognitive functioning on the model, we did conduct additional post hoc analyses (reported later in the Results section).
Table 3.
Path Regression Weights, p Values, and Control Variables in the Full Model
| Path | Standardized Regression Weight | p |
|---|---|---|
| Age → cognitive functioning at baseline | .305 | <.001*** |
| Age → cognitive functioning at 12-month follow-up | .100 | .082 |
| Age → cognitive functioning at 24-month follow-up | .106 | .054 |
| Age → cognitive functioning at 36-month follow-up | .051 | .337 |
| Education → cognitive functioning at baseline | −.171 | .009** |
| Gender → depressive symptoms at baseline | .171 | .003** |
| Financial strain → depressive symptoms at baseline | .215 | <.001*** |
Note:
p < .05;
p < .01;
p < .001.
Overall Test of Directionality of the Cognition–Depression Effect
Once the full model was finalized, we performed tests of two alternative models to directly compare the overall effect of depressive symptoms on subsequent cognitive functioning with the overall effect of cognitive functioning on subsequent depressive symptoms. First, we tested a depression-to-cognition model, which paralleled the full model except that the cross-lagged paths from cognition to depression at each time point were fixed at zero (i.e., the only cross-lagged paths estimated were those from depression to subsequent cognition). Then we tested a cognition-to-depression model in which the cross-lagged paths from depression to cognition at each time point were fixed at zero (i.e., the only cross-lagged paths estimated were those from cognition to subsequent depression). Finally, we compared these two alternative models with the full model by using chi-square difference tests. By assessing the relative fit of these models, these analyses provided an additional test of the possible directionality of the relationships between cognitive functioning and depressive symptoms over time.
Results of these analyses are found in Table 4, including model fit statistics for each of the models and results from the comparisons of the full model with each of the alternative models. Although all three models had similar fit indices, the chi-square difference test (Δχ2) comparing the depression-to-cognition model with the full model was statistically significant. In other words, eliminating the three prospective paths from cognition at one time point to depression at the next time point resulted in a significantly poorer fit. This indicates that the cognition-to-depression paths as a whole must be included in the model. In contrast, a similar test comparing the cognition-to-depression model with the full model was not statistically significant, indicating that eliminating the three prospective paths from depression at one time point to cognition at the next time point did not negatively impact the model. In other words, these are superfluous.
Table 4.
Fit Indices for the Three Models With Model Fit Comparisons
| Model | χ2 | χ2/df | CFI | RMSEA | Δχ2 vs Full Model |
|---|---|---|---|---|---|
| Full | χ2(137) = 275.153* | 2.01 | 0.922 | 0.061 | — |
| Depression to cognition | χ2(140) = 291.789* | 2.08 | 0.914 | 0.063 | χ2(3) = 16.64* |
| Cognition to depression | χ2(140) = 277.442* | 1.98 | 0.922 | 0.060 | χ2(3) = 2.29** |
Notes: CFI = Comparative Fit Index; RMSEA = root mean square error of approximation. For the depression-to-cognition model, the cognition → depression paths are set to zero. For the cognition-to-depression model, the depression → cognition paths are set to zero.
p < .001;
p > .50.
This is consistent with the pattern seen among the individual time-specific cross-lagged effects. Figure 1 shows that none of the path coefficients between depressive symptoms and cognitive functioning at subsequent time points were statistically significant. In contrast, the path coefficients between cognitive functioning and depressive symptoms across time were all significant and all negative. This suggests that poorer cognitive functioning predicted higher levels of depressive symptoms as measured at the subsequent time points.
Post Hoc Analyses
We conducted two additional post hoc analyses to assess for the potential impact of (a) initial or baseline risk of cognitive impairment and (b) missing data caused by deaths during the study. First, to determine whether the findings were driven by those older adults evidencing the greatest risk of cognitive impairment, we reanalyzed the full model by including only the 182 participants who had baseline MMSE scores of 24 or higher. As indicated, this study’s entry criteria required participants to have MMSE scores of 17 or above, a cutoff point considered to be more appropriate for immigrant, non-English-speaking groups with low educational levels. This post hoc analysis using the more conservative cutoff point of 24 provided a test of the study’s model with a subgroup at lower risk for cognitive impairment.
The cross-lagged path coefficients for this model showed a similar pattern to those from the full model. Specifically, none of the depression-to-cognition paths were statistically significant (i.e., baseline depression → 12-month cognition = −0.03, p = .66; 12-month depression → 24-month cognition = −0.06, p = .44; 24-month depression → 36-month cognition = −0.003, p = .97), yet two of the three cognition-to-depression paths were significant (baseline cognition → 12-month depression = −0.27, p < .001; 12-month cognition → 24-month depression = −0.05, p = .58; 24-month cognition → 36-month depression = −0.20, p = .02).
Second, to determine whether the loss of participants who had died during the study influenced the results, we reanalyzed the full model by including only the 237 participants who remained alive through the 36-month follow-up assessment. Again, these results showed a pattern similar to the full model’s findings, with none of the depression-to-cognition paths being significant (i.e., baseline depression → 12-month cognition = −0.04, p = .48; 12-month depression → 24-month cognition = −0.05, p = .41; 24-month depression → 36-month cognition = −0.01, p = .81), and all cognition-to-depression paths being significant (baseline cognition → 12-month depression = −0.20, p = .002; 12-month cognition → 24-month depression = −0.14, p = .04; 24-month cognition → 36-month depression = −0.17, p = .02).
DISCUSSION
Although the hypothesized reciprocal relationship between cognitive functioning and depressive symptoms was not supported by the data, poorer cognitive functioning among older adults did predict greater depressive symptoms at all subsequent time points. It may be that as older adults perceive symptoms of cognitive decline and decreased control over aspects of their lives, depressive symptoms can develop or become exacerbated (Bierman et al., 2007; Zunzunegui, Béland, Llácer, & León, 1998). For community-dwelling older adults, distress may stem from perceived threats to their ability to continue living independently, one of the greatest sources of anxiety among older adults (Quine & Morrell, 2007), and particularly salient among those with limited financial resources. Alternatively, growing cognitive impairments may impair older adults’ capacity to regulate mood, mobilize sources of social support, and engage in activities that can prevent depressive symptoms or promote coping (Fisher, Segal, & Coolidge, 2003). Cognitive changes, such as a decline in memory or inability to plan activities, can limit participation in reinforcing activities and interpersonal relationships, which may in turn lead to depression (Fagan, 2003). Cognitive impairments may also make individuals more prone to cognitive distortions (e.g., unrealistic expectations, overgeneralized reactions to negative life events), which can further promote depression (Beck, 1987; Rabin et al., 2007; Sachs-Ericsson & Blazer, 2006; Shifren et al., 1999).
As suggested, structural and functional brain changes associated with cognitive decline may produce behavioral changes, such as those that accompany, or are manifested by, depressive symptoms. Changes may include frontostriatal dysfunction, with related executive impairment and psychomotor retardation, which have been associated with the presence, chronicity, and recurrence of geriatric depression (Alexopolous et al., 2002; Butters et al., 2004). Subcortical cerebrovascular disease may underlie such deficits (Alexopolous et al.; Butters et al.). Brain imaging research may help clarify these relationships in the near future.
In contrast, this study found that older adults with more depressive symptoms were no more or less likely to experience cognitive functioning changes at later time points than were those older adults with fewer symptoms. Although some researchers have found evidence that higher levels of depression are related to later cognitive declines (Barnes et al., 2006; Paterniti et al., 2002), findings have been inconsistent (Barnes et al.; Bassuk et al., 1998; Ganguli et al., 2006), perhaps owing to study design and sample differences. The present study’s comparatively short follow-up period of 3 years may have limited the ability to detect a significant prospective relationship between depression and later cognition. Community samples of older adults tend to evidence low rates of severe cognitive decline (Ganguli et al.), and longer follow-up periods may be needed to detect cognitive changes among these healthier groups (Wilson et al., 2004). At least two other studies similarly failed to find a prospective relationship between older adults’ depression and cognition over a 3-year period (Dufouil et al., 1996; Henderson et al., 1997). In contrast, several studies that found a relationship between depressive symptoms and future cognitive declines did so over longer periods, such as 5 years or more (Barnes et al.; Gatz et al., 2005; Wilson et al.; but see also Ganguli et al., who did not find a prospective relationship between depressive symptoms and cognitive function over 12 years).
The absence of a relationship between depressive symptoms and consequent cognitive functioning in this study may have been influenced by the sample’s relatively low educational levels. At least one earlier study found that depression influenced later cognitive decline only among more educated older adults (Geerlings et al., 2000). Alternatively, depression may have to be chronic or severe to impact cognitive functioning, given that some studies have documented a relationship between clinical levels of depression (e.g., major depression or persistent depression) and later cognitive impairments, including Alzheimer’s disease (Ownby et al., 2006; Paterniti et al., 2002). The present study assessed depressive symptoms through self-reports of recently experienced symptoms; thus, symptom duration is unknown.
Assessing depression and cognitive functioning as continuous variables may also have influenced the findings. Several studies have found a prospective relationship between depressive symptoms and cognitive functioning over time when these variables were treated categorically (i.e., using cutoff points or clinical diagnoses; see Barnes et al., 2006; Fuhrer, Dufouil, & Dartigues, & the PAQUID Study Group, 2003; Gatz et al., 2005; Paterniti et al., 2002). However, at least two studies identified a prospective relationship between continuously measured depressive symptoms and cognitive functioning over time (Wilson et al., 2002, 2004). The present strategy to analyze depression and cognitive functioning continuously represents a strength to the degree that it allows evaluation of smaller changes in depression or cognition. This is important at a community and public health level, partly because subclinical symptoms of depression and cognitive decline are more common than clinical diagnoses, and these symptoms often cause substantial disturbances in functioning and quality of life (Artero, Touchon, & Ritchie, 2001).
Results of the post hoc analyses accounting for missing data caused by deaths or risk of severe cognitive impairment showed a pattern similar to the results to the full model. These findings speak to the consistency of the results despite attrition. The post hoc model that removed older adults with lower MMSE scores provides important evidence that the study’s findings were not driven by participants evidencing the greatest risk of cognitive impairment.
The use of multiple time points and a cross-lagged design provides a further strength to the study. A cross-lagged panel design is an effective way of examining the relative predictive association between two variables over time, controlling for the effects at earlier time points. By examining the association between each variable at an earlier time point with the other variable at a later time point, one can establish a temporal precedence and a correlational association—both of which are required for a true causal effect. However, it should be noted that a cross-lagged design in and of itself cannot address the possibility that the observed correlations are nonetheless spurious, as a result of other unmeasured factors. Furthermore, the absence of a cross-lagged effect may reflect a causal influence operating on a significantly different time frame than that which is used in the study. For example, the effect of a negative factor may take greater time to manifest itself in the second variable, or the effect may be more immediate and then dissipate with time.
A limitation of this study is that we examined neither participants’ medical history nor antidepressant use in the analyses. Although various conditions have been related to depression and cognition, especially cardiovascular disease (Fuhrer et al., 2003), the impact of medical history and antidepressant use in cognition and depression analyses has not been consistently significant (Barnes et al., 2006). A further limitation is that over 40% of older adults randomly selected for participation were not enrolled, primarily as a result of refusals, deaths, and moves away from the study area.
From a clinical perspective, this study’s findings support the need for medical and mental health professionals to be vigilant for even mild cognitive declines among older adults, and to provide cognitive interventions and compensatory mechanisms for older adults to minimize the loss of cognitive capabilities (see, e.g., Belleville et al., 2006; Charness, 2007). The results of the present study suggest that these actions might decrease the severity of depressive symptoms in older adults.
ACKNOWLEDGMENTS
This research was supported by the National Institute of Mental Health/National Institute of Environmental Health Sciences under Grant MH 63709 (J. Szapocznik, Principal Investigator; A. Spokane, Coprincipal Investigator), by the National Institute on Aging under Grant AG 027527 (J. Szapocznik, Principal Investigator; S. Brown, Coprincipal Investigator), and by the Robert Wood Johnson Foundation under Grant 037377. We thank Monica Zarate, Rosa Verdeja, Tatiana Clavijo, Aleyda Marcos, and Patricia Thomas for their help in conducting this study.
REFERENCES
- Alexopolous GS, Buckwalter K, Olin J, Martinez R, Wainscott C, Krishnan KRR. Comorbidity of late life depression: An opportunity for research on mechanisms and treatment. Biological Psychiatry. 2002;52:543–558. doi: 10.1016/s0006-3223(02)01468-3. [DOI] [PubMed] [Google Scholar]
- Arbuckle JL. Full information estimation in the presence of incomplete data. In: Marcoulides GA, Schumacker RE, editors. Advanced structural equation modeling: Issues and techniques. Mahwah, NJ: Erlbaum; 1996. pp. 243–277. [Google Scholar]
- Artero S, Touchon J, Ritchie K. Disability and mild cognitive impairment: A longitudinal population-based study. International Journal of Geriatric Psychiatry. 2001;16:1092–1097. doi: 10.1002/gps.477. [DOI] [PubMed] [Google Scholar]
- Baltes MM, Lang FR. Everyday functioning and successful aging: The impact of resources. Psychology and Aging. 1997;12(3):433–443. doi: 10.1037//0882-7974.12.3.433. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Alexopoulos GS, López OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: Findings from the Cardiovascular Health Study. Archives of General Psychiatry. 2006;63:273–280. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Berkman L, Wypij D. Depressive symptomatology and incident cognitive decline in an older adult community sample. Archives of General Psychiatry. 1998;63:1073–1081. doi: 10.1001/archpsyc.55.12.1073. [DOI] [PubMed] [Google Scholar]
- Baune BT, Suslow T, Arolt V, Berger K. The relationship between psychological dimensions of depressive symptoms and cognitive functioning in the elderly—The MEMO Study. Journal of Psychiatric Research. 2006;41:247–254. doi: 10.1016/j.jpsychires.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Beck A. Cognitive model of depression. Journal of Cognitive Psychotherapy. 1987;1:2–27. [Google Scholar]
- Belleville S, Gilbert B, Fontaine F, Gagnon L, Ménard E, Gauthier S. Improvement of episodic memory in persons with mild cognitive impairment and healthy older adults: Evidence from a cognitive intervention program. Dementia and Geriatric Cognitive Disorders. 2006;22(5–6):486–499. doi: 10.1159/000096316. [DOI] [PubMed] [Google Scholar]
- Bierman EJM, Comijs HC, Jonker C, Beekman ATF. Effects of anxiety versus depression on cognition in later life. American Journal of Geriatric Psychiatry. 2005;13:686–693. doi: 10.1176/appi.ajgp.13.8.686. [DOI] [PubMed] [Google Scholar]
- Bierman EJM, Comijs HC, Jonker C, Beekman ATF. Symptoms of anxiety and depression in the course of cognitive decline. Dementia and Geriatric Cognitive Disorders. 2007;24(3):213–219. doi: 10.1159/000107083. [DOI] [PubMed] [Google Scholar]
- Black SA, Espino DV, Mahurin R, Lichtenstein MJ, Hazuda HP, Fabrizio D, et al. The influence of noncognitive factors on the Mini-Mental State Examination in older Mexican Americans: Findings from the Hispanic EPESE–Established Population for the Epidemiologic Study of the Elderly. Journal of Clinical Epidemiology. 1999;52:1095–1102. doi: 10.1016/s0895-4356(99)00100-6. [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, et al. The nature and determinants of neuropsychological functioning in late-life depression. Archives of General Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Charness N, editor. Journal of Gerontology: Psychological Sciences. special issue I. 62B. 2007. Cognitive interventions and aging; pp. P5–P96. [Google Scholar]
- Cho MJ, Moscicki EK, Narrow WE, Rae DS, Locke BZ, Regier DA. Concordance between two measures of depression in the Hispanic Health and Nutrition Examination Survey. Social Psychiatry and Psychiatric Epidemiology. 1993;28:156–163. doi: 10.1007/BF00797317. [DOI] [PubMed] [Google Scholar]
- D’Elia LF, Satz P, Uchiyama CL, White T. Color Trails Test Professional Manual. Odessa, FL: Psychological Assessment Resources; 1996. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test—Second edition: Adult version manual. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Dufouil C, Fuhrer R, Dartigues JF, Alpérovitch A. Longitudinal analysis of the association between depressive symptomatology and cognitive deterioration. American Journal of Epidemiology. 1996;144:634–641. doi: 10.1093/oxfordjournals.aje.a008974. [DOI] [PubMed] [Google Scholar]
- Espino DV, Lichtenstein MJ, Palmer RF, Hazuda HP. Ethnic differences in Mini-Mental State Examination (MMSE) scores: Where you live makes a difference. Journal of the American Geriatrics Society. 2001;49:538–548. doi: 10.1046/j.1532-5415.2001.49111.x. [DOI] [PubMed] [Google Scholar]
- Fagan LA. Challenging the mind: Activities and socialization. In: Radin L, Radin G, editors. What if it’s not Alzheimer’s? A caregiver’s guide to dementia. Amherst, NY: Prometheus Books; 2003. pp. 169–180. [Google Scholar]
- Falcón LM, Tucker KL. Prevalence and correlates of depressive symptoms among Hispanic elders in Massachusetts. Journal of Gerontology: Social Sciences. 2000;55B:S108–S116. doi: 10.1093/geronb/55.2.s108. [DOI] [PubMed] [Google Scholar]
- Fisher BM, Segal DL, Coolidge FL. Assessment of coping in cognitively impaired older adults: A preliminary study. Clinical Gerontologist. 2003;26(3/4):3–12. [Google Scholar]
- Folstein M, Folstein S, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fuhrer R, Dufouil C, Dartigues JF the PAQUID Study Group. Exploring sex differences in the relationship between depressive symptoms and dementia incidence: Prospective results from the PAQUID Study. Journal of the American Geriatrics Society. 2003;51:1178–1180. doi: 10.1046/j.1532-5415.2003.51352.x. [DOI] [PubMed] [Google Scholar]
- Fuld PA. Fuld object memory evaluation manual. Wood Dale, IL: Shoelting Press; 1977. [Google Scholar]
- Ganguli M, Du Y, Dodge HH, Ratcliff GG, Chang CH. Depressive symptoms and cognitive decline in late life: A prospective epidemiological study. Archives of General Psychiatry. 2006;63:153–160. doi: 10.1001/archpsyc.63.2.153. [DOI] [PubMed] [Google Scholar]
- Gatz JL, Tyas SL, St. John P, Montgomery P. Do depressive symptoms predict Alzheimer’s disease and dementia? Journal of Gerontology: Medical Sciences. 2005;60A:744–747. doi: 10.1093/gerona/60.6.744. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, Schoevers RA, Beekman ATF, Jonker C, Deeg DJH, Schmand B, et al. Depression and risk of cognitive decline and Alzheimer’s disease: Results of two prospective community-based studies in the Netherlands. British Journal of Psychiatry. 2000;176:568–575. doi: 10.1192/bjp.176.6.568. [DOI] [PubMed] [Google Scholar]
- Henderson AS, Korten AE, Jacomb PA, MacKinnon AJ, Jorm AF, Christensen H, et al. The course of depression in the elderly: A longitudinal community-based study in Australia. Psychological Medicine. 1997;27:119–129. doi: 10.1017/s0033291796004199. [DOI] [PubMed] [Google Scholar]
- Lamar M, Resnick SM, Zonderman AB. Longitudinal changes in verbal memory in older adults: Distinguishing the effects of age from repeat testing. Neurology. 2003;60:82–86. doi: 10.1212/wnl.60.1.82. [DOI] [PubMed] [Google Scholar]
- La Rue A, Romero LJ, Ortiz IE, Liang HC, Lindeman RD. Neuropsychological performance of Hispanic and non-Hispanic older adults: An epidemiologic survey. Clinical Neuropsychology. 1999;13(4):474–486. doi: 10.1076/1385-4046(199911)13:04;1-Y;FT474. [DOI] [PubMed] [Google Scholar]
- La Rue A, Swan GE, Carmelli D. Cognition and depression in a cohort of aging men: Results from the Western Collaborative Group Study. Psychology and Aging. 1995;10:30–33. doi: 10.1037//0882-7974.10.1.30. [DOI] [PubMed] [Google Scholar]
- Lichtenberg PA, Ross T, Millis SR, Manning CA. The relationship between depression and cognition in older adults: A cross-validation study. Journal of Gerontology: Psychological Sciences. 1995;50B:P25–P32. doi: 10.1093/geronb/50b.1.p25. [DOI] [PubMed] [Google Scholar]
- Loewenstein DA, Argüelles T, Acevedo A, Freeman RQ, Mendelssohn E, Ownby RL, et al. The utility of a modified object memory test in distinguishing between different age groups of Alzheimer’s disease patients and normal controls. Journal of Mental Health and Aging. 2001;7:317–324. [Google Scholar]
- Lyketsos CG, López O, Jones B, Fitzpatrick AL, Breitner J, DeKovsky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the Cardiovascular Health Study. Journal of the American Medical Association. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- O’Brien JT. Vascular cognitive impairment. American Journal of Geriatric Psychiatry. 2006;14:724–733. doi: 10.1097/01.JGP.0000231780.44684.7e. [DOI] [PubMed] [Google Scholar]
- Ostrosky-Solis F, López-Arango G, Ardila A. Sensitivity and specificity of the Mini-Mental State Examination in a Spanish-speaking population. Applied Neuropsychology. 2000;7:25–31. doi: 10.1207/S15324826AN0701_4. [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer’s disease: Systematic review, meta-analysis, and meta-regression analysis. Archives of General Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterniti S, Verdier-Taillefer MH, Dufouil C, Alpérovitch A. Depressive symptoms and cognitive decline in elderly people. British Journal of Psychiatry. 2002;181:406–410. doi: 10.1192/bjp.181.5.406. [DOI] [PubMed] [Google Scholar]
- Perrino T, Brown SC, Mason CA, Szapocznik J. Depressive symptoms among urban Hispanic older adults: Prevalence and sociodemographic correlates. Clinical Gerontologist. doi: 10.1080/07317110802478024. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quine S, Morrell S. Fear of loss of independence and nursing home admission in older Australians. Health and Social Care in the Community. 2007;15:212–220. doi: 10.1111/j.1365-2524.2006.00675.x. [DOI] [PubMed] [Google Scholar]
- Rabin LA, Borgos MJ, Saykin AJ, Wishart HA, Crane PK, Nutter-Upham KE, et al. Judgment in older adults: Development and psychometric evaluation of the Test of Practical Judgment (TOP-J) Journal of Clinical and Experimental Neuropsychology. 2007;29:752–767. doi: 10.1080/13825580601025908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;3:385–401. [Google Scholar]
- Sachs-Ericsson N, Blazer DG. Depression in late life: Etiology, diagnosis and treatment. In: John Pathy MS, Sinclair AJ, Morley JE, editors. Principles and practice of geriatric medicine. 4th ed. Chichester, England: Wiley; 2006. [Google Scholar]
- Shifren K, Park DC, Bennett JM, Morrrell RW. Do cognitive processes predict mental health in individuals with rheumatoid arthritis? Journal of Behavioral Medicine. 1999;22:529–547. doi: 10.1023/a:1018782211847. [DOI] [PubMed] [Google Scholar]
- SPSS, Inc. Amos 6.0: Structural equation modeling to test relationships. Chicago: Author; 2005. [Google Scholar]
- Tang M-X, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, et al. The APOE-ε4 allele and the risk of Alzheimer disease among African Americans, Whites, and Hispanics. Journal of the American Medical Association. 1998;279:751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Mendes de Leon CF, Aggarwal NT, Schneider JS, Bach J, et al. Depressive symptoms, cognitive decline and risk of AD in older persons. Neurology. 2002;59:364–370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Mendes de Leon CF, Bennett DA, Bienias JL, Evans DA. Depressive symptomatology and cognitive decline in a community population of older persons. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75:126–129. [PMC free article] [PubMed] [Google Scholar]
- Zunzunegui MV, Béland F, Llácer A, León V. Gender differences in depressive symptoms among Spanish elderly. Social Psychiatry and Psychiatric Epidemiology. 1998;33:195–205. doi: 10.1007/s001270050043. [DOI] [PubMed] [Google Scholar]