Abstract
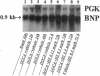
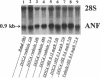
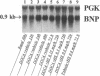
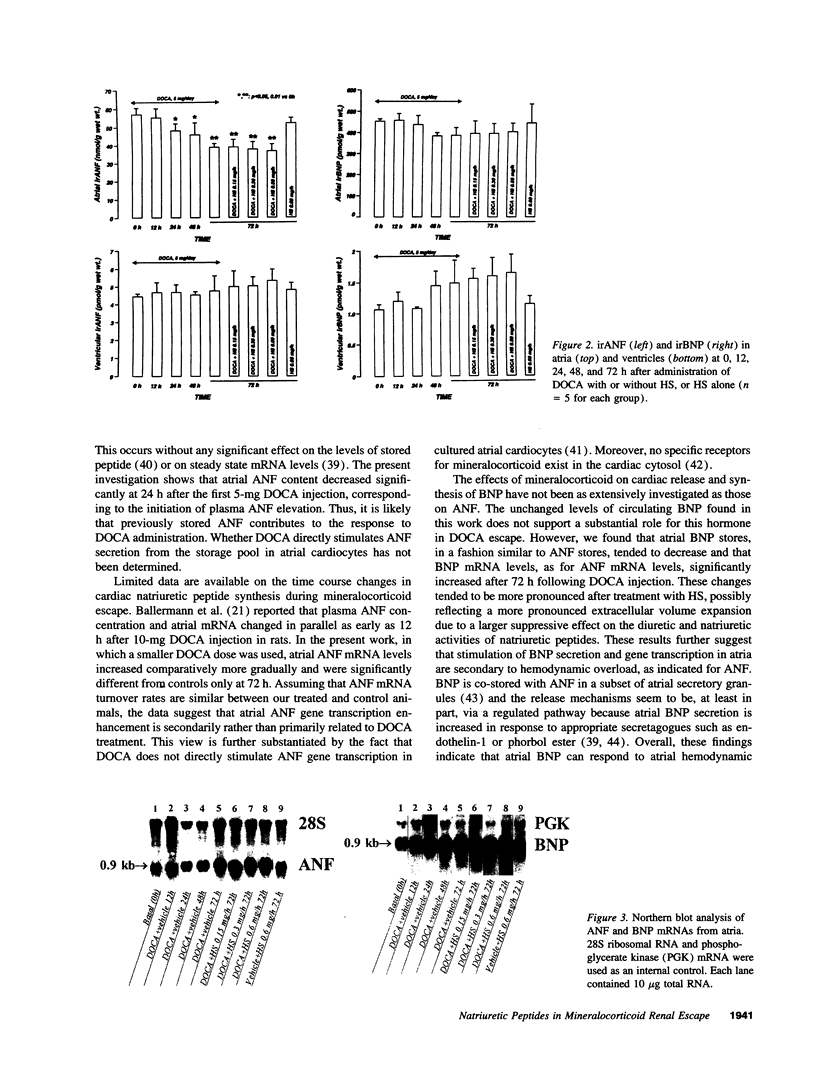
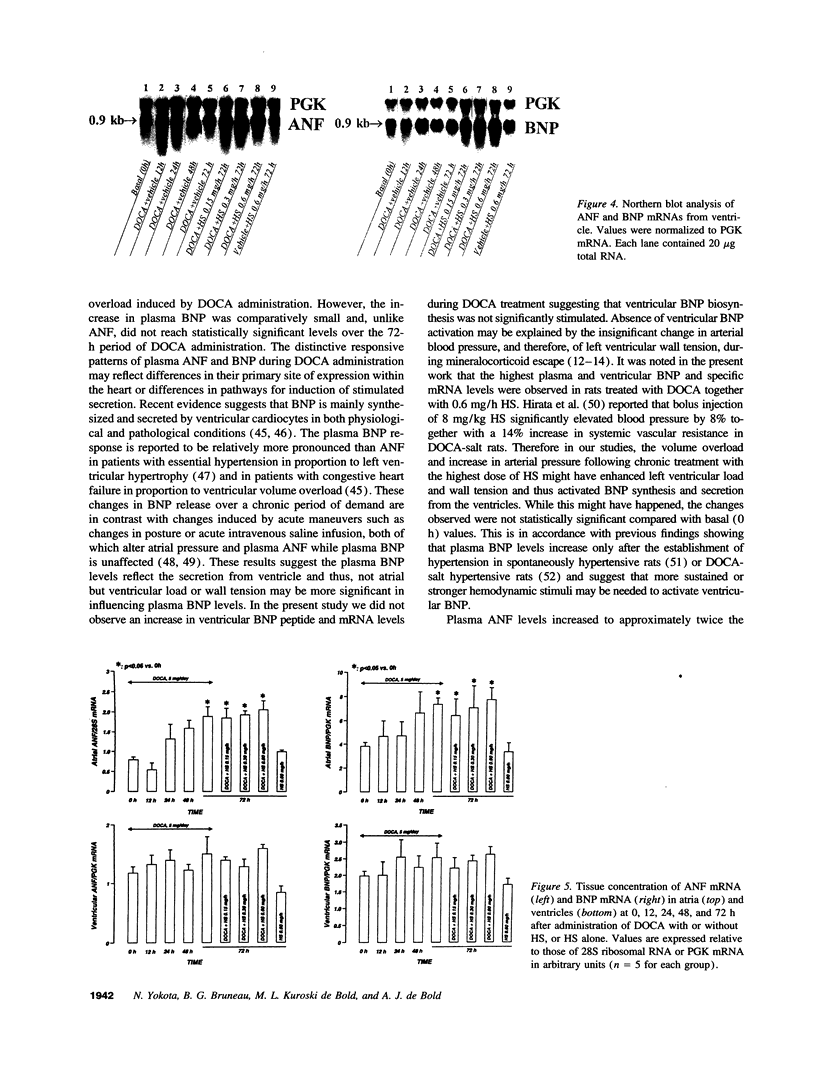
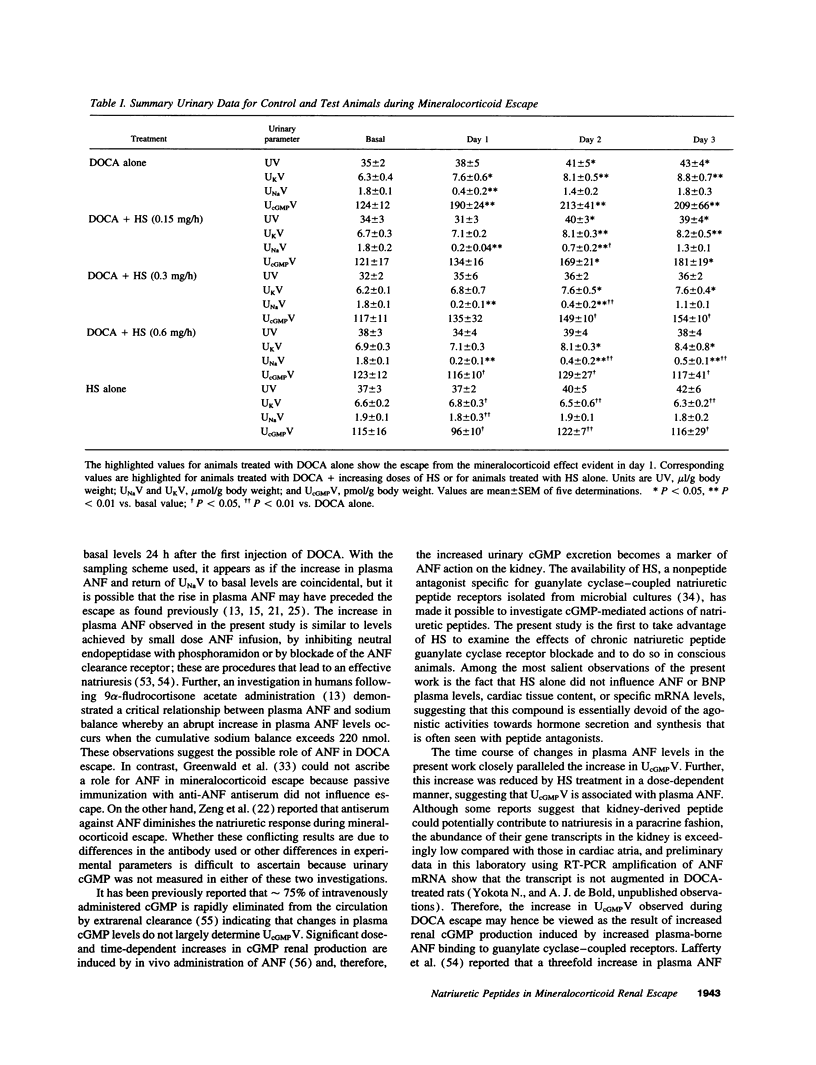
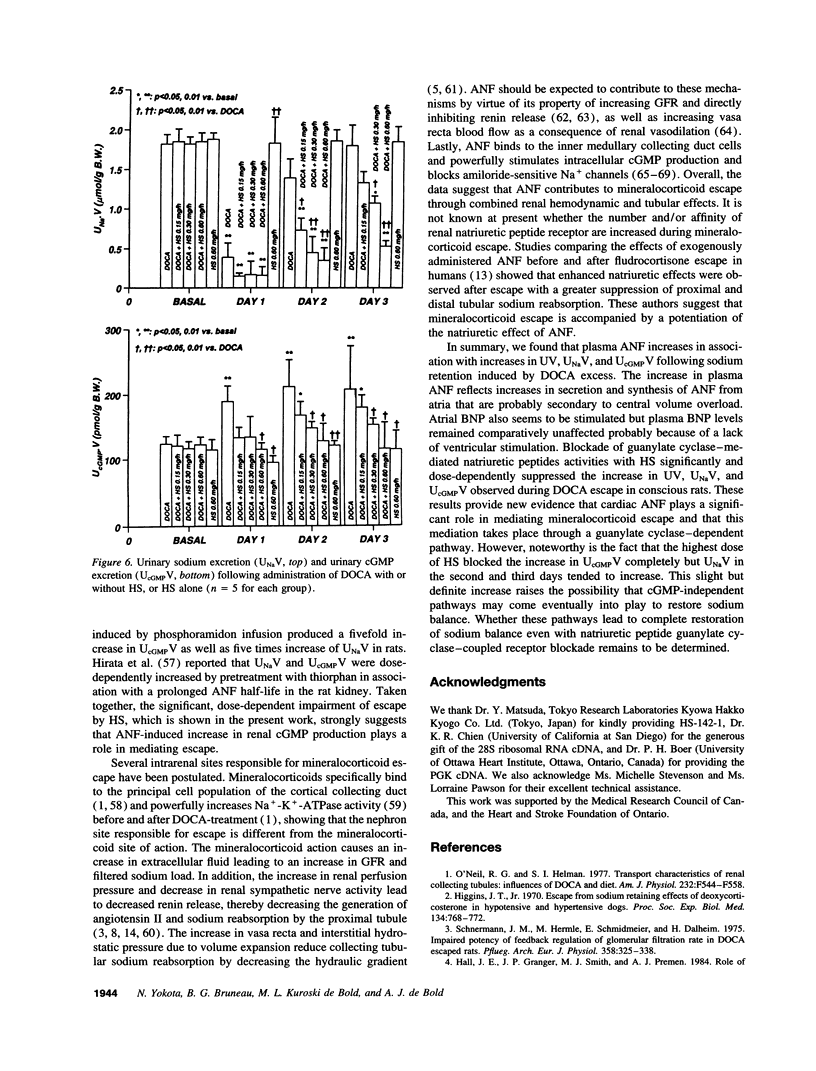
The mechanism underlying the mineralocorticoid escape phenomenon remains unknown. To assess the possible contribution of natriuretic peptides to mineralocorticoid escape, rats were injected with 5 mg deoxycorticosterone acetate for 3 d. Plasma atrial natriuretic factor (ANF) rose to twice basal levels and atrial ANF content decreased significantly by 24 h of treatment. This coincided with renal escape and with a significant increase in urinary cGMP excretion. Plasma ANF remained elevated and atrial ANF content continued to decline by 48 and 72 h while atrial ANF mRNA levels increased significantly only at 72 h. Plasma brain natriuretic peptide did not increase during escape although atrial brain natriuretic peptide mRNA levels increased significantly. Chronically administered HS-142-1 (HS), a specific antagonist of the guanylate cyclase-coupled natriuretic peptide receptors, significantly and dose-dependently impaired the escape phenomenon. The highest dose of HS completely suppressed the increase in urinary cGMP. Despite the continued suppression, partial escape was observed by the end of the observation period. HS alone influenced neither plasma nor tissue or urine parameters. These findings show that despite activation of atrial ANF, blockade of the guanylate cyclase-coupled natriuretic peptide receptors impairs the ability of the kidney to escape the Na+ retaining effect of excess mineralocorticoid in a dose-dependent fashion. Later-acting, unknown mechanisms eventually come into play to mediate the escape phenomenon through a guanylate cyclase-independent pathway. Therefore, ANF of cardiac origin appears to be a major factor initiating mineralocorticoid escape through a guanylate cyclase-dependent pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballermann B. J., Bloch K. D., Seidman J. G., Brenner B. M. Atrial natriuretic peptide transcription, secretion, and glomerular receptor activity during mineralocorticoid escape in the rat. J Clin Invest. 1986 Sep;78(3):840–843. doi: 10.1172/JCI112650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biollaz J., Dürr J., Brunner H. R., Porchet M., Gavras H. Escape from mineralocorticoid excess: the role of angiotensin II. J Clin Endocrinol Metab. 1982 Jun;54(6):1187–1193. doi: 10.1210/jcem-54-6-1187. [DOI] [PubMed] [Google Scholar]
- Broadus A. E., Kaminsky N. I., Hardman J. G., Sutherland E. W., Liddle G. W. Kinetic parameters and renal clearances of plasma adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in man. J Clin Invest. 1970 Dec;49(12):2222–2236. doi: 10.1172/JCI106441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett J. C., Jr, Granger J. P., Opgenorth T. J. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol. 1984 Nov;247(5 Pt 2):F863–F866. doi: 10.1152/ajprenal.1984.247.5.F863. [DOI] [PubMed] [Google Scholar]
- Burnett J. C., Jr, Haas J. A., Larson M. S. Renal interstitial pressure in mineralocorticoid escape. Am J Physiol. 1985 Sep;249(3 Pt 2):F396–F399. doi: 10.1152/ajprenal.1985.249.3.F396. [DOI] [PubMed] [Google Scholar]
- Cappuccio F. P., Markandu N. D., Buckley M. G., Sagnella G. A., Shore A. C., MacGregor G. A. Changes in the plasma levels of atrial natriuretic peptides during mineralocorticoid escape in man. Clin Sci (Lond) 1987 May;72(5):531–539. doi: 10.1042/cs0720531. [DOI] [PubMed] [Google Scholar]
- Chang M. S., Lowe D. G., Lewis M., Hellmiss R., Chen E., Goeddel D. V. Differential activation by atrial and brain natriuretic peptides of two different receptor guanylate cyclases. Nature. 1989 Sep 7;341(6237):68–72. doi: 10.1038/341068a0. [DOI] [PubMed] [Google Scholar]
- Dickstein G. M., Woodson J. F., Lamb N. E., Rose C. E., Jr, Peach M. J., Carey R. M. Escape from the sodium-retaining action of intrarenal angiotensins II and III in the conscious dog. Endocrinology. 1985 Nov;117(5):2160–2169. doi: 10.1210/endo-117-5-2160. [DOI] [PubMed] [Google Scholar]
- Dürr J., Favre L., Gaillard R., Riondel A. M., Vallotton M. B. Mineralocorticoid escape in man: role of renal prostaglandins. Acta Endocrinol (Copenh) 1982 Mar;99(3):474–480. doi: 10.1530/acta.0.0990474. [DOI] [PubMed] [Google Scholar]
- Düsing R., Wilke R., Körber A., Klingmüller D., Kramer H. J. Inner medullary osmolality and sodium concentration are decreased in rats during escape from DOCA-induced salt retention. Clin Sci (Lond) 1981 Apr;60(4):467–469. doi: 10.1042/cs0600467. [DOI] [PubMed] [Google Scholar]
- Farman N. Steroid receptors: distribution along the nephron. Semin Nephrol. 1992 Jan;12(1):12–17. [PubMed] [Google Scholar]
- Funder J. W., Duval D., Meyer P. Cardiac glucocorticoid receptors: the binding of tritiated dexamethasone in rat and dog heart. Endocrinology. 1973 Dec;93(6):1300–1308. doi: 10.1210/endo-93-6-1300. [DOI] [PubMed] [Google Scholar]
- Gaillard C. A., Koomans H. A., Rabelink T. J., Braam B., Boer P., Dorhout Mees E. J. Enhanced natriuretic effect of atrial natriuretic factor during mineralocorticoid escape in humans. Hypertension. 1988 Oct;12(4):450–456. doi: 10.1161/01.hyp.12.4.450. [DOI] [PubMed] [Google Scholar]
- Gardner D. G., Gertz B. J., Deschepper C. F., Kim D. Y. Gene for the rat atrial natriuretic peptide is regulated by glucocorticoids in vitro. J Clin Invest. 1988 Oct;82(4):1275–1281. doi: 10.1172/JCI113726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Campoy J. M., Kachelski J., Burnett J. C., Jr, Romero J. C., Granger J. P., Knox F. G. Proximal tubule response in aldosterone escape. Am J Physiol. 1989 Jan;256(1 Pt 2):R86–R90. doi: 10.1152/ajpregu.1989.256.1.R86. [DOI] [PubMed] [Google Scholar]
- Granger J. P., Burnett J. C., Jr, Romero J. C., Opgenorth T. J., Salazar J., Joyce M. Elevated levels of atrial natriuretic peptide during aldosterone escape. Am J Physiol. 1987 May;252(5 Pt 2):R878–R882. doi: 10.1152/ajpregu.1987.252.5.R878. [DOI] [PubMed] [Google Scholar]
- Greenwald J. E., Sakata M., Michener M. L., Sides S. D., Needleman P. Is atriopeptin a physiological or pathophysiological substance? Studies in the autoimmune rat. J Clin Invest. 1988 Apr;81(4):1036–1041. doi: 10.1172/JCI113414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grekin R. J., Ling W. D., Shenker Y., Bohr D. F. Immunoreactive atrial natriuretic hormone levels increase in deoxycorticosterone acetate-treated pigs. Hypertension. 1986 Jun;8(6 Pt 2):II16–II20. doi: 10.1161/01.hyp.8.6_pt_2.ii16. [DOI] [PubMed] [Google Scholar]
- Higgins J. T., Jr Escape from sodium-retaining effects of deoxycorticosterone in hypotensive and hypertensive dogs. Proc Soc Exp Biol Med. 1970 Jul;134(3):768–772. doi: 10.3181/00379727-134-34879. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Matsuoka H., Hayakawa H., Sugimoto T., Suzuki E., Sugimoto T., Kangawa K., Matsuo H. Role of endogenous atrial natriuretic peptide in regulating sodium excretion in spontaneously hypertensive rats. Effects of neutral endopeptidase inhibition. Hypertension. 1991 Jun;17(6 Pt 2):1025–1032. doi: 10.1161/01.hyp.17.6.1025. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Matsuoka H., Suzuki E., Hayakawa H., Sugimoto T., Matsuda Y., Morishita Y., Kangawa K., Minamino N., Matsuo H. Role of endogenous atrial natriuretic peptide in DOCA-salt hypertensive rats. Effects of a novel nonpeptide antagonist for atrial natriuretic peptide receptor. Circulation. 1993 Feb;87(2):554–561. doi: 10.1161/01.cir.87.2.554. [DOI] [PubMed] [Google Scholar]
- Holmes S. J., Espiner E. A., Richards A. M., Yandle T. G., Frampton C. Renal, endocrine, and hemodynamic effects of human brain natriuretic peptide in normal man. J Clin Endocrinol Metab. 1993 Jan;76(1):91–96. doi: 10.1210/jcem.76.1.8380606. [DOI] [PubMed] [Google Scholar]
- Kohno M., Horio T., Yokokawa K., Murakawa K., Yasunari K., Akioka K., Tahara A., Toda I., Takeuchi K., Kurihara N. Brain natriuretic peptide as a cardiac hormone in essential hypertension. Am J Med. 1992 Jan;92(1):29–34. doi: 10.1016/0002-9343(92)90011-y. [DOI] [PubMed] [Google Scholar]
- Koller K. J., Lowe D. G., Bennett G. L., Minamino N., Kangawa K., Matsuo H., Goeddel D. V. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science. 1991 Apr 5;252(5002):120–123. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- Koseki C., Hayashi Y., Torikai S., Furuya M., Ohnuma N., Imai M. Localization of binding sites for alpha-rat atrial natriuretic polypeptide in rat kidney. Am J Physiol. 1986 Feb;250(2 Pt 2):F210–F216. doi: 10.1152/ajprenal.1986.250.2.F210. [DOI] [PubMed] [Google Scholar]
- Lafferty H. M., Gunning M., Silva P., Zimmerman M. B., Brenner B. M., Anderson S. Enkephalinase inhibition increases plasma atrial natriuretic peptide levels, glomerular filtration rate, and urinary sodium excretion in rats with reduced renal mass. Circ Res. 1989 Sep;65(3):640–646. doi: 10.1161/01.res.65.3.640. [DOI] [PubMed] [Google Scholar]
- Lang C. C., Choy A. M., Turner K., Tobin R., Coutie W., Struthers A. D. The effect of intravenous saline loading on plasma levels of brain natriuretic peptide in man. J Hypertens. 1993 Jul;11(7):737–741. doi: 10.1097/00004872-199307000-00009. [DOI] [PubMed] [Google Scholar]
- Light D. B., Schwiebert E. M., Karlson K. H., Stanton B. A. Atrial natriuretic peptide inhibits a cation channel in renal inner medullary collecting duct cells. Science. 1989 Jan 20;243(4889):383–385. doi: 10.1126/science.2463673. [DOI] [PubMed] [Google Scholar]
- Maack T., Camargo M. J., Kleinert H. D., Laragh J. H., Atlas S. A. Atrial natriuretic factor: structure and functional properties. Kidney Int. 1985 Apr;27(4):607–615. doi: 10.1038/ki.1985.54. [DOI] [PubMed] [Google Scholar]
- Maack T., Suzuki M., Almeida F. A., Nussenzveig D., Scarborough R. M., McEnroe G. A., Lewicki J. A. Physiological role of silent receptors of atrial natriuretic factor. Science. 1987 Oct 30;238(4827):675–678. doi: 10.1126/science.2823385. [DOI] [PubMed] [Google Scholar]
- Mangat H., de Bold A. J. Stretch-induced atrial natriuretic factor release utilizes a rapidly depleting pool of newly synthesized hormone. Endocrinology. 1993 Sep;133(3):1398–1403. doi: 10.1210/endo.133.3.8365374. [DOI] [PubMed] [Google Scholar]
- Metzler C. H., Gardner D. G., Keil L. C., Baxter J. D., Ramsay D. J. Increased synthesis and release of atrial peptide during DOCA escape in conscious dogs. Am J Physiol. 1987 Jan;252(1 Pt 2):R188–R192. doi: 10.1152/ajpregu.1987.252.1.R188. [DOI] [PubMed] [Google Scholar]
- Miyamori I., Ikeda M., Matsubara T., Okamoto S., Koshida H., Morise T., Takeda R. Human atrial natriuretic polypeptide during escape from mineralocorticoid excess in man. Clin Sci (Lond) 1987 Oct;73(4):431–436. doi: 10.1042/cs0730431. [DOI] [PubMed] [Google Scholar]
- Morishita Y., Sano T., Ando K., Saitoh Y., Kase H., Yamada K., Matsuda Y. Microbial polysaccharide, HS-142-1, competitively and selectively inhibits ANP binding to its guanylyl cyclase-containing receptor. Biochem Biophys Res Commun. 1991 May 15;176(3):949–957. doi: 10.1016/0006-291x(91)90374-g. [DOI] [PubMed] [Google Scholar]
- Morishita Y., Sano T., Kase H., Yamada K., Inagami T., Matsuda Y. HS-142-1, a novel nonpeptide atrial natriuretic peptide (ANP) antagonist, blocks ANP-induced renal responses through a specific interaction with guanylyl cyclase-linked receptors. Eur J Pharmacol. 1992 Mar 12;225(3):203–207. doi: 10.1016/0922-4106(92)90021-m. [DOI] [PubMed] [Google Scholar]
- Mukoyama M., Nakao K., Hosoda K., Suga S., Saito Y., Ogawa Y., Shirakami G., Jougasaki M., Obata K., Yasue H. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991 Apr;87(4):1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil R. G., Helman S. I. Transport characteristics of renal collecting tubules: influences of DOCA and diet. Am J Physiol. 1977 Dec;233(6):F544–F558. doi: 10.1152/ajprenal.1977.233.6.F544. [DOI] [PubMed] [Google Scholar]
- Ogawa Y., Nakao K., Mukoyama M., Hosoda K., Shirakami G., Arai H., Saito Y., Suga S., Jougasaki M., Imura H. Natriuretic peptides as cardiac hormones in normotensive and spontaneously hypertensive rats. The ventricle is a major site of synthesis and secretion of brain natriuretic peptide. Circ Res. 1991 Aug;69(2):491–500. doi: 10.1161/01.res.69.2.491. [DOI] [PubMed] [Google Scholar]
- Overlack A., Bäcker-Kreutz E., Ressel C., Müller H. M., Kolloch R., Stumpe K. O. Mineralocorticoid escape during kallikrein inhibition. Clin Sci (Lond) 1986 Jan;70(1):13–17. doi: 10.1042/cs0700013. [DOI] [PubMed] [Google Scholar]
- Reinhardt H. W., Boemke W., Palm U., Kaczmarczyk G. What causes escape from sodium retaining hormones? Acta Physiol Scand Suppl. 1990;591:12–17. [PubMed] [Google Scholar]
- Richards A. M., Crozier I. G., Espiner E. A., Yandle T. G., Nicholls M. G. Plasma brain natriuretic peptide and endopeptidase 24.11 inhibition in hypertension. Hypertension. 1993 Aug;22(2):231–236. doi: 10.1161/01.hyp.22.2.231. [DOI] [PubMed] [Google Scholar]
- Romero J. C., Knox F. G., Opgenorth T. J., Granger J. P., Keiser J. A. Contribution of sympathetic neural reflexes to mineralocorticoid escape. Fed Proc. 1985 May;44(8):2382–2387. [PubMed] [Google Scholar]
- Sano T., Morishita Y., Matsuda Y., Yamada K. Pharmacological profile of HS-142-1, a novel nonpeptide atrial natriuretic peptide antagonist of microbial origin. I. Selective inhibition of the actions of natriuretic peptides in anesthetized rats. J Pharmacol Exp Ther. 1992 Feb;260(2):825–831. [PubMed] [Google Scholar]
- Sarda I. R., de Bold M. L., de Bold A. J. Optimization of atrial natriuretic factor radioimmunoassay. Clin Biochem. 1989 Feb;22(1):11–15. doi: 10.1016/s0009-9120(89)80063-3. [DOI] [PubMed] [Google Scholar]
- Sasaki A., Kida O., Kangawa K., Matsuo H., Tanaka K. Involvement of sympathetic nerves in cardiosuppressive effects of alpha-human atrial natriuretic polypeptide (alpha-hANP) in anesthetized rats. Eur J Pharmacol. 1986 Jan 29;120(3):345–349. doi: 10.1016/0014-2999(86)90475-9. [DOI] [PubMed] [Google Scholar]
- Schnermann J., Hermle M., Schmidmeier E., Dahlheim H. Impaired potency for feedback regulation of glomerular filtration rate in DOCA escaped rats. Pflugers Arch. 1975 Aug 12;358(4):325–338. doi: 10.1007/BF00580530. [DOI] [PubMed] [Google Scholar]
- Schulz S., Singh S., Bellet R. A., Singh G., Tubb D. J., Chin H., Garbers D. L. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell. 1989 Sep 22;58(6):1155–1162. doi: 10.1016/0092-8674(89)90513-8. [DOI] [PubMed] [Google Scholar]
- Sudoh T., Kangawa K., Minamino N., Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988 Mar 3;332(6159):78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- Sudoh T., Minamino N., Kangawa K., Matsuo H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990 Apr 30;168(2):863–870. doi: 10.1016/0006-291x(90)92401-k. [DOI] [PubMed] [Google Scholar]
- Suga S., Nakao K., Hosoda K., Mukoyama M., Ogawa Y., Shirakami G., Arai H., Saito Y., Kambayashi Y., Inouye K. Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology. 1992 Jan;130(1):229–239. doi: 10.1210/endo.130.1.1309330. [DOI] [PubMed] [Google Scholar]
- Suzuki E., Hirata Y., Kohmoto O., Sugimoto T., Hayakawa H., Matsuoka H., Sugimoto T., Kojima M., Kangawa K., Minamino N. Cellular mechanisms for synthesis and secretion of atrial natriuretic peptide and brain natriuretic peptide in cultured rat atrial cells. Circ Res. 1992 Nov;71(5):1039–1048. doi: 10.1161/01.res.71.5.1039. [DOI] [PubMed] [Google Scholar]
- Terada Y., Knepper M. A. Na+-K+-ATPase activities in renal tubule segments of rat inner medulla. Am J Physiol. 1989 Feb;256(2 Pt 2):F218–F223. doi: 10.1152/ajprenal.1989.256.2.F218. [DOI] [PubMed] [Google Scholar]
- Terada Y., Moriyama T., Martin B. M., Knepper M. A., Garcia-Perez A. RT-PCR microlocalization of mRNA for guanylyl cyclase-coupled ANF receptor in rat kidney. Am J Physiol. 1991 Dec;261(6 Pt 2):F1080–F1087. doi: 10.1152/ajprenal.1991.261.6.F1080. [DOI] [PubMed] [Google Scholar]
- Trippodo N. C., Cole F. E., MacPhee A. A., Pegram B. L. Biologic mechanisms of atrial natriuretic factor. J Lab Clin Med. 1987 Feb;109(2):112–119. [PubMed] [Google Scholar]
- Tunny T. J., Higgins B. A., Gordon R. D. Plasma levels of atrial natriuretic peptide in man in primary aldosteronism, in Gordon's syndrome and in Bartter's syndrome. Clin Exp Pharmacol Physiol. 1986 Apr;13(4):341–345. doi: 10.1111/j.1440-1681.1986.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Wong K. R., Xie M. H., Shi L. B., Liu F. Y., Huang C. L., Gardner D. G., Cogan M. G. Urinary cGMP as biological marker of the renal activity of atrial natriuretic factor. Am J Physiol. 1988 Dec;255(6 Pt 2):F1220–F1224. doi: 10.1152/ajprenal.1988.255.6.F1220. [DOI] [PubMed] [Google Scholar]
- Yokota N., Yamamoto Y., Kitamura K., Kangawa K., Minamino N., Matsuo H., Eto T. Alterations in circulating and cardiac tissue concentrations of brain natriuretic peptide in spontaneously hypertensive rats. Cardiovasc Res. 1993 Jul;27(7):1312–1315. doi: 10.1093/cvr/27.7.1312. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Seifter J. L., Lear S., Brenner B. M., Silva P. Atrial peptides inhibit oxygen consumption in kidney medullary collecting duct cells. Am J Physiol. 1986 Aug;251(2 Pt 2):F379–F383. doi: 10.1152/ajprenal.1986.251.2.F379. [DOI] [PubMed] [Google Scholar]
- Zeng Z. P., Naruse M., Naruse K., Kato Y., Shi Y. F., Demura H., Shizume K. Antiserum against homologous atrial natriuretic peptide diminishes the natriuretic response during mineralocorticoid escape in rats. Endocrinology. 1991 Jan;128(1):226–230. doi: 10.1210/endo-128-1-226. [DOI] [PubMed] [Google Scholar]
- Zimmerman R. S., Edwards B. S., Schwab T. R., Heublein D. M., Burnett J. C., Jr Atrial natriuretic peptide during mineralocorticoid escape in the human. J Clin Endocrinol Metab. 1987 Mar;64(3):624–627. doi: 10.1210/jcem-64-3-624. [DOI] [PubMed] [Google Scholar]
- de Bold A. J. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985 Nov 15;230(4727):767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- de Bold A. J., Borenstein H. B., Veress A. T., Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981 Jan 5;28(1):89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]