Abstract
We recently observed that social interactions influence morphine responsiveness in adolescent males. Given sex-related differences in both social interactions and responses to morphine, the present study examines social influences on morphine sensitization in adolescent female mice. Four experimental groups were examined: [1] morphine-treated mice (twice daily, 10–40 mg/kg, s.c.) housed physically and visually separated from saline-treated mice (‘morphine only’), [2] morphine-treated mice housed together with saline-treated mice (‘morphine cage-mates (of saline)’), [3] saline-treated mice housed together with morphine-treated mice (‘saline cage-mates (of morphine)’), and [4] saline-treated mice housed physically and visually separated from morphine-treated mice (‘saline only’). Following the treatment period, mice were tested individually for their locomotor response to 20 mg/kg morphine (s.c.). There were no significant differences in morphine-induced hyper-locomotion between saline only and saline cage-mates (of morphine) female adolescent mice. Notably, morphine only mice exhibited significantly greater morphine sensitization as compared to morphine cage-mates (of saline). Thus, this study demonstrates social influences on morphine sensitization in adolescent females. Drug use during early adolescence is a key predictor of later drug abuse and dependence during adulthood. Thus, understanding the specific vulnerabilities to drug use in this age group may represent a first step in helping develop more effective treatment programs.
Keywords: Opioid, Locomotion, Peer-influences, Drugs of abuse
1. INTRODUCTION
Drug use during adolescence is particularly problematic because it is a major predictor of later drug abuse and dependence during adulthood (Chen et al., 2009; Grant and Dawson, 1998; Hawkins et al., 1997; Odgers et al., 2008). Additionally, an increased rate of comorbidity of anxiety and mood disorders was demonstrated when drug use began at a young age (Caspi et al., 2005; Degenhardt et al., 2007; Gfroerer et al., 2002; Mathers et al., 2006; Tucker et al., 2006). A recent survey revealed that nonmedical use of opioid prescription pain relievers (such as hydrocodone, oxycodone, and morphine) is the second most common form of illicit drug use in the United States after marijuana (SAMHSA, 2009). This clearly illustrates the need for more research and a better understanding of the effects of opiate exposure during adolescence.
Peer influences are among the strongest predictors of adolescents’ drug use. While most studies focus on humans, some studies suggest that in rodents there may also be a social effect on alcohol preference and consumption from interaction with intoxicated peers (Fernández-Vidal and Molina, 2004; Hunt et al., 2001). Similarly, we recently observed that social interactions with morphine-treated mice resulted in an enhanced hyper-locomotion response to morphine in drug-naïve adolescent male mice (Hodgson et al., 2010).
In both humans and rodents, there are sex-related differences in social interactions, including in play fighting and display of aggression (Meaney, 1989; Pinna et al., 2004). Additionally, there are also sex differences in the response to stress. Female rats exhibit fewer behavioral deficits than males after acute stressors but adapt more slowly to chronic stress (Dalla et al., 2007; Haleem et al., 1988; Mitsushima et al., 2003; Steenbergen et al., 1990). Moreover, there are sex-specific differences in the potency or efficacy of opioids as reinforcers, where females are more vulnerable to the acquisition of opioids, administer larger quantities, and work harder to obtain it (Cicero et al., 2003; Lynch and Carroll, 1999). Furthermore, differences in the emotional response to morphine withdrawal were observed between male and female mice (Hodgson et al., 2009a; Hodgson et al., 2009b). Given sex-related differences in both social interactions and in the responses to morphine, this study examines vulnerabilities to social influences on morphine locomotor sensitization in female adolescent mice.
2. METHODS
2.1 Animals
All procedures were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, and were approved by the TAMU Institutional Animal Care and Use Committee. Female C57BL/6 mice, purchased from Harlan Lab (Houston, TX), were housed 4 per cage in a temperature-controlled vivarium with a 12 h/12 h light/dark cycle (light on at 07:00) and food and water ad lib. In this study, mice were injected during what is considered the late phase of their prepubescent period, and were tested during their mid-adolescence/periadolescent period (Spear, 2000). Accordingly, mice arrived at postnatal day 22 (PND 22). They were acclimated to the vivarium until PND 28, when morphine injections began, and behavioral testing was performed on PND 42.
2.2 Morphine treatment regimen
The different experimental groups are summarized in Table 1. Female adolescent (PND 28, n=10–12) mice were treated twice daily (9 a.m. and 5 p.m.) for 6 consecutive days with increasing doses of morphine (10–40 mg/kg, 10 ml/kg, s.c.) or saline for a total of 12 injections. Specifically, on days 1 and 2, the mice were injected with 10 mg/kg morphine or saline. On days 3 and 4, they were injected with 20 mg/kg morphine or saline. On days 5 and 6, they were injected with 40 mg/kg morphine or saline. This morphine regimen was selected based on both our previous studies (Buckman et al., 2009; Eitan et al., 2003; Hodgson et al., 2008; Hodgson et al., 2009a; Hodgson et al., 2010; Hodgson et al., 2009b) as well as other investigators (Contet et al., 2008; el-kadi and Sharif, 1994; Kest et al., 2001; Matthes et al., 1996; Spanagel et al., 1994) demonstrating that such doses induce significant antinociceptive tolerance, sensitization, dependence and withdrawal. Following the injections, mice were returned to their respective home cages. Morphine sulfate was purchased from Sigma (St. Louis, MO).
Table 1.
Summary of the experimental groups
| Experimental group | Housed together with mice |
Cage configuration* |
Exp days 1–6 |
Exp days 7–14 |
Exp day 15** |
n |
|---|---|---|---|---|---|---|
| Saline only | Saline-injected | SSSS | Saline | No treatment |
20 mg/kg Morphine |
12 |
| Saline cage-mates (of morphine) | Morphine-injected | SS(MM) | 10 | |||
| Morphine cage-mates (of saline) | Saline-injected | MM(SS) | Morphine (10–40 mg/kg) |
10 | ||
| Morphine only | Morphine-injected | MMMM | 12 |
S – Saline-injected mouse; M- Morphine-injected mouse
Locomotion recording
Mice were group-housed in one of two conditions referred to as ‘only’ and ‘cage-mates’. ‘Morphine only’ mice are morphine-treated mice housed physically and visually separated from saline-treated mice (i.e. all 4 mice in the cage received morphine). Similarly, ‘saline only’ mice are saline-treated mice housed physically and visually separated from morphine-treated mice (i.e. all 4 mice in the cage received saline). There was also a group of morphine- and saline-treated mice that were housed together (i.e. 2 mice receiving morphine and 2 mice receiving saline per cage). They represent two different treatment conditions. The saline-treated mice of this group are referred to as ‘saline cage-mates (of morphine)’ (i.e. each saline cage-mate (of morphine) mouse had 2 morphine-treated and 1 saline-treated cage-mates). The morphine-treated mice are referred to as ‘morphine cage-mates (of saline)’ (i.e. each morphine cage-mate (of saline) mouse had 2 saline-treated and 1 morphine-treated cage-mates).
2.3 Morphine-induced hyper-locomotion
Nine days following the final pretreatment dose of morphine or saline, locomotion was recorded in the second part of the light phase, which is between 2 pm and 6 pm. Mice were habituated to the room for at least 30 minutes prior to testing and then placed separately (one mouse per apparatus) into an opaque upright cylindrical container (261 mm in diameter and 355 mm high). Each behavioral room contained 4 cylinders, thus 4 mice were individually recorded in the same room at the same time. Note that the mice were physically and visually separated during the locomotion test. Baseline locomotion activity was recorded for 60 minutes by an overhead camera. All mice were then injected with 20 mg/kg morphine and recorded for another 60 minutes. Each cylindrical container was cleaned thoroughly with water, dried completely and aired for at least 24 hours before being used again. Total distance traveled in cm (locomotion) was scored using EthoVision 3.1 (Noldus Information Technology, Leesburg, VA).
2.4 Data analyses
Similar to our previous study (Eitan et al., 2003), the locomotor sensitization study represents a split-plot design. Separate analyses of variance were computed for the total distance traveled scores (sum of baseline 60 minutes, and sum of post-morphine 60 minutes) and a within-group factor of time (1–120 minutes summed in 5 minute intervals). Additional post-hoc contrasts between each treatment group were computed using Bonferroni’s post-hoc procedure. Differences with p-values less than 0.05 were deemed statistically significant. Results are presented as mean ± SEM.
3. RESULTS
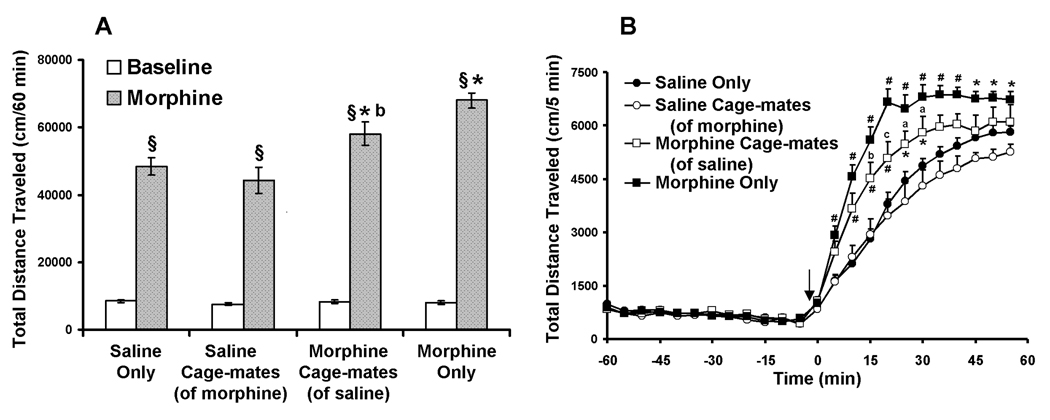
The results are presented in Fig 1. For the baseline and post-morphine total distance traveled scores (Fig 1A), two way ANOVA revealed a main effect of experimental group (F(3, 80)=13.58, p<0.0001), a main effect of treatment (F(1, 80)=1059, p<0.0001), and a significant interaction between experimental group and treatment (F(3, 80)=12.95, p<0.0001). Similarly, for the total distance traveled scores summed in 5 minute intervals over the 120 minute test (Fig 1B), two way ANOVA revealed a main effect of experimental group (F(3, 960)=103.3, p<0.0001), a main effect of time (F(23, 960)=469.5, p<0.0001), and a significant interaction between experimental group and time (F(69, 960)=6.04, p<0.0001). Bonferroni’s post-hoc comparisons revealed no significant differences in baseline locomotor activity (the first 60 minutes) between the different experimental groups. In all four experimental groups, locomotion increased following morphine administration (for each experimental groups’ baseline vs. post-morphine, p<0.001). There were no significant differences in morphine-induced hyper-locomotion between saline only and saline cage-mates (of morphine) female adolescent mice. As expected, both morphine-treated groups exhibited locomotion sensitization (morphine only vs. saline only, p<0.001; and morphine cage-mates (of saline) vs. saline only, p<0.01). Notably, significant differences in morphine sensitization were found between morphine cage-mates (of saline) and morphine only mice, i.e. a significantly higher hyper-locomotion response to morphine was observed in the morphine only female mice as compared to the morphine cage mate (of saline) mice (morphine cage-mates (of saline) vs. morphine only, p<0.01).
Fig. 1. Morphine locomotor sensitization in adolescent female mice.
(A) Total distance traveled (cm) in the 60 minutes prior to morphine administration (Baseline, White bars) and in the 60 minutes following 10 mg/kg morphine injection (Morphine, Gray bars). (§) indicates a significant difference from baseline (p<0.001); (*) indicates a significant difference from saline only mice (p<0.01); (b) indicates a significant difference between the morphine cage-mates (of saline) and morphine only mice (p<0.01). (B) Total distance traveled (cm) during the entire 120 minute test, segmented into 5 minute intervals. Arrow indicates time of morphine administration. (*) indicates a significant difference from saline only mice (p<0.05); (#) indicates a significant difference from saline only mice (p<0.001); (a) indicates a significant difference between the morphine cage-mates (of saline) and morphine only mice (p<0.05); (b) indicates a significant difference between the morphine cage-mates (of saline) and morphine only mice (p<0.01); (c) indicates a significant difference between the morphine cage-mates (of saline) and morphine only mice (p<0.001). Results are presented as mean ± SEM.
4. DISCUSSION
This study demonstrates social influences on morphine locomotor sensitization in adolescent female mice. Drug responsiveness was studied in group-housed mice. The response of mice housed in mixed treatment cages, where morphine-and saline-treated mice were housed together, was compared to that of mice housed in non-mixed cages, consisting of only morphine-treated or only saline-treated mice. There were no significant differences in morphine-induced hyper-locomotion between saline cage-mates (of morphine) and saline only female mice. As expected, both morphine-treated groups exhibited locomotor sensitization as compared to saline-injected mice. However, there was a significant difference in the response to morphine between the morphine cage-mates (of saline) and morphine only mice.
In this study, four mice were tested at a time. The cylindrical test containers were aired for at least 24 hours between each use. Thus, it is unlikely that significant odor cues remained from a previously tested mouse to affect the behaviors of the next mouse examined in that particular cylinder. The mice were physically and visually separated from each other during the test; nonetheless we cannot rule out the possibility that other cues transferred between the mice during the test period had some effect on their behaviors. As was demonstrated for ‘pain empathy’ (Langford et al., 2006), it is possible that the cues will have a greater effect on the cage-mates compared to unfamiliar mice. Although odor or vocal cues transferred during the test might play some role, a more likely explanation is that interactions prior to the locomotion test play a significant role in the behavioral outcome observed.
Olfactory cues transmitted during encounters with alcohol-intoxicated peers can affect subsequent alcohol preference and consumption in periadolescent mice (Fernández-Vidal and Molina, 2004; Hunt et al., 2001). Thus, perhaps pheromones secreted by the morphine- and/or saline-treated mice during their interactions (i.e. while housed together) do affect their peers. Each morphine only mouse is exposed to three morphine-treated cage-mates but not to any saline-treated cage-mates, while each morphine cage-mate (of saline) mouse interacts with only one other morphine-treated mouse and is exposed to two saline-treated cage-mates. If pheromones secreted by morphine- and/or saline-treated mice have a modulating effect, this might result in differences in morphine sensitization levels between the morphine cage-mates (of saline) and the morphine only mice.
Housing conditions were previously reported to affect drug responsiveness. Social isolation was demonstrated to enhance morphine sensitization while re-grouping reversed this effect (Frances et al., 2000). This was suggested to be mediated by stress levels, as indicated by the effects of isolation and re-grouping on plasma corticosterone levels. Stress can also be instigated as a result of increased aggressive behaviors between peers. Indeed, morphine withdrawal was demonstrated to increase aggressive behaviors in males (Felip et al., 2000; Rodríguez-Arias et al., 1999; Sukhotina, 2001). Thus, the morphine only mice might be exposed to more incidents of aggressive behaviors from morphine-treated cage-mates as compared to the morphine cage-mates (of saline). However, this study used female mice. Although we cannot rule out the possibility that the females display recordable levels of aggression (resulting in increased stress in their cage-mates), aggressive behaviors are not generally exhibited by non-lactating females (Moyer, 1968). Moreover, if morphine withdrawal indeed results in increased aggression and fighting, it is expected to affect all the mice in the cage including the saline cage-mates. As mentioned earlier, stress is known to affect the responsiveness to morphine, thus the lack of a significant difference between the saline cage-mates (of morphine) and saline only mice, even though each saline cage-mate (of morphine) was exposed to two morphine-treated mice, suggests that the morphine-treated females do not display significant levels of aggressive behaviors.
In contrast to isolation, environmental enrichment was demonstrated to decrease the rewarding properties of opioids in mice (El Rawas et al., 2009). This effect was suggested to result from blunting of the hypothalamo-pituitary-adrenal (HPA) stress axis (Stairs and Bardo, 2009). Thus, perhaps the exposure to the saline-treated mice by the morphine cage-mates (of saline), but not by morphine only mice, also results in blunting of the HPA stress axis, resulting in a reduced morphine sensitization in the morphine cage-mates (of saline) as compared to the morphine only mice. However, environmental enrichment had no effect on the activating effects of opioids (El Rawas et al., 2009). Thus, further studies of environmentally and/or socially enriched mice as compared to isolated mice are required to better understand specific age and developmental vulnerabilities to drug use.
This study demonstrates social influences on locomotor sensitization in morphine-treated females. Multiple behavioral assays are used to index abuse potential (Ator and Griffiths, 2003; Carter and Griffiths, 2009), including the capacity of a drug to induce hyper-locomotion (Wise and Bozarth, 1987), the capacity to induce conditioned place preference (Bardo and Bevins, 2000), and the capacity to support intravenous drug self-administration (Brady and Griffiths, 1976). Intravenous self-administration is considered to be the “gold-standard” for abuse liability, whereas the capacity of a drug to stimulate locomotion is an indicator of abuse potential. Thus, the interpretation of the present results, namely the extrapolation from a measure of rodent response to humans, should be performed with caution. Further studies are required to identify the nature of the social interactions that cause this phenomenon (i.e. which sensory modality mediates the social effect on morphine sensitization - physical, visual, auditory and/or olfactory), to establish the duration of the effect, to determine possible social effects on other drug-induced behaviors (such as reward and self-administration), and to reveal the generality of the phenomenon to other drugs of abuse. Moreover, the molecular underpinnings of this social effect have yet to be determined. Understanding the mechanisms underlying adolescents’ vulnerabilities to the social influences on drug use may represent a first step in helping develop more effective treatment programs.
Acknowledgments
Funding source: KWR is supported by NIH (P50DA05010).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest: The authors have no financial interests to disclose.
REFERENCES
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;70:S55–S72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Brady JV, Griffiths RR. Behavioral procedures for evaluating the relative abuse potential of cns drugs in primates. Fed Proc. 1976;35:2245–2253. [PubMed] [Google Scholar]
- Buckman SG, Hodgson SR, Hofford RS, Eitan S. Increased elevated plus maze open-arm time in mice during spontaneous morphine withdrawal. Behav Brain Res. 2009;197:454–456. doi: 10.1016/j.bbr.2008.09.035. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend. 2009;105:S14–S25. doi: 10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, Poulton R, Craig IW. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-o-methyltransferase gene: Longitudinal evidence of a gene × environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Chen C-Y, Storr CL, Anthony JC. Early-onset drug use and risk for drug dependence problems. Addictive Behaviors. 2009;34:319–322. doi: 10.1016/j.addbeh.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, Meyer ER. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav. 2003;74:541–549. doi: 10.1016/s0091-3057(02)01039-0. [DOI] [PubMed] [Google Scholar]
- Contet C, Filliol D, Matifas A, Kieffer BL. Morphine-induced analgesic tolerance, locomotor sensitization and physical dependence do not require modification of mu opioid receptor, cdk5 and adenylate cyclase activity. Neuropharmacology. 2008;54:475–486. doi: 10.1016/j.neuropharm.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Dalla C, Edgecomb C, Whetstone AS, Shors TJ. Females do not express learned helplessness like males do. Neuropsychopharmacology. 2007;33:1559–1569. doi: 10.1038/sj.npp.1301533. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Coffey C, Moran P, Carlin JB, Patton GC. The predictors and consequences of adolescent amphetamine use: Findings from the victoria adolescent health cohort study. Addiction. 2007;102:1076–1084. doi: 10.1111/j.1360-0443.2007.01839.x. [DOI] [PubMed] [Google Scholar]
- Eitan S, Bryant CD, Saliminejad N, Yang YC, Vojdani E, Keith D, Jr, Polakiewicz R, Evans CJ. Brain region-specific mechanisms for acute morphine-induced mitogen-activated protein kinase modulation and distinct patterns of activation during analgesic tolerance and locomotor sensitization. J Neurosci. 2003;23:8360–8369. doi: 10.1523/JNEUROSCI.23-23-08360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-kadi AO, Sharif SI. The influence of various experimental conditions on the expression of naloxone-induced withdrawal symptoms in mice. Gen Pharmacol. 1994;25:1505–1510. doi: 10.1016/0306-3623(94)90181-3. [DOI] [PubMed] [Google Scholar]
- El Rawas R, Thiriet N, Lardeux V, Jaber M, Solinas M. Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacology. 2009;203:561–570. doi: 10.1007/s00213-008-1402-6. [DOI] [PubMed] [Google Scholar]
- Felip CM, Rodríguez-Arias M, Espejo EF, Miñarro J, Stinus L. Naloxone-induced opiate withdrawal produces long-lasting and context-independent changes in aggressive and social behaviors of postdependent male mice. Behav Neurosci. 2000;114:424–430. doi: 10.1037//0735-7044.114.2.424. [DOI] [PubMed] [Google Scholar]
- Fernández-Vidal JM, Molina JC. Socially mediated alcohol preferences in adolescent rats following interactions with an intoxicated peer. Pharmacol Biochem Behav. 2004;79:229–241. doi: 10.1016/j.pbb.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Frances H, Graulet A-M, Debray M, Coudereau J-P, Gueris J, Bourre J-M. Morphine-induced sensitization of locomotor activity in mice: Effect of social isolation on plasma corticosterone levels. Brain Res. 2000;860:136–140. doi: 10.1016/s0006-8993(00)02053-9. [DOI] [PubMed] [Google Scholar]
- Gfroerer JC, Wu LT, Penne MA. DHHS Publication No. SMA 02-3711, Analytic Series A-17. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2002. Initiation of marijuana use: Trends, patterns, and implications. [Google Scholar]
- Grant BF, Dawson DA. Age of onset of drug use and its association with dsmiv drug abuse and dependence: Results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse. 1998;10:163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- Haleem DJ, Kennett G, Curzon G. Adaptation of female rats to stress: Shift to male pattern by inhibition of corticosterone synthesis. Brain Res. 1988;458:339–347. doi: 10.1016/0006-8993(88)90476-3. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. Journal of Studies on Alcohol. 1997;58:280–290. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Norris CJ, Eitan S. Increased elevated plus maze open-arm time in mice during naloxone-precipitated morphine withdrawal. Behav Pharmacol. 2008;19:805–811. doi: 10.1097/FBP.0b013e32831c3b57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Roberts KW, Eitan D, Wellman PJ, Eitan S. Sex differences in affective response to opioid withdrawal during adolescence. J Psychopharmacol. 2009a doi: 10.1177/0269881109106976. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Roberts KW, Wellman PJ, Eitan S. Socially-induced morphine pseudo-sensitization in adolescent mice. Behav Pharmacol. 2010 doi: 10.1097/FBP.0b013e328337be25. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Wellman PJ, Eitan S. Different affective response to opioid withdrawal in adolescent and adult mice. Life Sci. 2009b;84:52–60. doi: 10.1016/j.lfs.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PS, Holloway JL, Scordalakes EM. Social interaction with an intoxicated sibling can result in increased intake of ethanol by periadolescent rats. Dev Psychobiol. 2001;38:101–109. doi: 10.1002/1098-2302(200103)38:2<101::aid-dev1002>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A. Assessment of acute and chronic morphine dependence in male and female mice. Pharmacol Biochem Behav. 2001;70:149–156. doi: 10.1016/s0091-3057(01)00600-1. [DOI] [PubMed] [Google Scholar]
- Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, Chanda ML, Levitin DJ, Mogil JS. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Mathers M, Toumbourou JW, Catalano RF, Williams J, Patton GC. Consequences of youth tobacco use: A review of prospective behavioural studies. Addiction. 2006;101:948–958. doi: 10.1111/j.1360-0443.2006.01438.x. [DOI] [PubMed] [Google Scholar]
- Matthes HWD, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. The sexual differentiation of social play. Psychiatr Dev. 1989;7:247–261. [PubMed] [Google Scholar]
- Mitsushima D, Masuda J, Kimura F. Sex differences in the stress-induced release of acetylcholine in the hippocampus and corticosterone from the adrenal cortex in rats. Neuroendocrinology. 2003;78:234–240. doi: 10.1159/000073707. [DOI] [PubMed] [Google Scholar]
- Moyer KE. Kinds of aggression and their physiological basis. Commun Behav Biol. 1968;2:65–87. [Google Scholar]
- Odgers CL, Caspi A, Nagin DS, Piquero AR, Slutske WS, Milne BJ, Dickson N, Poulton R, Moffitt TE. Is it important to prevent early exposure to drugs and alcohol among adolescents? Psychological Science. 2008;19:1037–1044. doi: 10.1111/j.1467-9280.2008.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Agis-Balboa RC, Doueiri MS, Guidotti A, Costa E. Brain neurosteroids in gender-related aggression induced by social isolation. Crit Rev Neurobiol. 2004;16:75–82. doi: 10.1615/critrevneurobiol.v16.i12.80. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Arias M, Pinazo J, Miñarro J, Stinus L. Effects of sch 23390, raclopride, and haloperidol on morphine withdrawal-induced aggression in male mice. Pharmacol Biochem Behav. 1999;64:123–130. doi: 10.1016/s0091-3057(99)00067-2. [DOI] [PubMed] [Google Scholar]
- SAMHSA. The nsduh report: Trends in nonmedical use of prescription pain relievers: 2002 to 2007. Rockville, MD: Substance Abuse and Mental Health Services Administration: Office of Applied Studies; 2009. [Google Scholar]
- Spanagel R, Almeida OF, Bartl C, Shippenberg TS. Endogenous kappa-opioid systems in opiate withdrawal: Role in aversion and accompanying changes in mesolimbic dopamine release. Psychopharmacology (Berlin) 1994;115:121–127. doi: 10.1007/BF02244761. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochem Behav. 2009;92:377–382. doi: 10.1016/j.pbb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen HL, Heinsbroek RPW, Van Hest A, Van de Poll NE. Sex-dependent effects of inescapable shock administration on shuttlebox-escape performance and elevated plus-maze behavior. Physiol Behav. 1990;48:571–576. doi: 10.1016/0031-9384(90)90302-k. [DOI] [PubMed] [Google Scholar]
- Sukhotina IA. Morphine withdrawal-facilitated aggression is attenuated by morphine-conditioned stimuli. Pharmacol Biochem Behav. 2001;68:93–98. doi: 10.1016/s0091-3057(00)00429-9. [DOI] [PubMed] [Google Scholar]
- Tucker JS, Ellickson PL, Collins RL, Klein DJ. Does solitary substance use increase adolescents' risk for poor psychosocial and behavioral outcomes? A 9-year longitudinal study comparing solitary and social users. Psychol Addict Behav. 2006;20:363–372. doi: 10.1037/0893-164X.20.4.363. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]