Abstract
The giant fiber system (GFS) of Drosophila is a well-characterized neuronal circuit that mediates the escape response in the fly. It is one of the few adult neural circuits from which electrophysiological recordings can be made routinely. This article describes a simple procedure for stimulating the giant fiber neurons directly in the brain of the adult fly and obtaining recordings from the output muscles of the giant fiber system.
Overview
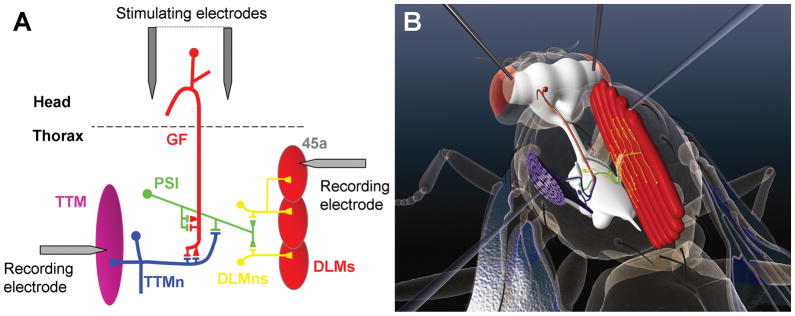
The giant fiber system (GFS) mediates a fast escape behavior in adult flies (Allen et al. 2006). Behaviorally, it is characterized by an initial extension of the mesothoracic leg, to propel the flies off the substrate, followed by a wing downbeat to initiate flight. The efferent (output) pathways of the GFS have been well defined (Figure 1) for the most part by work from Wyman and others in the 1980’s using a combination of dye-injection, EM and electrophysiological techniques (Ikeda et al. 1980; King and Wyman 1980; Koto et al. 1981). The two largest interneurons in the fly, the aptly named giant fibers (GFs), relay the signal from the brain to the mesothoracic neuromere where each makes two identified synapses. The first is to a large motorneuron (TTMn) that drives the tergotrochanteral “jump” muscle (TTM), which is also referred to in the literature as the tergal depressor of trochanter or TDT. This GF-TTMn synapse is the largest central synapse in the fly and is a mixed synapse with the electrical gap-junction component encoded by the shaking-B (shakB) gene and the chemical component using acetylcholine as its neurotransmitter (Blagburn et al. 1999; Allen and Murphey 2007; Phelan et al. 2008). The second identified synapse of the GF is to another interneuron, the peripherally synapsing interneuron (PSI), which exits the ganglion via the posterior dorsal medial nerve (PDMN) and synapses with dorsal longitudinal motorneurons (DLMns) within the PDMN. The DLMns drive the large indirect flight muscles (DLMs). Electrophysiological recordings can be made from the GFS in a simple non-invasive manner to determine the function of the central synapses within the circuit. Using combinations of adult viable mutants and/or GAL4 lines that express in its neurons, the GFS has provided a useful model circuit to investigate the role of several molecules in the formation of central synapses including Glued, Rac1, Robo, Semaphorin1a and Neuroglian (Allen et al. 1999; Allen et al. 2000; Godenschwege et al. 2002a; Godenschwege et al. 2002b; Godenschwege et al. 2006). The GFS has also been used to investigate the effects of aging, sensitivity to anesthetics, the effects of neurodegeneration and the molecular basis of habituation (Engel and Wu 1996; Lin and Nash 1996; Engel and Wu 1998; Martinez et al. 2007; Watson et al. 2008).
Figure 1.
The giant fiber system: neurons and muscles A) Schematic indicating the neurons and connections of the GFS. For clarity, only one half of the bi-lateral circuit is shown. The giant fiber (GF, Red) relays information from the brain to the thoracic ganglia where it makes an electro-chemical synapse to the tergotrochanteral motorneuron (TTMn, Blue) which innervates the tergotrochanteral muscle (TTM). It also makes an electro-chemical synapse to the peripherally synapsing interneuron (PSI, Green) which, in turn, makes chemical synapses to the dorsal longitudinal motorneurons (DLMns, Yellow) that innervate the dorsal longitudinal muscles (DLMs). The relative positions of the stimulating and recording electrodes are indicated. Adapted from Allen et al., (2006). B) Artists impression of the GFS showing the CNS within the fly’s body. The neurons and muscles of the GFS are shown in their approximate positions and the best positions for the stimulating and recording electrodes are depicted.
Stimulating and recording from the GFS
The GFs can be activated directly with brain stimulation and the two output pathways can be monitored by recording simultaneously from the TTM and DLMs. The original rationale was that by placing the stimulating electrodes into the brain and slowly increasing the stimulation voltage, a point would be reached where only the GF interneurons would propagate an action potential since their large size would mean they have the least resistance and thus the lowest threshold. While this may theoretically be true, in practice accurate positioning of the electrodes is hard to achieve so the stimulation voltage given is much above threshold. This ensures that the GFs are activated directly and not by upstream neurons (unless that is desired, see below). Though many neurons in the brain may be activated, the only route to the TTMs and DLMs from the brain activated by this procedure seems to be via the GFs. This is suggested by the findings that genetic ablation of the GFs, or abrogation of the electro-chemical synapses between the GF and the TTMn and PSI, results in total loss of TTM and DLM responses upon brain stimulation (Allen et al. 2000; Allen and Murphey 2007). However, both TTMn and the DLMns have other unidentified inputs, one of which is triggered by looming stimuli (Fotowat et al. 2009). Once direct activation of the GFs is achieved, recordings from TTM monitor the function of the GF-TTMn central synapse along with the neuromuscular junction (NMJ) and recordings from DLM monitor the function of the GF-PSI and PSI-DLMns synapses as well as the NMJ.
Standard tests of synaptic function
The most commonly used tests for the GFS are the response latency, the refractory period and the ability to follow to high frequency stimulation. These will be described in turn.
Response latency
This is the time taken for the output muscle to respond to a single stimulus activating the GFs. In the TTM of wild type flies this is ~0.8 ms after GF activation and is via the monosynaptic pathway through the large electro-chemical GF-TTMn synapse. The response in a DLM, through the disynaptic pathway, is seen ~1.2 ms after GF activation. These latencies correspond to the escape behavior where the jump always occurs before the wing downbeat. This robust short-latency (SL) response is a good indicator of synaptic function and any abnormalities in the synapses of the GFS will result in an increase in the latency or a loss of the response. For example, loss of gap junctions or structural malformations of the synapse that alter its shape or size (Thomas and Wyman 1984; Oh et al. 1994; Allen et al. 1999; Allen et al. 2000; Godenschwege et al. 2002a; Godenschwege et al. 2002b; Godenschwege et al. 2006; Allen and Murphey 2007; Uthaman et al. 2008).
In addition to SL responses, intermediate-latency (IL) responses (TTM ~1.8 ms, DLM ~2.2 ms), and long-latency (LL) responses (TTM ~3.9 ms, DLM ~4.3 ms) can be elicited by simply reducing the voltage during brain stimulation, or providing a light-off stimulus to a tethered fly. All these responses are still conducted through the GF; note the delay between the TTM and DLM response is always ~0.4 ms, indicating the disynaptic pathway from GF to DLM via the PSI and DLMn. The longer IL and LL responses, during low-voltage electrical stimulation or a light-off stimulus, are attributed to indirect activation of the GF by the afferent neurons in the brain. These neurons still remain unidentified but have interesting properties as they show both sensitivity to anesthetics and habituation to repeated stimuli (Engel and Wu 1996; Lin and Nash 1996; Engel and Wu 1998).
Refractory period
In this test twin stimuli are given, initially 10 ms apart, and the responses from both TTM and DLM recorded. The interval between the two stimuli is then gradually reduced until the second stimulus fails to elicit a response. The shortest time between two stimuli that still produces two responses is defined as the refractory period. For TTM this is ~3 ms and DLM is ~5 ms due to the greater time needed for the PSI-DLMn chemical synapses to replenish their synaptic vesicles. This test is less common than the other two as similar information can be gleaned if you observe the responses to the first two stimuli in the “following at high frequencies” test (see below).
Following at high frequencies
In this test a train of ten stimuli are given to the preparation at high frequency and the number of responses is recorded. These trains of stimuli are usually given at 100, 200 & 250 or 300 Hz. At 100 Hz (stimuli 10 ms apart) both TTM and DLM should respond 1:1 and give ten responses. At the higher frequencies e.g. 250 Hz (stimuli 4 ms apart), TTM will still respond 1:1 due to the robust GF-TTMn electro-chemical synapse, however, DLM recordings will start to show failures as the time between stimuli is less than the refractory period of the PSI-DLMns synapses. An alternative way of performing the test is to gradually increase the frequency of the stimuli until the response rates fall below 50% (5 out of 10). This is described as the Following Frequency50 (FF50) (Gorczyca and Hall 1984). This test will often reveal an abnormality in synaptic function that does not cause an abnormal response latency (Allen et al. 1999), although it usually confirms an aberrant response latency.
PROTOCOL
Recording from TTM and DLM - the outputs of the giant fiber system
ABSTRACT
This protocol is a standard method for recording from the giant fiber system of Drosophila. It is a relatively non-invasive method that allows the investigator to stimulate the giant fibers in the brain and assay the function of several central synapses within this neural circuit by recording from the thoracic musculature.
MATERIALS
Reagents
3M KCL or saline
Soft dental wax (available from most dental product suppliers)
Slide or mounting tray. These can be made in a variety of ways including from a small Petri dish filled with tooth carding wax (shown in figure 2B), from a piece of plexiglass or a coin, or from a small piece of wood.
Drosophila melanogaster wild type/control flies (e.g. Oregon R, w1118, bendless/+; shakB2/+) and mutant strains (e.g. bendless, shakB2).
Figure 2.
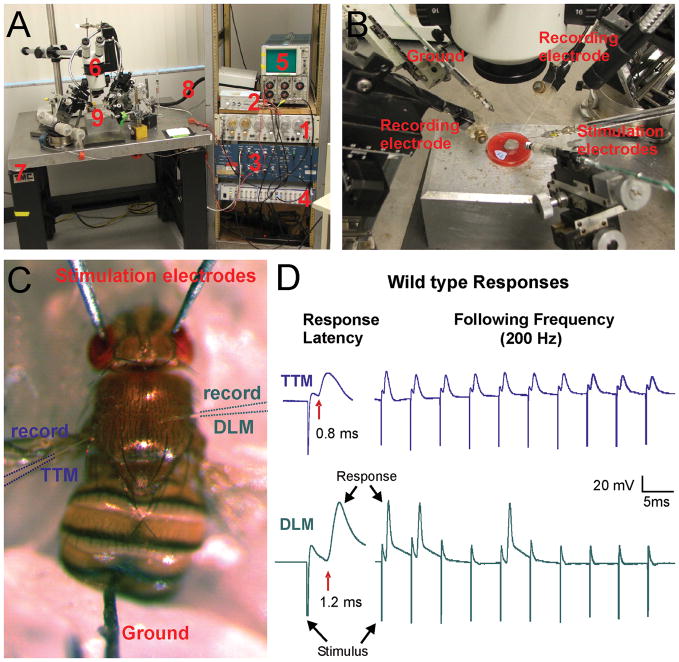
Electrophysiology of the Giant fiber system. A) Components of the electrophysiological rig. 1: Stimulator (S48 Square Pulse Stimulator, Grass instruments); 2: Stimulation isolation unit (SIU5 RF Transformer Isolation Unit, Grass instruments); 3: Two Intracellular amplifier (Model 5A Microelectrode Amplifier, Getting Instruments); 4: Data acquisition system (Digidata 1440A, Molecular devices) & Computer with software (not shown); 5: Storage Oscilloscope 5111A (Tektronix); 6: Stereomicroscope (Wild M5) on a boom stand; 7: Vibration isolation table (TMC); 8: Light source (Fostec). 9: Recording platform with 5 manual multi axis micromanipulators (Narashigi, Sutter and World Precision Instruments). B) Magnification of 9 in Figure 2A. Around the recording tray are arranged: Two stimulation electrodes (sharp tungsten electrodes), two recording electrodes (glass electrodes filled with saline) and one ground electrode (sharp tungsten electrodes). C) Drosophila melanogaster impaled with stimulation electrodes through the eyes in the brain and a ground in the abdomen. Two glass electrodes are placed the thorax for recording of responses from the TTM and DLM. D) Sample electrophysiological traces from recordings of the TTM and DLM upon brain stimulation of a wild type fly. The response latency of the GF-TTM pathway is 0.8 ms and it can follow stimuli one to one at 200 Hz. In contrast, the response latency of the GF-DLM pathway is 1.2 ms and responses are not seen after every stimulus when given 10 stimuli at 200 Hz.
Equipment
Electrophysiological rig set up (Figure 2A, B)
Stimulator (e.g. S48 Square Pulse Stimulator, Grass instruments, Figure 2A, 1)
Stimulation isolation unit (e.g. SIU5 RF Transformer Isolation Unit, Grass instruments, Figure 2A, 2)
Intracellular amplifier, 2 channels (eg. 1× Axoclamp 2B or 2× Axopatch 200B, Molecular Devices or 2 × Model 5A Microelectrode Amplifier, Getting Instruments, Figure 2A, 3)
Data acquisition system (e.g. Digidata 1440A, Molecular devices, Figure 2A, 4) & Computer with software (e.g. pClamp 10, Molecular Devices) and/or storage Oscilloscope (e.g Tektronix 5111, Figure 2A, 5)
Stereomicroscope (6–50x or higher) on a boom stand (Figure 2A, 6)
Vibration isolation table (Figure 2A, 7)
Light source (Figure 2A, 8)
Recording platform with 5 manual multi axis micromanipulators (e.g. MM-3 Micromanipulator, Narashigi or M3301, World Precision Instruments (Figure 2A, 9; Figure 2B)
3 sharp tungsten electrodes (1× Ground and 2× Stimulation electrodes). (Figure 2A, 9; Figure 2B) These can be fabricated from .005” diameter tungsten wire sharpened electrolytically using 4 M NaOH. Alternatively commercially available tungsten electrodes can be used.
2 glass recording electrodes with a resistance of 40–60 MΩ (Figure 2A, 9; Figure 2B) These are fabricated using a good glass microelectrode puller e.g. a Sutter P-95. Again pre-formed micro-electrodes can be purchased if desired.
Faraday cage (optional).
METHOD
Mounting flies
-
1. Anaesthetize the fly on ice or with CO2.
The fly should be left for 20–30 min after mounting if CO2 is used since occasionally it can affect recordings (see troubleshooting). This is not a problem when using ice; however, the fly must be secured in the wax more quickly as recovery from cooling can be quite rapid.
1. Using forceps transfer the anaesthetized fly to the wax by its legs and mount it into soft wax on a slide or tray with the ventral side down, pushing the legs into the wax to secure.
-
2. Pull the proboscis outwards and push into the wax so that the head lies slightly forward and down upon the surface.
This step is important as the head needs to be secure and not move when the stimulating electrodes are inserted (step 6). Keeping the head slightly stretched in front of the thorax will also help prevent inadvertent stimulation of the ventral nerve cord (see troubleshooting).
-
3. Pull the wings outwards, away from the thorax, and secure. Ensure that the fly cannot move its thorax and that the areas of the DLM and TTM (Figure 3, dotted areas) are visible and accessible.
If the fly is mounted incorrectly or not securely it becomes very difficult to obtain recordings so it is advisable to practice these steps several times before proceeding with the protocol.
Figure 3.
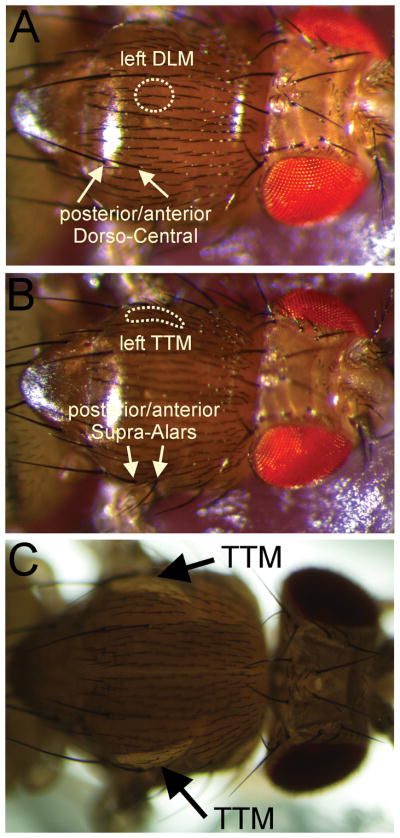
Identification of the TTM and DLM localization using bristles and illumination. A) There are six pairs of indirect flight muscles, but only the Dorsal Longitudinal Muscle pair 45a (also called the dorsal median muscle or muscle number 6) are innervated by the DLM motor neurons that receive input via the PSI from the contra-lateral GF (Demerec 1994). The attachment site of the DLM 45a muscles are under the cuticle between the anterior Dorso-Central setae (yellow arrows) and the midline of the animal (yellow circle indicates site of left DLM). B) The TTM is underneath the cuticle, just dorsal of the anterior and posterior Supra-Alars setae (yellow arrows) as indicated by the area circled by a dotted line (Demerec 1994). C) As the TTM fibers are running along the dorsal-ventral axis they can be nicely visualized when a light source is placed underneath the fly (black arrows).
Placement of electrodes
General Note: Successful recording from the GFS relies on being able to arrange the 5 micromanipulators so that the electrodes can be placed within several mm of each other. It is worth spending some time moving and adjusting these before a preparation is introduced so that minimal adjustment is required when recordings are needed.
4. Place the ground electrode into the posterior end of the abdomen (Figure 2C).
-
5. Place the stimulating electrodes through the eyes into the brain (Figure 2C).
The brain sits at the back of the head capsule but that electrodes pushed in too far may traverse the head capsule and enter the thorax where they may stimulate the ventral nerve cord directly (see troubleshooting)
6. Give single pulses of 30–60 V for 0.03 ms and check for successful activation of the giant fiber system by looking for movement of the wings and/or TTM muscle upon stimulation.
7. Place the saline (or 3 M KCl) filled glass electrode for intracellular recordings into the left (or right) DLM muscle fiber 45a, which is immediately below the cuticle (see Figures 2C, 3A).
Stimulation and recording
-
8. Give single stimuli as in step 4 and modulate the stimulus strength by varying the voltage to determine the threshold for eliciting a response.
The response of a good DLM recording is around 50–70 mV and has a latency of approximately 1.2–1.4 ms (Figure 2D). Set the voltage 5–10 V above the determined threshold for the remainder of the experiment.
-
9. Place the second intracellular recording electrode in the right (or left) TTM muscle on the contra lateral side with respect to the recording electrode for the DLM (see Figure 2C, Figure 3B).
The TTM muscle fibers are much smaller than the DLM muscle fibers and hence it is more difficult to obtain and maintain a good recording. The response of a good recording from the TTM is around 30–50 mV and has a latency of approximately 0.8 ms (Figure 2D). Protocols can be programmed in software such as pCLAMP to capture 10 ms sweeps to collect data.
-
10. Once you have good recordings from the TTM and DLM give 10 single stimuli with an intermittence of approximately 5 seconds between the stimuli and determine the average response latency for both giant fiber outputs.
For this step a separate software protocol that captures 120 ms sweeps can be used to collect the data.
11. Finally, determine the following frequency by giving ten trains of 10 stimuli at 100 Hz, with an intermittence of approximately 2 seconds between the trains. Calculate the % of the total responses. Do the same assay for trains of stimuli given at 200 Hz and 300 Hz.
12. Compare the TTM and DLM response latencies as well as the following frequencies at 100, 200 and 300 Hz between wild type and mutant flies.
TROUBLESHOOTING
Problem
The recording electrodes are sliding on the cuticle without being able to pierce it on the appropriate spot in order to impale the correct muscle.
Solution
The more perpendicular the electrode is to the cuticle, the easier it is for the electrode to get through the cuticle. Move the electrode to a slightly different area within the target area, change the angle of the micromanipulator itself, try for the muscle on the contra lateral side or re-mount the fly in a differently angled position.
Problem
The recording electrodes are indenting the cuticle or the recording electrodes are bending without piercing the cuticle.
Solution
Make sure your electrode is not broken and has the appropriate shape. The tip of your electrode should have the approximate shape and size similar to the posterior Supra-Alars setae (Figure 3B). In case the electrode is not broken and has the appropriate shape, try gently tapping on the back of the forward moving knob of the micromanipulator (once there is slight indentation) to encourage penetration through the cuticle.
Problem
No stimulation artifact and no response.
Solution
Check whether all equipment is turned on. Double-check whether the fly is responding upon stimulation (step 4). If it doesn’t there is something wrong with your stimulation (check stimulation electrodes, ground and stimulator settings etc.). If the fly does respond then there is something wrong with your recording (check recording electrodes and amplifier settings etc.).
Problem
The muscle response has an unusual shape with multiple peaks.
Solution
This occurs when the microelectrode is not recording from a single muscle cell. This can occur in recordings from either muscle but is more common in TTM recordings since this muscle is composed of many small fibers and maintaining the position of the electrode after several muscle contractions is problematic. An unusual shaped or multi-peaked response trace does not affect the data since response latencies and followings will still be preserved.
Problem
There is a very large stimulation artifact obscuring the muscle repsonse and/or recordings of multiple stimuli are drifting on the recording monitor.
Solution
Double-check whether the ground electrode is properly in the fly and double-check the voltage and duration of the stimuli given. Also, when the hemolymph dries up around the ground wire it results in its loss of conductance. This can be prevented and recovered with a little drop of saline on the fly where the ground electrode enters the abdomen.
Problem
Obtaining long latencies or no responses in wild type flies.
Solution
Double-check whether you are in the correct target area for the appropriate muscle. Alternatively, your electrode might have gone in too far and you pierced through the correct muscle. Both muscles are just underneath cuticle. An approximate measure is that the cuticle is no thicker than 2–3x of the thickness of a posterior Supra-Alars setae at it thickest visible point (Figure 3B). Alternatively your stimulation is below threshold, therefore try increasing the voltage (duration). If you have used CO2 to anaesthetize the fly, either leave the fly to recover from CO2 longer before testing or anaesthetize flies using ice. Finally, your wild type fly may be a mutant.
Problem
Obtaining very short latencies for both, TTM (<0.7 ms) and DLM (< 1 ms).
Solution
This occurs if the ventral nerve cord, and thus the TTMn and DLMn motorneurons are being activated directly. Check the position of the stimulating electrodes and replace them in the brain if necessary.
DISCUSSION
In wild type flies average response latencies to a single stimulus are in the range 0.8 ms +/− 0.1 ms for the GF-TTM pathway and 1.4 ms +/− 0.3 ms for the GF-DLM pathway depending on genotype and genetic background. Similarly, with respect to following frequencies the GF-TTM path is able to follow 10 stimuli 1:1 up to 300 Hz and the GF-DLM pathway up to 100 Hz but variability between individual flies of different genotypes and genetic background have been observed. Hence, it is important to choose carefully the appropriate control flies when analyzing the electrophysiological phenotypes of mutants or targeted disruptions in the GFS. Two classical mutants that do affect the function of the GFS dramatically are shakB2 and bendless (Thomas and Wyman 1984; Blagburn et al. 1999; Allen and Murphey 2007; Phelan et al. 2008; Uthaman et al. 2008). In shakB2 flies the GF-TTMn synapse lacks the gap junctions but the chemical component is still present. The average response latency for the TTM in these flies is consistently increased to an average of 1.5 ms and it is not able to follow stimuli given at either 100, 200 or 300 Hz due the weak labile nature of resultant GF-TTMn synapse. In addition, no responses are obtained from the DLM when the GF is stimulated in the brain. Proof that the lack of responses are not due to a defect at the NMJ comes from the ability to record responses from the DLM muscle, when the motorneurons are stimulated directly by placing the stimulation electrodes in the thorax (Thomas and Wyman 1984). In contrast, in bendless flies the GF-DLM pathway remains unaffected when compared to wild type control flies. However, the GF-TTM connection is consistently increased to an average of more than 2 ms and is not able to following stimuli given at either 100, 200 or 300 Hz.
The reason that these indirect electrophysiological tests of these central synapses of the GFS are successful is that the NMJs at both TTM and the DLMs are large and extensive with many synaptic boutons. They rarely fail; the motorneurons can be stimulated directly at frequencies up to 500 Hz and the muscles will still show 1:1 responses to stimuli (MJA & TAG, unpublished data). Thus any effects seen on transmission through the pathways from the GF can be attributed to central synaptic defects. If defects are seen when testing it is always prudent to stimulate the motoneurons directly to confirm that the NMJs are functioning correctly in at least a few flies of the same genotype, because some mutants do affect the adult NMJ (Huang et al., 2006).
RECIPES
Saline (Gu and O'Dowd 2006)
101 mM NaCl
1 mM CaCl2
4 mM MgCl2
3 mM KCl
5 mMglucose
1.25 mM NaH2PO4
20.7 mM NaHCO3
pH 7.2
Acknowledgments
Work in the M.J.A. lab has been supported by the Wellcome Trust and the Leverhulme Trust. T.A.G. is supported by R01 HD050725. Thanks to Robin Konieczny for the artwork in Figure 1. We also owe much to R.K. Murphey for his enthusiasm and encouragement regarding the GFS.
Contributor Information
Marcus J Allen, Email: M.J.Allen@kent.ac.uk.
Tanja A Godenschwege, Email: godensch@fau.edu.
References
- Allen MJ, Godenschwege TA, Tanouye MA, Phelan P. Making an escape: development and function of the Drosophila giant fibre system. Semin Cell Dev Biol. 2006;17(1):31–41. doi: 10.1016/j.semcdb.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Allen MJ, Murphey RK. The chemical component of the mixed GF-TTMn synapse in Drosophila melanogaster uses acetylcholine as its neurotransmitter. The European journal of neuroscience. 2007;26(2):439–445. doi: 10.1111/j.1460-9568.2007.05686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MJ, Shan X, Caruccio P, Froggett SJ, Moffat KG, Murphey RK. Targeted expression of truncated glued disrupts giant fiber synapse formation in Drosophila. J Neurosci. 1999;19(21):9374–9384. doi: 10.1523/JNEUROSCI.19-21-09374.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MJ, Shan X, Murphey RK. A role for Drosophila Drac1 in neurite outgrowth and synaptogenesis in the giant fiber system. Mol Cell Neurosci. 2000;16(6):754–765. doi: 10.1006/mcne.2000.0903. [DOI] [PubMed] [Google Scholar]
- Blagburn JM, Alexopoulos H, Davies JA, Bacon JP. Null mutation in shaking-B eliminates electrical, but not chemical, synapses in the Drosophila giant fiber system: a structural study [In Process Citation] J Comp Neurol. 1999;404(4):449–458. [PubMed] [Google Scholar]
- Demerec M, editor. Biology of Drosophila. Cold Spring Harbor Laboratory press; New York: 1994. [Google Scholar]
- Engel JE, Wu CF. Altered habituation of an identified escape circuit in Drosophila memory mutants. J Neurosci. 1996;16(10):3486–3499. doi: 10.1523/JNEUROSCI.16-10-03486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JE, Wu CF. Genetic dissection of functional contributions of specific potassium channel subunits in habituation of an escape circuit in Drosophila. J Neurosci. 1998;18(6):2254–2267. doi: 10.1523/JNEUROSCI.18-06-02254.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotowat H, Fayyazuddin A, Bellen HJ, Gabbiani F. A Novel Neuronal Pathway for Visually Guided Escape in Drosophila Melanogaster. Journal of neurophysiology. 2009 doi: 10.1152/jn.00073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godenschwege TA, Hu H, Shan-Crofts X, Goodman CS, Murphey RK. Bi-directional signaling by Semaphorin 1a during central synapse formation in Drosophila. Nat Neurosci. 2002a;5(12):1294–1301. doi: 10.1038/nn976. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Kristiansen LV, Uthaman SB, Hortsch M, Murphey RK. A conserved role for Drosophila Neuroglian and human L1-CAM in central-synapse formation. Curr Biol. 2006;16(1):12–23. doi: 10.1016/j.cub.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Simpson JH, Shan X, Bashaw GJ, Goodman CS, Murphey RK. Ectopic expression in the giant fiber system of Drosophila reveals distinct roles for roundabout (Robo), Robo2, and Robo3 in dendritic guidance and synaptic connectivity. J Neurosci. 2002b;22(8):3117–3129. doi: 10.1523/JNEUROSCI.22-08-03117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczyca M, Hall JC. Identification of a cholinergic synapse in the giant fiber pathway of Drosophila using conditional mutations of acetylcholine synthesis. J Neurogenet. 1984;1(4):289–313. doi: 10.3109/01677068409107093. [DOI] [PubMed] [Google Scholar]
- Gu H, O'Dowd DK. Cholinergic synaptic transmission in adult Drosophila Kenyon cells in situ. J Neurosci. 2006;26(1):265–272. doi: 10.1523/JNEUROSCI.4109-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FD, Woodruff E, Mohrmann R, Broadie K. Rolling blackout is required for synaptic vesicle exocytosis. J Neurosci. 2006;26:2369–2379. doi: 10.1523/JNEUROSCI.3770-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Koenig JH, Tsuruhara T. Organization of identified axons innervating the dorsal longitudinal flight muscle of Drosophila melanogaster. J Neurocytol. 1980;9(6):799–823. doi: 10.1007/BF01205020. [DOI] [PubMed] [Google Scholar]
- King DG, Wyman RJ. Anatomy Of the Giant Fiber Pathway In Drosophila .1. 3 Thoracic Components Of the Pathway. Journal Of Neurocytology. 1980;9(6):753–770. doi: 10.1007/BF01205017. [DOI] [PubMed] [Google Scholar]
- Koto M, Tanouye MA, Ferrus A, Thomas JB, Wyman RJ. The Morphology Of the Cervical Giant Fiber Neuron Of Drosophila. Brain Research. 1981;221(2):213–217. doi: 10.1016/0006-8993(81)90772-1. [DOI] [PubMed] [Google Scholar]
- Lin MQ, Nash HA. Influence of general anesthetics on a specific neural pathway in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1996;93(19):10446–10451. doi: 10.1073/pnas.93.19.10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez VG, Javadi CS, Ngo E, Ngo L, Lagow RD, Zhang B. Age-related changes in climbing behavior and neural circuit physiology in Drosophila. Developmental neurobiology. 2007;67(6):778–791. doi: 10.1002/dneu.20388. [DOI] [PubMed] [Google Scholar]
- Oh CE, McMahon R, Benzer S, Tanouye MA. Bendless, a Drosophila Gene Affecting Neuronal Connectivity, Encodes a Ubiquitin-Conjugating Enzyme Homolog. Journal Of Neuroscience. 1994;14(5 Pt2):3166–3179. doi: 10.1523/JNEUROSCI.14-05-03166.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan P, Goulding LA, Tam JL, Allen MJ, Dawber RJ, Davies JA, Bacon JP. Molecular mechanism of rectification at identified electrical synapses in the Drosophila giant fiber system. Curr Biol. 2008;18(24):1955–1960. doi: 10.1016/j.cub.2008.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JB, Wyman RJ. Mutations Altering Synaptic Connectivity Between Identified Neurons In Drosophila. Journal Of Neuroscience. 1984;4(2):530–538. doi: 10.1523/JNEUROSCI.04-02-00530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthaman SB, Godenschwege TA, Murphey RK. A mechanism distinct from highwire for the Drosophila ubiquitin conjugase bendless in synaptic growth and maturation. J Neurosci. 2008;28(34):8615–8623. doi: 10.1523/JNEUROSCI.2990-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MR, Lagow RD, Xu K, Zhang B, Bonini NM. A drosophila model for amyotrophic lateral sclerosis reveals motor neuron damage by human SOD1. The Journal of biological chemistry. 2008;283(36):24972–24981. doi: 10.1074/jbc.M804817200. [DOI] [PMC free article] [PubMed] [Google Scholar]