Abstract
Background
—Oxidative stress is causally linked to the progression of heart failure, and mitochondria are critical sources of reactive oxygen species in failing myocardium. We previously observed that in heart failure, elevated cytosolic Na+ ([Na+]i) reduces mitochondrial Ca2+ ([Ca2+]m) by accelerating Ca2+ efflux via the mitochondrial Na+/Ca2+ exchanger. Because the regeneration of antioxidative enzymes requires NADPH, which is indirectly regenerated by the Krebs cycle, and Krebs cycle dehydrogenases are activated by [Ca2+]m, we speculated that in failing myocytes, elevated [Na+]i promotes oxidative stress.
Methods and Results
—We used a patch-clamp–based approach to simultaneously monitor cytosolic and mitochondrial Ca2+ and, alternatively, mitochondrial H2O2 together with NAD(P)H in guinea pig cardiac myocytes. Cells were depolarized in a voltage-clamp mode (3 Hz), and a transition of workload was induced by β-adrenergic stimulation. During this transition, NAD(P)H initially oxidized but recovered when [Ca2+]m increased. The transient oxidation of NAD(P)H was closely associated with an increase in mitochondrial H2O2 formation. This reactive oxygen species formation was potentiated when mitochondrial Ca2+ uptake was blocked (by Ru360) or Ca2+ efflux was accelerated (by elevation of [Na+]i). In failing myocytes, H2O2 formation was increased, which was prevented by reducing mitochondrial Ca2+ efflux via the mitochondrial Na+/Ca2+ exchanger.
Conclusions
—Besides matching energy supply and demand, mitochondrial Ca2+ uptake critically regulates mitochondrial reactive oxygen species production. In heart failure, elevated [Na+]i promotes reactive oxygen species formation by reducing mitochondrial Ca2+ uptake. This novel mechanism, by which defects in ion homeostasis induce oxidative stress, represents a potential drug target to reduce reactive oxygen species production in the failing heart.
Keywords: heart failure, sodium, calcium, free radicals, ion channels
Oxidative stress plays a fundamental role in many cardiovascular diseases and aging.1,2 In chronic heart failure, oxidative stress is causally linked to the progression of the disease,1,3,4 and mitochondria were identified as critical sources of reactive oxygen species (ROS) in the heart.5 ROS impair excitation-contraction (EC) coupling,6–8 cause arrhythmias,9 and contribute to cardiac remodeling by activating signaling pathways that induce hypertrophy, apoptosis, and necrosis.10–13 The precise mechanisms that regulate mitochondrial ROS formation, however, are incompletely understood.
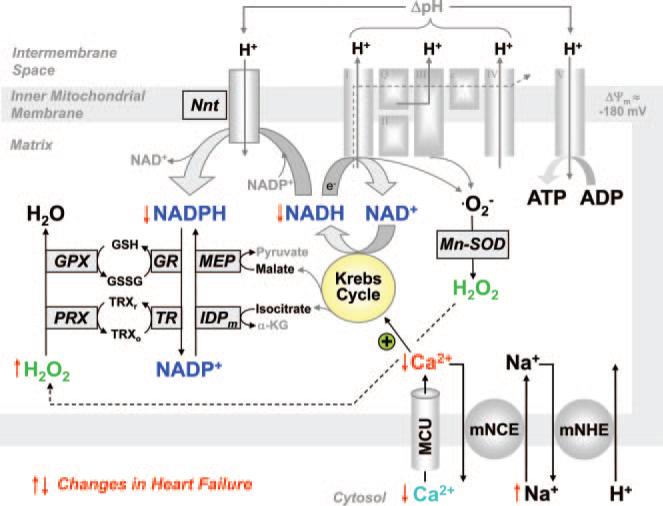
In cardiac myocytes, the processes of EC coupling consume large amounts of ATP, which is replenished by oxidative phosphorylation in mitochondria. Because the heart undergoes frequent changes in workload, precise matching of ATP supply and demand is essential to maintain cardiac function.14 Two key regulators of oxidative phosphorylation are ADP and Ca2+. When energy consumption increases (eg, during β-adrenergic stimulation), elevated ADP stimulates ATP production at the F1/F0-ATPase (Figure 1). This accelerates electron flux along the electron transport chain (ETC) and oxidizes the primary electron donor, NADH. To maintain the higher electron fluxes, a concomitant increase in NADH production must occur. This is accomplished by Ca2+-induced stimulation of rate-controlling dehydrogenases of the Krebs cycle.15–17 Ca2+ enters mitochondria via a Ca2+ uniporter (MCU) and is exported by a Na+/Ca2+ exchanger (mNCE). Although the kinetics of mitochondrial Ca2+ uptake are still debated (ie, slow uptake versus beat-to-beat oscillations), most studies agree that in response to an elevation of the frequency or amplitude of cytosolic Ca2+ transients (as occurs during β-adrenergic stimulation), steady state mitochondrial [Ca2+] ([Ca2+]m) increases.16 Thus, energy supply and demand matching is closely linked to the processes of EC coupling.
Figure 1.
Regulation of oxidative phosphorylation and ROS formation by NAD(P)H and Ca2+. The Krebs cycle is fueled by metabolic substrates (glucose, fatty acids) via acetyl-coenzyme A and mediates the recovery of oxidized NAD+ to NADH, which donates electrons to the ETC for oxidative phosphorylation of ATP. Regeneration of antioxidative capacity is also coupled to the Krebs cycle, because regeneration of NADPH requires products of the Krebs cycle (isocitrate, malate, NADH). Three rate-controlling enzymes of the Krebs cycle (pyruvate, isocitrate, and α-ketoglutarate dehydrogenase) are activated by Ca2+. ΔΨm indicates mitochondrial membrane potential; Nnt, nicotinamide nucleotide transhydrogenase; Mn-SOD, Mn2+-dependent superoxide dismutase; PRX, peroxiredoxin; GPX, glutathione peroxidase; TRXr/o, reduced/oxidized thioredoxin; GSH/GSSG, reduced/oxidized glutathione; TR, thioredoxin reductase; GR, glutathione reductase; IDPm, mitochondrial NADP+-dependent isocitrate dehydrogenase; MEP, mitochondrial malic enzyme; α-KG, α-ketoglutarate; and mNHE, mitochondrial Na+/H+ exchanger.
In chronic heart failure, perturbations of EC coupling cause contractile dysfunction.18,19 One major deficit is a decreased Ca2+ load of the sarcoplasmic reticulum, which reduces cytosolic Ca2+ transients.18,19 On the other hand, the cytosolic Na+ concentration ([Na+]i) is elevated, which facilitates cytosolic Ca2+ influx via the sarcolemmal Na+/Ca2+ exchanger during the action potential.20–23 Although this compensates in part for the decreased sarcoplasmic reticulum Ca2+ load, we previously reported that an elevation of [Na+]i negatively affects energy supply and demand matching.17,24 Because mitochondrial Ca2+ efflux is governed by a Na+/Ca2+ exchanger (mNCE), elevation of [Na+]i accelerates mitochondrial Ca2+ efflux, and reduced [Ca2+]m hampers the activation of Krebs cycle dehydrogenases. This results in pronounced oxidation of NADH to NAD+ during transitions of workload.17,24
Besides its elemental role in the regulation of oxidative phosphorylation via NADH, the Krebs cycle may also play a key role in recovery of the antioxidative capacity of the mitochondrial matrix. Under physiological conditions, 0.2% to 2% of oxygen is incompletely reduced to superoxide (•O2−) at the ETC, which is then dismutated to H2O2 by the matrix-located superoxide dismutase.2 H2O2, in turn, is eliminated by peroxiredoxin and glutathione peroxidase,2 and the regeneration of these enzymes requires reduced NADPH (Figure 1).25,26 Oxidized NADP+ is recovered to NADPH by NADP+-dependent isocitrate dehydrogenase, NADP+-dependent malic enzyme, and nicotinamide nucleotide transhydrogenase.25 Considering that all 3 NADPH-regenerating enzymes derive their substrates from the Krebs cycle (isocitrate, malate, and NADH; Figure 1), and the turnover rate of the Krebs cycle is regulated by Ca2+, we hypothesized that during transitions of workload, mitochondrial Ca2+ uptake maintains the antioxidative capacity of the matrix in a reduced state and thus controls mitochondrial levels of H2O2.
Using a patch-clamp based approach, we observed that during transitions of workload, transient oxidations of NAD(P)H were associated with increased formation of mitochondrial H2O2, whereas mitochondrial Ca2+ uptake recovered NAD(P)H and decreased H2O2 formation. In failing myocytes, elevated [Na+]i reduced mitochondrial Ca2+ uptake and potentiated mitochondrial H2O2 formation. The results reveal a novel mechanism by which defects in cellular Na+ homeostasis induce mitochondrial oxidative stress in the failing heart.
Methods
A detailed Methods section can be found in the online-only Data Supplement.
Heart Failure Model and Functional Evaluation
Aortic banding was performed in guinea pigs as described previously.24 Animals underwent echocardiography every 2 weeks after surgery. Left ventricular ejection fraction was calculated with VisualSonics V1.3.8 software from 2-dimensional long-axis views. When a decrease in ejection fraction was observed, animals were euthanized, heart weight/body weight was measured, and cardiomyocytes were isolated.
Cell Isolation
Cardiac myocytes were isolated from normal and failing guinea pig hearts by enzymatic digestion and stored in supplemented Dulbecco's modified Eagle's medium (Invitrogen, Karlsruhe, Germany) as described previously.17,24
Patch-Clamp Experiments
Myocytes were voltage clamped in the whole-cell configuration (37°C, pipette resistance 2 to 4 MΩ) and equilibrated with a physiological K+-glutamate–based pipette solution as described previously.17,24 For the experiments in Figures 2 through 5, myocytes were depolarized from −80 to 10 mV at 3 Hz for 80 ms, and isoproterenol (10 and 100 nmol/L) was used to increase workload via β-adrenergic stimulation. To inhibit the MCU, Ru360 (1 μmol/L) was added to the pipette solution. Alternatively, [Na+]in the pipette solution was raised from 5 to 15 mmol/L to accelerate mitochondrial Ca2+ efflux via the mNCE.17 For the protocol in Figure 6D, myocytes were depolarized at 4 Hz for 3 minutes in the presence of isoproterenol (100 nmol/L) as described previously.24
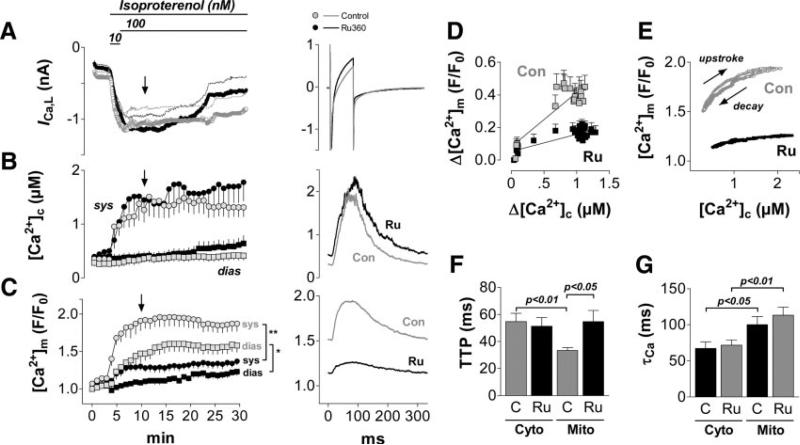
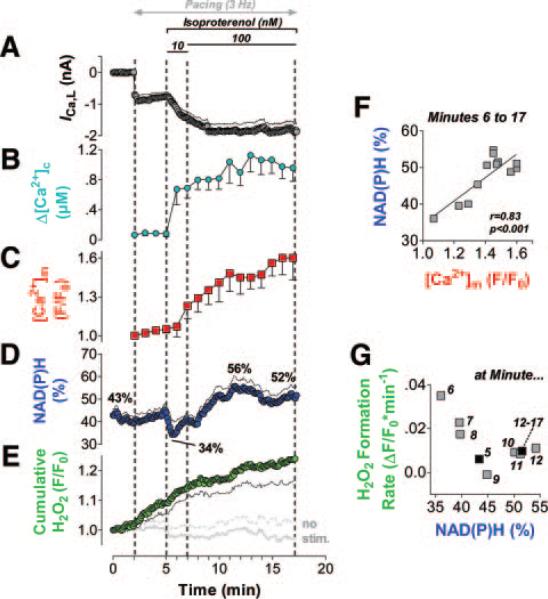
Figure 2.
Mitochondrial Ca2+ uptake during transitions of workload. Myocytes were depolarized from −80 to 10 mV for 80 ms at 3 Hz and superfused with isoproterenol. Pipette solution contained 5 mmol/L [Na+]i, in the absence (control, n=13) and presence of the MCU-blocker Ru360 (1 μmol/L; n=11). A–C, left panels: Time courses of L-type Ca2+ currents (ICa,L; A), [Ca2+]c (B), and [Ca2+]m (C) during the whole experiment; right panels, averaged currents, [Ca2+]c, and [Ca2+]m after 10 minutes of the protocol, respectively (see arrow in left panels). Con indicates control; sys, systole; and dias, diastole. D, Averaged amplitudes of [Ca2+]m (Δ[Ca2+]m) plotted against the respective Δ[Ca2+]c, in the absence (Con) and presence of Ru360 (Ru). E, [Ca2+]m plotted against [Ca2+]c during a single Ca2+ transient (averaged data at 10 minutes of the protocol). F, Time to peak (TTP) and G, time constant of decay (τ) of [Ca2+]c (Cyto) and [Ca2+]m (Mito) in the absence (C) and presence (Ru) of Ru360, respectively. *P<0.05 from minute 9 to 12 and P<0.01 from minute 12 to 30; **P<0.01 from minute 6 to 30 in C, and as indicated in F and G (2-way ANOVA, respectively).
Figure 5.
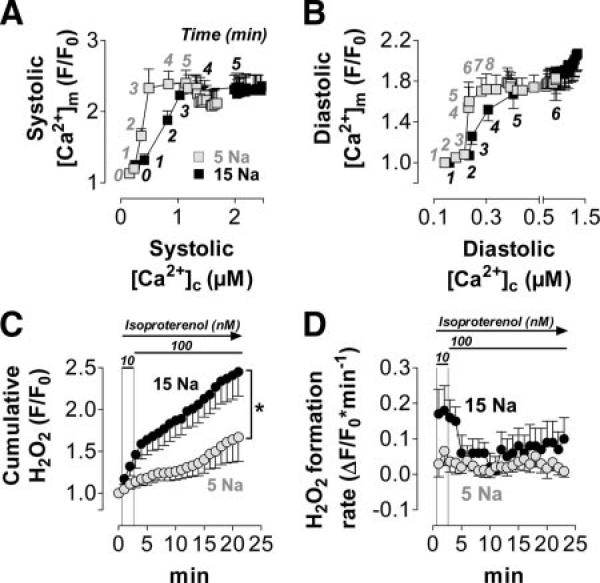
Elevated [Na+]i increases ROS production in normal myocytes. A similar protocol as in Figure 2 was performed, except that isoproterenol was washed in starting 40 seconds after the onset of pacing. Pipette solution was 5 or 15 mmol/L [Na+]i as indicated. A and B, [Ca2+]c and [Ca2+]m were measured in normal myocytes (5 mmol/L [Na+]i, n=15; 15 mmol/L [Na+]i, n=20). A, Systolic [Ca2+]m plotted against systolic [Ca2+]c; B, diastolic [Ca2+]m plotted against diastolic [Ca2+]c; the numbers indicate the time (in minutes) after the start of the experiment. C and D, Net H2O2 formation was determined by CM-DCF in a separate set of cells (n=15/12). CM-DCF oxidation, indicating levels of H2O2, is given as F/F0 (C) or ΔF/F0 per minute (D). *P<0.05 for 5 vs 15 mmol/L [Na+]i at minute 6 to 18 (1-way ANOVA).
Figure 6.
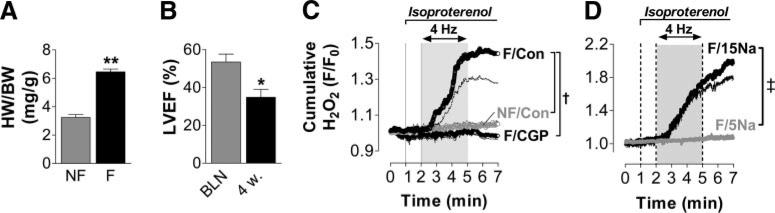
Increased ROS production in failing myocytes is related to deficient mitochondrial Ca2+ uptake. A, Heart weight/body weight (HW/BW) ratio in guinea pigs 4 weeks after ascending aortic constriction (failing, F; n=3) compared with age- and sex-matched control animals (nonfailing, NF; n=4). B, In vivo left ventricular ejection fraction (LVEF) before (BLN) and 4 weeks after (4w) aortic banding (n=3). C, H2O2 levels in intact, field-stimulated myocytes (4 Hz) from normal (NF, n=8) or failing (F) myocytes, in the absence (Con; n=8) or presence (CGP) of CGP-37157 (1 μmol/L; n=6), an inhibitor of the mNCE. D, Cumulative H2O2 formation in failing myocytes that were voltage clamped (4 Hz) and equilibrated with a pipette solution that contained either 5 or 15 mmol/L [Na+] (n=7/3). *P<0.05 F vs NF; **P<0.01 4w vs BLN; †P<0.05 F/Con vs NF/Con and F/CGP vs F/Con at minutes 4 to 7, respectively; ‡P<0.001 for 5 vs 15 mmol/L [Na+]i at minutes 3 to 7 by unpaired t test (A), paired t test (B), and 1-way ANOVA (C and D), respectively.
Fluorescence Recordings to Determine [Ca2+]c, [Ca2+]m, NAD(P)H, and H2O2
To monitor [Ca2+]c together with [Ca2+]m, myocytes were loaded with the cell-permeable Ca2+ indicator rhod-2 acetoxymethyl esther (rhod-2 AM, 3 μmol/L; Invitrogen), which locates primarily to mitochondria, and then dialyzed with a pipette solution that contained indo-1 salt to monitor [Ca2+]c, as described previously.17 Alternatively, myocytes were loaded with the H2O2-sensitive 5-(-6)-chloromethyl-2′,7′-dichlorohydrofluorescein diacetate (CM-H2DCFDA; Invitrogen), which locates primarily to mitochondria,27 and dialyzed with dye-free pipette solution. The fluorescent product CM-DCF was monitored together with the autofluorescence of NAD(P)H (online-only Data Supplement Figures I through III).
Field Stimulation in Failing and Nonfailing Myocytes
For the experiments in Figure 6C, myocytes were field stimulated in a heated chamber (37°C) at 4 Hz in the presence of isoproterenol as described previously,24 and H2O2 formation was recorded by CM-DCF fluorescence in the absence and presence of CGP-37157 (1 μmol/L), respectively.
Statistical Analysis
Values are given as mean±SEM. Statistical analysis was performed with 2-way ANOVA (Figures 2F and 2G) and 1-way (Figures 4B through 4D, 5C, 6C, and 6D) or 2-way (Figure 2C) ANOVA for repeated measures, respectively. An unpaired or paired t test was applied for Figures 6A and 6B, respectively, and linear regression analysis was applied in Figures 3F and 4E. Analysis was performed with SPSS (ANOVA) and GraphPad Prism (t tests, regression analysis; version 3.00 for Windows, GraphPad Software; San Diego, Calif).
Figure 4.
Inhibition of mitochondrial Ca2+ uptake increases mitochondrial ROS production. The same protocol was used as in Figures 2 and 3, respectively. Amplitudes of [Ca2+]c (Δ[Ca2+]c; A) and diastolic [Ca2+]m (B), in the absence (Con, n=13) and presence (Ru, n=11; 1 μmol/L in the pipette solution) of Ru360 are displayed. Ca2+ data are taken from the series of experiments in Figure 2. NAD(P)H (C) and H2O2 (D) in the absence (n=16) and presence of Ru360 (n=15; 1 μmol/L in the pipette solution). E, NAD(P)H plotted against diastolic [Ca2+]m. F, Net mitochondrial formation of H2O2 plotted versus NAD(P)H. *P<0.05 at minute 8 to 10 and P<0.01 from minute 12 to 17; **P<0.05 from minute 10 to 15; †P<0.05 at minute 14 to 17 and P<0.07 at minute 7 to 14 (1-way ANOVA, respectively).
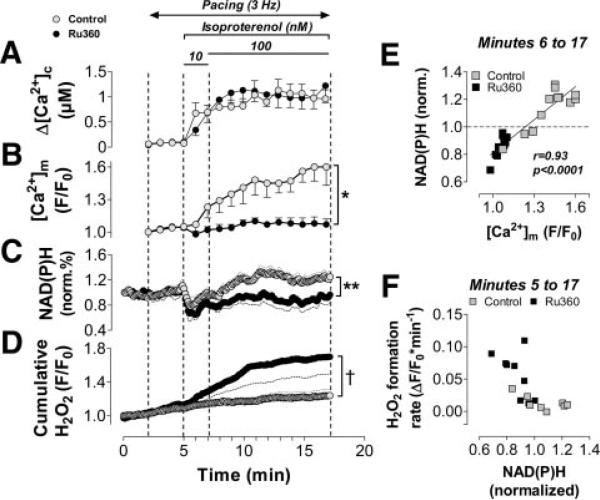
Figure 3.
Dynamic regulation of mitochondrial ROS by [Ca2+]m and NAD(P)H. Myocytes (n=16) were loaded with CM-H2DCF to monitor H2O2 together with the autofluorescence of NAD(P)H. A similar protocol as in Figure 2 was performed (voltage-clamp pulses from −80 to 10 mV at 3 Hz, [Na+]i=5 mmol/L). Changes in ICa,L (A), NAD(P)H (D), and H2O2 (E) are displayed together with changes in Δ[Ca2+]c (B) and diastolic [Ca2+]m (C) from the experiments in Figure 2 (control group). The gray trace in E indicates H2O2 in unpatched cells that were not paced. F, NAD(P)H correlated to diastolic [Ca2+]m after β-adrenergic stimulation and initial NAD(P)H oxidation (starting at minute 6). G, Rates of CM-DCF oxidation, indicating the net H2O2 formation (ΔF/F0×min−1), averaged over 1 minute, respectively, and correlated to the respective (averaged) NAD(P)H levels after β-adrenergic stimulation with isoproterenol at the indicated time points. For minutes 12 to 17, 1 average value was calculated. Stim indicates stimulation.
Results
Beat-to-Beat Oscillation of [Ca2+]m During Cytosolic Ca2+ Transients
Guinea pig myocytes were voltage clamped and depolarized at 3 Hz. Isoproterenol increased the L-type Ca2+ current (ICa,L) and the amplitude of cytosolic Ca2+ transients (Δ[Ca2+]c; Figures 2A and 2B). During cytosolic Ca2+ transients, rapid mitochondrial Ca2+ transients were recorded (Figure 2C), with a linear relationship between Δ[Ca2+]m and Δ[Ca2+]c (Figure 2D). The time to peak of [Ca2+]m was shorter than that of [Ca2+]c, whereas the decay of [Ca2+]m was slower than that of [Ca2+]c (Figures 2E through 2G). When Δ[Ca2+]c increased, diastolic [Ca2+]m accumulated, whereas diastolic [Ca2+]c was maintained (Figures 2B and 2C). The addition of Ru360, a selective inhibitor of the MCU,28 to the pipette solution reduced Δ[Ca2+]m by approximately two thirds and prevented diastolic accumulation of [Ca2+]m (Figures 2C through 2E). Conversely, [Ca2+]c was slightly (although insignificantly) increased by Ru360. Time to peak of [Ca2+]m, but not of [Ca2+]c, was prolonged by Ru360, whereas the decays of [Ca2+]m and [Ca2+]c were unchanged.
Dynamic Regulation of Mitochondrial H2O2 by [Ca2+]m and NAD(P)H
To relate the changes in [Ca2+]c and [Ca2+]m to the mitochondrial redox state of NAD(P)H/NAD(P)+ and ROS production, we determined the autofluorescence of NAD(P)H while monitoring the mitochondrial oxidation rate of an H2O2-sensitive probe (CM-DCF; Figures 3D and 3E; online-only Data Supplement Figures I through III). This probe will sense the balance between H2O2 production and scavenging, and thus, it reports the biologically relevant amount of H2O2 that actually exerts its effects within or outside mitochondria. Because the experimental protocol and conditions were identical to the protocol that determined [Ca2+]m and [Ca2+]c (Figure 2), the amplitudes of [Ca2+]c transients (Figure 3B) and changes of diastolic [Ca2+]m (Figure 3C) were included in this Figure, which facilitates cross comparisons with the analysis of NAD(P)H and H2O2. CM-DCF oxidation increased immediately on electrical stimulation of myocytes (Figure 3E), whereas the redox state of NAD(P)H/NAD(P)+ was maintained during the first 3 minutes of stimulation (Figure 3D). After β-adrenergic stimulation, when ICa,L and Δ[Ca2+]c increased rapidly, NAD(P)H was oxidized within the first minute (Figures 3A, 3B, and 3D). At the same time, the rate of CM-DCF oxidation increased ≈6-fold (Figures 3E and 3G). The degrees of NAD(P)H and CM-DCF oxidation both correlated with the absolute increase of ICa,L within the first 30 seconds after isoproterenol exposure (r=0.58 and −0.65, P<0.05, respectively; not shown). This indicates that the more abrupt the transition of workload was, the more NAD(P)H was temporarily oxidized and the more H2O2 increased.
The initial oxidation of NAD(P)H was followed by its continuous recovery over the next 5 minutes (Figure 3D). This recovery correlated closely with the increase in diastolic [Ca2+]m (Figures 3C, 3D, and 3F), in line with the concept that [Ca2+]m activates rate-controlling dehydrogenases of the Krebs cycle to accelerate the regeneration of NAD(P)H.14–17 The rate of mitochondrial formation of H2O2 peaked 1 minute after application of isoproterenol (at minute 6 of the protocol), when NAD(P)H was maximally oxidized (Figure 3G). With the subsequent Ca2+-induced recovery of NAD(P)H, net H2O2 formation decreased in parallel, reaching a new steady state at a rate comparable to the level before β-adrenergic stimulation, albeit at more reduced NAD(P)H/NAD(P)+ (Figure 3G).
Blocking Mitochondrial Ca2+ Uptake Increases H2O2
To causally relate the regulation of NAD(P)H/NAD(P)+ and H2O2 to mitochondrial Ca2+ uptake, the MCU was blocked by the addition of Ru360 (1 μmol/L) to the pipette solution. Despite a similar increase in ICa,L and Δ[Ca2+]c after β-adrenergic stimulation (Figures 2A and 4A), the lack of mitochondrial Ca2+ accumulation potentiated the initial oxidation and blunted the recovery of NAD(P)H, which resulted in more oxidized NAD(P)H/NAD(P)+ than in control conditions (Figures 4B and 4C). This was associated with an elevated rate of H2O2 formation (Figure 4D). When both groups were combined, the NAD(P)H/NAD(P)+ redox state correlated positively with [Ca2+]m (Figure 4E) but inversely with H2O2 (Figure 4F).
Elevation of [Na+]i Increases H2O2
In cardiac hypertrophy and failure, [Na+]i is elevated,20–22 and Ca2+ export from mitochondria, governed by the mNCE, consequently is increased, which limits [Ca2+]m accumulation and oxidizes NAD(P)H.17,24 Thus, we speculated that elevating [Na+]i would increase mitochondrial H2O2 levels. This was tested by use of a similar protocol with either 5 or 15 mmol/L [Na+]i in the pipette solution (Figure 5). The relationship between systolic (or diastolic) [Ca2+]m and the respective [Ca2+]c was shifted in the direction of lower [Ca2+]m per [Ca2+]c at 15 versus 5 mmol/L [Na+]i during the first 5 to 8 minutes of the protocol (Figures 5A and 5B), which indicates that elevated [Na+]i promotes mitochondrial Ca2+ efflux via the mNCE.17,24,29 At 15 mmol/L [Na+]i, H2O2 levels were elevated compared with 5 mmol/L [Na+]i, especially during the first 4 minutes of β-adrenergic stimulation (Figures 5C and 5D), which resembled the time frame in which the relation between [Ca2+]m and [Ca2+]c diverged between 5 and 15 mmol/L [Na+]i (Figures 5A and 5B).
Deficient Mitochondrial Ca2+ Uptake Accounts for Increased ROS in Heart Failure
In a guinea pig model of heart failure (induced by ascending aortic banding), [Na+]i increased from 5 to 17 mmol/L in failing versus nonfailing myocytes, and NAD(P)H oxidized in response to an abrupt increase in workload.24 After 4 weeks, aortic banding induced left ventricular hypertrophy and contractile dysfunction in vivo (Figures 6A and 6B). Myocytes from failing hearts displayed a 20-fold increase in the rate of CM-DCF oxidation compared with control myocytes after abruptly enhancing workload in vitro (Figure 6C). This marked increase in H2O2 was prevented by the inhibition of the mNCE with CGP-37157 (Figure 6C), which potentiates mitochondrial Ca2+ accumulation in nonfailing and failing cells17,24 and prevents NAD(P)H oxidation in failing myocytes.24
To corroborate the link between increased H2O2 formation and elevated [Na+]i, the cytosol of failing myocytes was equilibrated with pipette solutions that contained either 15 or 5 mmol/L [Na+]i and depolarized at 4 Hz in voltage-clamp mode. Indeed, by lowering [Na+]i, H2O2 formation was normalized to levels that occurred in nonfailing myocytes with endogenous [Na+]i of ≈5 mmol/L (Figure 6D).
Discussion
The main findings of the present study are that during transitions of workload, (1) transient oxidations of NAD(P)H in cardiac myocytes are associated with increased mitochondrial formation of H2O2, (2) mitochondrial Ca2+ uptake controls net H2O2 formation by recovering NAD(P)H through activation of Krebs cycle dehydrogenases, and (3) elevated [Na+]i reduces mitochondrial Ca2+ uptake and promotes formation of H2O2 in failing cardiac myocytes.
Oxidative Stress in Heart Failure
Increased oxidative stress is observed in the plasma and myocardium of patients with heart failure and is related to an impairment of left ventricular function.30–32 Indeed, animal models suggest a causal link between oxidative stress and the progression of contractile dysfunction to overt heart failure.1,3,4 Besides NADPH oxidase, xanthine oxidase, and uncoupled nitric oxide synthases, mitochondria are important sources of ROS.1,5,31 The relative contribution of these different sources to overall oxidative stress in the failing heart, however, is presently unknown. Owing to the short half-life and high reactivity of ROS, microdomains of ROS may exist with differential effects on cell function, depending on their subcellular localization and source. For instance, although NADPH oxidase–derived ROS are involved in the development of cardiac hypertrophy,33,34 mitochondrial ROS induce energetic deficits,27 apoptosis,12 an impairment of EC coupling,7 and arrhythmias.9 However, the precise regulation of mitochondrial ROS production under physiological and pathological conditions, especially in intact cells, is incompletely understood.
Regulation of Mitochondrial ROS Production
In studies on isolated mitochondria, electrons leak from complexes I and III of the ETC to O2, producing •O2−. A greater reduction of these complexes (for example, when NADH is highly reduced in the absence of ADP at low rates of respiration [“state 4”]) increases the probability of electron leak and ROS production.2,35 Initiation of NADH oxidation by the addition of ADP (“state 3” respiration) or partial uncoupling of mitochondria (eg, with chemical protonophores) can decrease ROS production.36,37 According to this model, one might expect that an increase in work (and thus, oxygen consumption) would favor a decrease of ROS levels.2 However, this model of ROS production is largely based on the behavior of isolated mitochondria under specific in vitro conditions, including the use of inhibitors of the ETC, which may not reflect the physiological situation in intact cells. Within the cellular lattice of intact myocytes, close association of mitochondria with Ca2+ stores and ATP-consuming sites (so-called microdomains) plays a key role in Ca2+- and ADP-mediated regulation of mitochondrial function.38,39 Furthermore, because the heart never stops beating, there is always a certain level of ADP-induced respiration, and thus, pure state 4 respiration never occurs.14 Therefore, in the present study, we analyzed ROS production under more physiological conditions (ie, during state 3 respiration), with mitochondria spatially and functionally integrated into their native environment.
Using a novel technique in which patch clamping was combined with fluorescence microscopy in working myocytes, we observed that initiation of work (imposed by EC coupling) increased mitochondrial ROS production. Although this is in contrast to what would be expected from results on isolated mitochondria,2,35–37 it is in line with a report on intact cardiac myocytes, in which an increase of the stimulation frequency potentiated intracellular ROS production.40 Furthermore, we observed an inverse relation between the redox state of NAD(P)H and mitochondrial net formation of H2O2 (Figure 4F). Again, this is in contrast to results from isolated mitochondria, in which under state 4 conditions, the redox state of NAD(P)H correlated positively with ROS formation,37 but it is in line with our previous observations in quiescent cardiac myocytes, where rapid dissipations of the mitochondrial membrane potential (ΔΨm) resulted in oxidation of NAD(P)H and increased ROS production.27 In the latter study, the increase in ROS production was related to acceleration of electron flow through the ETC and thus an increased turnover rate in the Q cycle of complex III, a major source of •O2− in mitochondria.27 Accordingly, in the present study, ROS production was accelerated when the ETC was uncoupled with FCCP [carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone], which results in increased electron flux along the ETC until NADH is completely consumed, but it was reduced when electron flux was blocked by cyanide (online-only Data Supplement Figure III).
One major conclusion derived from the present experiments is that in working myocytes, ROS formation not only depends on the rate of electron flux along the ETC but is also dynamically regulated by the redox state of the mitochondrial matrix. Although the amplitude of [Ca2+]c transients (which correlates with the amount of work and thus electron flux along the ETC14) was comparable 1 and 4 minutes after isoproterenol application (ie, at minutes 6 and 9 of the protocol in Figure 3B), net H2O2 formation was substantially higher at the earlier time point, when NAD(P)H was more oxidized (Figure 3G). Because the elimination of H2O2 is governed by peroxiredoxin and glutathione peroxidase, which require electrons from reduced NAD(P)H and glutathione, respectively (Figure 1),25 these results suggest that fluctuations in the NAD(P)H/NAD(P)+ redox state translate into variations in the antioxidative capacity of the matrix and thus net H2O2 formation. These conclusions are supported by our previous observations in quiescent cardiac myocytes, in which NAD(P)H correlated positively with the glutathione redox state but inversely with ROS formation.26 Inhibition of the nicotinamide nucleotide transhydrogenase or glutathione reductase, 2 key enzymes that mediate electron and proton transfer from NADH to NADPH and from NADPH to glutathione, respectively (Figure 1), potentiated the formation of H2O2.26 Also in isolated mitochondria, the glutathione redox state correlated closely with net H2O2 formation.41
After the initial oxidation of NAD(P)H to NAD(P)+, the subsequent recovery of NAD(P)H was causally linked to progressive mitochondrial Ca2+ accumulation, because inhibition of the MCU blunted this recovery. In line with the inverse correlation between NAD(P)H and H2O2, blocking mitochondrial Ca2+ uptake potentiated net H2O2 formation. These data indicate that mitochondrial Ca2+ uptake is not only important for matching energy supply and demand but also for the recovery of the antioxidative capacity of the mitochondrial matrix to control net formation of H2O2. The close link between mitochondrial Ca2+ uptake and H2O2 formation is readily explained by the observations that (1) mitochondrial Ca2+ uptake accelerates the turnover rate of the Krebs cycle by stimulating rate-limiting dehydrogenases,15–17 and (2) the regeneration of antioxidative enzymes depends on NADPH, which in turn is regenerated by substrates derived from the Krebs cycle (Figure 1).25,26
Regulation of H2O2 by [Na+]i
In heart failure, [Na+]i is elevated,20–22 which favors mitochondrial Ca2+ export via the mNCE, reduces steady state [Ca2+]m, and oxidizes NAD(P)H.17,24 In the present study, ROS formation was increased substantially in failing compared with nonfailing myocytes. Of note, this difference occurred only in working but not quiescent myocytes. An inhibitor of the mNCE, which blocks Na+-induced Ca2+ exportation and thus potentiates mitochondrial Ca2+ accumulation in failing and nonfailing myocytes,17,24 prevented increased ROS formation. Moreover, sole elevation of [Na+]i in normal myocytes per se potentiated ROS formation, whereas lowering [Na+]i in failing myocytes prevented it. Thus, it can be concluded that elevated [Na+]i and deficient mitochondrial Ca2+ uptake contribute to increased ROS formation in failing myocytes.
Besides the difference in [Na+]i, other factors may contribute to reduced mitochondrial Ca2+ uptake or increased ROS formation in failing myocytes. In a recent patch-clamp study on isolated mitochondria, the activity of mitochondrial Ca2+ channels (termed mCa1 and mCa2) was decreased in mitochondria from failing compared with nonfailing human myocardium.42 Furthermore, ΔΨm, the driving force for mitochondrial Ca2+ uptake via the MCU (or mCa1/mCa2,42 respectively), was decreased in mitochondria from failing hearts.43 All of these changes in concert would predict deficient mitochondrial Ca2+ uptake in heart failure and, according to the present results, could potentiate mitochondrial ROS production.
Pathophysiological Implications and Conclusions
H2O2 activates the late Na+ current (INa) and increases [Na+]i in cardiac myocytes.44 One potential mechanism for this activation is that Ca2+/calmodulin kinase II is activated by ROS-induced methionine oxidation,10 and Ca2+/calmodulin kinase II interacts with the Na+ channel, increasing late INa and [Na+]i.45 Thus, in heart failure, a vicious circle of elevated [Na+]i and oxidative stress may be established. Together with ROS-induced inhibition of sarcoplasmic reticulum Ca2+ ATPase,6 activation of ryanodine receptors,7 and the sarcolemmal Na+/Ca2+ exchanger (in its reverse mode),46 this vicious circle may sustain defects of EC coupling typically observed in heart failure.
In conclusion, we have identified a previously unrecognized role of mitochondrial Ca2+ uptake for the control of mitochondrial H2O2 levels and revealed a pathophysiological mechanism by which elevated [Na+]i increases mitochondrial H2O2 in myocytes (Figure 1). Given that in addition to their negative effects on EC coupling and energetics,6,7,10,27,44–46 mitochondrial ROS trigger apoptosis12 and cardiac arrhythmias,9 and given that oxidative stress has been linked to cardiac remodeling by inducing hypertrophic growth through activation of MAP kinases,1 Ca2+/calmodulin kinase II,10 and histone-deacetylase 4,11 therapeutic strategies aimed at correcting [Na+]i or mitochondrial Ca2+ uptake in heart failure may be beneficial via a reduction in oxidative stress. Finally, this mechanism may also be of relevance for ischemia/ reperfusion, during which a massive [Na+]i overload occurs23 and oxidative stress and cell death develop.
CLINICAL PERSPECTIVE.
Oxidative stress plays a fundamental role in cardiovascular diseases and aging. In patients with heart failure, oxidative stress is causally linked to the progression of the disease, and mitochondria were identified as critical sources of reactive oxygen species (ROS) in the heart. ROS impair cardiac contractility, cause arrhythmias, and contribute to cardiac remodeling by inducing hypertrophy, apoptosis, and necrosis. The precise mechanisms that regulate mitochondrial ROS formation, however, are incompletely understood. Here, we identified a mechanism by which an elevation of cytosolic sodium ([Na+]i), as occurs in failing cardiac myocytes, increases mitochondrial ROS formation. A key role is played by the Krebs cycle, which produces NADH, the main electron donor for ATP production at the respiratory chain. A less appreciated role of the Krebs cycle, however, is to indirectly support the regeneration of NADPH, which serves to maintain the antioxidative capacity of the mitochondrial matrix. During transitions of workload (eg, during β-adrenergic stimulation), mitochondrial Ca2+ uptake activates rate-controlling enzymes of the Krebs cycle to adapt NADH production to an increased energetic demand. The elevated [Na+]i in failing myocytes induces Ca2+ exportation from mitochondria via an Na+/Ca2+ exchanger, which hampers regeneration of NADH and NADPH, resulting in energetic mismatch and oxidative stress. The effects of cytosolic Na+ on mitochondrial function described in the present study indicate that this mechanism could play a role in the toxicity of cardiac glycosides (which further increase [Na+]i) and suggest a potential therapeutic application for drugs that lower [Na+]i during the progression of heart failure.
Supplementary Material
Supplemental Methods
Cell isolation
Adult female guinea-pigs (Hartley; 200-250 g; Charles River, Germany) were anesthetized with pentobarbital (~350 mg/kg BW i.p.), and after thoracotomy, the heart was removed, the aorta cannulated and mounted on a Langendorff perfusion apparatus. Retrograde perfusion was started with a Ca2+-buffered Normal Tyrode's (NT) solution containing (in mmol/L) NaCl 130, KCl 5, MgCl2 1, Na-HEPES 10, glucose 10, Na-pyruvate 2, ascorbic acid 0.3 and Na-EGTA 0.05, pH 7.4., for 5 min at 37°C and a flow rate of 8.4 ml/min. For enzymatic digestion, the heart was perfused with the same NT solution lacking EGTA, but containing 100 μmol/L CaCl2, collagenase type II (136 U/ml; Worthington, USA) and protease (0.4 U/ml; Sigma, Germany) for 6 min. Finally, the heart was perfused with a “High [K+]” solution containing (in mmol/L) K-glutamate 120, KCl 25, MgCl2 1, HEPES 10, Na-EGTA 0.05, pyruvate 2 and ascorbic acid 0.3, pH 7.4, for 5 min. The ventricles were minced carefully in High [K+] solution and filtered into a Falcon tube. After 15 min, the pellet was resuspended and stored in Dulbecco's Modification of Eagle's Medium (DMEM; Invitrogen, Germany) supplemented with 5% fetal bovine serum (FBS; PAN, Germany), 1% penicillin-streptomycin (Invitrogen), and 15 mmol/L HEPES (Invitrogen), pH 7.4, in a 5% CO2 incubator at 37°C.
Whole-cell patch-clamp and fluorescence measurements in cardiac myocytes
Simultaneous measurements of [Ca2+]c and [Ca2+]m
To simultaneously measure [Ca2+]c and [Ca2+]m in cardiac myocytes, we combined the patch-clamp technique with dual (alternating) excitation of 2 different fluorescent probes as described previously.1 Myocytes were loaded with the cell-permeable Ca2+-indicator rhod-2 acetoxymethyl esther (rhod-2 AM, 3 μmol/L; Invitrogen; dissolved in DMSO +10% pluronic acid) in DMEM for 1h at 37°C, and then washed for 1h in rhod-2-free DMEM to allow deesterification. Due to its positive charge, rhod-2 accumulates primarily in the mitochondrial matrix.1-3 Rhod-2 was excited at λexc=540 nm, and fluorescence (F) recorded at λem=605 nm. Fluorescence was normalized to F at time=0 of the respective experiment (F/F0). To eliminate cytosolic traces of rhod-2 that may contaminate the mitochondrial signal, we patch-clamped myocytes in the whole-cell configuration (pipette resistances, 2-4 MΩ) and equilibrated the cytosol for 6-8 min with rhod-2-free pipette solution containing (in mmol/L) K-glutamate 130, KCl 19, MgCl2 0.5, Na-HEPES 5, HEPES 10, Mg-ATP 5, pH 7.2, resulting in [Na+]i = 5 mmol/L. In experiments with [Na+]i = 15 mmol/L, the pipette solution contained 15 mmol/L Na-HEPES and no HEPES free acid. To measure [Ca2+]c together with [Ca2+]m, pipette solution contained cell-impermeable indo-1 penta-K+ salt (75 μmol/L; Molecular Probes). Indo-1 was excited at λexc=360 nm, and emission collected at λem1= 405 nm and λem2= 485 nm. Cellular autofluorescence was recorded before rupturing the cell-attached patch and subtracted before determining R (ratio of emission at 405 over 485 nm). [Ca2+]c was calculated according to the equation [Ca2+]c = Kd×β×[(R−Rmin)/(Rmax − R)],4 using a Kd of 844 nmol/L,5 and experimentally determined Rmin=0.27, Rmax=2.02, and β=2.14. We have previously established this method and verified specific compartmentalization of rhod-2 and indo-1 to mitochondrial and cytosolic compartments, respectively, in various control experiments.1
Myocytes were placed on cover slips that were mounted in a heated recording chamber (37°C; Warner Instruments, USA) on the stage of an epifluorescence microscope (Nikon TE2000) and superfused with NT solution (composition see above, except lacking EGTA, but containing 2 mmol/L CaCl2). Currents were recorded in whole-cell voltage clamp mode (EPC-10 amplifier, Patchmaster software, HEKA Elektronik, Lambrecht/Pfalz, Germany) with 2-4 MΩ pipettes, to give typical total series resistances of <10 MΩ. Electrophysiological signals were acquired, stored and analyzed using Fitmaster software (HEKA, Germany).
After rupturing the cell-attached patch, myocytes were equilibrated with pipette solution for >6 min (Supplementary Figure S1C,D). Prior to the start of the experiment, the holding potential (EH) was polarized in 10 mV steps from 0 mV to -80 mV over ~1 min. For the actual protocol, cells were depolarized from -80 mV to +10 mV for 80 ms at a frequency of 3 Hz for 30 min. We determined the resulting currents at three time-points: before (Idias), 10-12 ms after depolarization (Iearly) and in the last 5 ms of the pulse (=75 to 80 ms after depolarization; Ilate; Supplementary Figure S2B). Since we used a protocol in which no currents were intentionally blocked (i.e., by inhibitors or voltage steps), and thus, the early inward current (Figure 1A, Supplementary Figure S2B) was mostly related to the current of voltage-gated Na+-channels (INa), we calculated the difference between Ilate and Iearly as a rough estimate of ICa,L, where Iearly was measured at a time-point when INa was mostly inactivated. The estimation of ICa,L served to control for isoproterenol-induced activation of ICa,L (especially in the CM-DCF/NAD(P)H protocol, where no [Ca2+]c was measured; see below) rather than to absolutely quantify ICa,L.
For fluorescence measurements, a mercury based lamp (EXFO X-CITE 120, Exfo Life Sciences, Mississauga, Ontario, Canada) was used as a light source. Excitation filters were rapidly switched by a filter wheel (LB-10-BS; Sutter Instruments, USA). Emitted fluorescence was recorded by a custom-built 3-fold photomultiplier system (3×PTI, Model 814; Photon Technology International, Birmingham, NJ, USA). Customized polychroic filters (Chroma Technology Corp., Rockingham, VT, USA) were used to separate the 2 excitation- (360 nm, 540 nm) from the 3 emission wavelengths (405, 485 and 605 nm).
Simultaneous measurements of NAD(P)H and reactive oxygen species (ROS)
To monitor NAD(P)H together with the formation of H2O2, cardiac myocytes were loaded with 5-(-6)-chloromethyl-2',7'-dichlorohydrofluorescein diacetate (CM-H2DCFDA). The acetate group of CM-H2DCFDA is hydrolyzed by esterases when it enters the cell and is trapped inside as the nonfluorescent 5-(-6)-chloromethyl-2',7'-dichlorohydrofluorescein (CM-H2DCFH). CM-H2DCFH is well retained in cells, where it locates primarily to the mitochondrial matrix.6 Oxidation of CM-H2DCFH particularly by H2O2, but also hydroxyl radicals, yields the fluorescent product CM-DCF.7 CM-DCF was excited at λexc=485 nm, and fluorescence was recorded at λem=525 nm. The fluorescence of CM-DCF was measured together with the autofluorescence of endogenous NAD(P)H, which derives primarily from mitochondria.6 NAD(P)H was excited at λexc=360 nm, and fluorescence was recorded at λem=450 nm.
We noted that the fluorescence of CM-DCF (FDCF) was variable at baseline (Supplementary Fig. S1A). This may be related to different loading efficiencies of the dye, but also to the fact that CM-H2DCFH is irreversibly oxidized by H2O2. Thus, since already before the onset of the experiment, endogenous H2O2 may be formed, CM-H2DCFH was already oxidized to CM-DCF by different degrees that should depend on i) the amount of basal endogenous ROS and ii) the time between completion of loading and the onset of the experiment. Therefore, we used cells within 2 hours of DCF-loading and carefully analyzed the baseline FDCF of every cell before attempting to patch it. We selected cells that were notably loaded with CM-DCF (usually with a minimum F>200 mV as the PMT output), but not oxidized to a higher degree yet (e.g., F<1000 mV), with an average FDCF of 604±85 mV (n=56), which resembled the mean F of all cells screened (n=854; Supplementary Fig. S1E). After rupturing the cell membrane and establishing a stable access with the pipette (indicated by stable currents induced by the test pulse; Supplementary Fig. S1C,D), the cytosol was equilibrated with dye-free pipette solution (composition as indicated above, but lacking indo-1) for 6 min. During this time, FDCF decayed by 11±2% over 6 min (Supplementary Fig. S1F). Since CM-H2DCFH locates primarily to the mitochondrial matrix,6 it can be assumed that by cell dialysis, cytosolic traces of CM-DCF were eliminated by this technique. It is of note that only in cells with considerable FDCF (on average, 692±100 mV), an exponential decay of FDCF was observed with a τ-value of 3.0±0.4 min, whereas in cells with lower FDCF (239±67 mV), no detectable decay was observed (Supplementary Fig. S1G, H).
Voltage clamping was performed as described above, except that during the CM-DCF/NAD(P)H protocol, in the initial 2 min of the protocol, the cells were held at -80 mV, and then depolarized to +10 mV for 80 ms at a frequency of 3 Hz for 15 min (Supplementary Fig. 2). After 3 min of pacing, the β-adrenergic agonist isoproterenol was washed-in (at 10 nmol/L for 2 min and 100 nmol/L for 10 min; Supplementary Fig. 2A). After a total time of 17 min, pacing was abruptly stopped and cells held at EH= -80 mV. After 45s, FCCP (5 μmol/L) was washed in for ~1 min to uncouple the ETC and thus, obtain complete oxidation of NAD(P)H to NAD(P)+ (Fmin; =0% reduced NAD(P)H; Supplementary Fig. S2C; see also ref1). Subsequently, Na-cyanide (4 mmol/L) was used to block complex IV of the ETC and thus, induce maximal reduction of NAD(P)H (Fmax; =100% reduced NAD(P)H). It is of note that during Na-cyanide, a large but reversible inward current occurred (at -80 mV; Supplementary Fig. S2A) which may be related to a shift of the ATP/ADP ratio and thus, opening of ATP-dependent K+-channels in the cell membrane (IKATP). All NAD(P)H fluorescence values during the experiment were related to their individual Fmax and Fmin values, and the NAD(P)H/NAD(P)+ redox state expressed as % reduced NAD(P)H. After wash-out of Na-cyanide, the NAD(P)H/NAD(P)+ redox state slightly oxidized towards its initial values. 1.5-2 min after Na-cyanide wash-out, H2O2 (10 mmol/L) was washed in to verify the specificity of FDCF. Only cells that showed a considerable increase of FDCF in response to exogenous H2O2 were used for analysis of FDCF. All cells were still intact and alive at the end of this protocol.
Heart failure model and functional evaluation
Adult male Hartley guinea-pigs (250-300 g) were anesthetized and intubated. Aortic banding was produced by tying a suture around the ascending aorta using an 18-gauge needle as a spacer, which was then removed. When animals were breathing spontaneously after the procedure, buprenex (0.05mg/kg) was administered for analgesia and animals were observed until full recovery.
Field stimulation in failing and nonfailing myocytes
To monitor the formation of H2O2 in failing myocytes during increased work, cells were loaded with CM-H2DCFDA and placed on cover slips that were mounted on a heated field-stimulation chamber (37°C; Warner Instruments, USA). CM-DCF fluorescence was recorded for 1 min without stimulation in NT solution (containing 2 mmol/L CaCl2), and then for 1 min in the presence of isoproterenol, with or without 1 μmol/L CGP-37157. After these first 2 min, cells were field-stimulated at 4 Hz for 3 min, and then monitored for 2 min after stopping stimulation. Cardiac myocytes from age and sex matched animals were used as control cells.
Figure S1: Experimental protocol for simultaneous determination of NAD(P)H and H2O2. Cardiac myocytes were loaded with the H2O2-sensitive fluorescent dye CM-H2DCFDA, and fluorescence of CM-DCF (FDCF) was monitored together with autofluorescence of NAD(P)H (FNAD(P)H) in the same cells, respectively. A total of n=854 cells was screened and their fluorescence collected. A, distribution of baseline FDCF and FNAD(P)H in these 854 cells, respectively. B, FDCF plotted against FNAD(P)H in all cells. Of n=854 cells screened, n=56 cells were patched (voltage-clamp) and equilibrated for 6 min with a dye-free pipette solution. During these 6 min, cells were held at 0 mV and depolarized to +10 mV at 0.25 Hz. Original test-pulse (C) and the averaged currents (n=56) at pre-pulse, peak and during the pulse (D), as indicated in C, over the 6 min equilibration phase. E, Averaged baseline FDCF of screened and patched cells, respectively. F, FDCF in all patched cells during the 6 min equilibration phase. G and H, FDCF at baseline (G) and during the 6 min equilibration phase in cells in which a decay of fluorescence was observed, compared to cells where no decay occurred.
Figure S2: Calibration of mitochondrial NAD(P)H and DCF fluorescence in guinea-pig cardiac myocytes. Cardiac myocytes were loaded with CM-H2DCFDA, and the fluorescence of CM-DCF was collected together with the autofluorescence of NAD(P)H as described in Figure S1. The cells were held at -80 mV for 2 min, and then depolarized in voltage-clamp mode from -80 to +10 mV for 80 ms (B) at 3 Hz for 15 min. After 3 min of pacing, isoproterenol was washed in at 10 nmol/L for 2 min, and then at 100 nmol/L for 10 min. After 15 min of pacing (at t=17 min), depolarization was stopped and cells held at -80 mV. After 45 s, FCCP (5 μmol/L) was washed in for ~1 min (in the absence of isoproterenol), and then Na-cyanide (CN, 4 mmol/L) for ~1.5 min. After this, cells were superfused with drug-free control solution for ~1.5-2 min, before H2O2 (10 mmol/L) was washed in. The given time points varied slightly depending on the individual response of the myocytes to FCCP and CN, respectively. A, Idias, ICa,L and Ilate, as indicated in the original trace in B, over the course of the experiment. ICa,L was estimated as the difference between Ilate and Iearly. Note the consistent, reversible inward current at -80 mV during the exposure to CN, and an outward current during the exposure to H2O2. C, Fluorescence of H2-DCF and autofluorescence of NAD(P)H during the experiments. Maximal oxidation of NAD(P)H by FCCP was considered as 0% reduced NAD(P)H, whereas maximal reduction of NAD(P)H by CN was defined as 100% reduced NAD(P)H in all individual cells. Since the time points at which these values were reached varied slightly between cells, the average data for NAD(P)H do not completely span from 0 to 100%.
Figure S3: Pronounced net formation of H2O2 during complete NAD(P)H oxidation. a, Net formation of H2O2 and the redox state of NAD(P)H/NADP+ in all myocytes taken from Figure S2 (n=16) during the application of FCCP (5 μmol/L) and Na-cyanide (CN, 4 mmol/L), respectively. During this time of the protocol, myocytes were held at -80 mV in voltage-clamp mode and were not depolarized. The arrow indicates the time at which depolarization to +10 mV at 3 Hz was abruptly stopped (note the typical overshoot of NAD(P)H after cessation of stimulation8, 9). b, Similar plot as in Figure 3G of the manuscript, except that the 2 data points for the conditions in the presence of FCCP or CN, respectively, were added. The values were calculated as the averages of values during the indicated time-frames for FCCP and CN, respectively. It can be observed that the rate of H2O2 formation increases when NAD(P)H is oxidized by FCCP. When NADH is completely consumed, H2O2 formation decreases again, since no more electrons are available for the ETC, substantially limiting electron flux. When NADH is reduced in the presence of cyanide, H2O2 formation slightly increases (despite the lack of electron flow along the ETC), potentially due to reduction of ETC complexes and thus, slippage of electrons to O2 (as observed in isolated mitochondria in state 4 conditions10-13).
Supplemental References
1. Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O'Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006;99(2):172-182.
2. Pacher P, Csordas P, Schneider T, Hajnoczky G. Quantification of calcium signal transmission from sarco-endoplasmic reticulum to the mitochondria. J Physiol. 2000;529 Pt 3:553-564.
3. Pacher P, Thomas AP, Hajnoczky G. Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc Natl Acad Sci U S A. 2002;99(4):2380-2385.
4. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260(6):3440-3450.
5. Bassani JW, Bassani RA, Bers DM. Calibration of indo-1 and resting intracellular [Ca]i in intact rabbit cardiac myocytes. Biophys J. 1995;68(4):1453-1460.
6. Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278(45):44735-44744.
7. Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol. 1997;29(9):2571-2583.
8. Brandes R, Bers DM. Intracellular Ca2+ increases the mitochondrial NADH concentration during elevated work in intact cardiac muscle. Circ Res. 1997;80(1):82-87.
9. Cortassa S, Aon MA, Marban E, Winslow RL, O'Rourke B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys J. 2003;84(4):2734-2755.
10. Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416(1):15-18.
11. Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem. 2003;86(5):1101-1107.
12. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(Pt 2):335-344.
13. Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483-495.
Acknowledgments
We thank Michelle Gulentz and Angela Zimmer for technical assistance, Stefan Gräber for statistical consultation, and Sonia Cortassa, Miguel Aon, and Ulrich Laufs for discussion of the manuscript.
Sources of Funding
The study was supported by the Emmy Noether Program and the Klinische Forschergruppe KFO-196 by the Deutsche Forschungsgemeinschaft (to Dr Maack). Dr O'Rourke and Dr Liu are supported by the National Institutes of Health (P01-HL081427).
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.109.914911/DC1.
Disclosures
None.
References
- 1.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura R, Egashira K, Machida Y, Hayashidani S, Takeya M, Utsumi H, Tsutsui H, Takeshita A. Probucol attenuates left ventricular dysfunction and remodeling in tachycardia-induced heart failure: roles of oxidative stress and inflammation. Circulation. 2002;106:362–367. doi: 10.1161/01.cir.0000021430.04195.51. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 5.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 6.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 7.Yan Y, Liu J, Wei C, Li K, Xie W, Wang Y, Cheng H. Bidirectional regulation of Ca2+ sparks by mitochondria-derived reactive oxygen species in cardiac myocytes. Cardiovasc Res. 2008;77:432–441. doi: 10.1093/cvr/cvm047. [DOI] [PubMed] [Google Scholar]
- 8.Maack C, Dabew ER, Hohl M, Schäfers HJ, Böhm M. Endogenous activation of mitochondrial KATP channels protects human failing myocardium from hydroxyl radical-induced stunning. Circ Res. 2009;105:811–817. doi: 10.1161/CIRCRESAHA.109.206359. [DOI] [PubMed] [Google Scholar]
- 9.Akar FG, Aon MA, Tomaselli GF, O'Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005;115:3527–3535. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, Vatner SF, Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 12.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Halestrap A. Biochemistry: a pore way to die. Nature. 2005;434:578–579. doi: 10.1038/434578a. [DOI] [PubMed] [Google Scholar]
- 14.Balaban RS. Domestication of the cardiac mitochondrion for energy conversion. J Mol Cell Cardiol. 2009;46:832–841. doi: 10.1016/j.yjmcc.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandes R, Bers DM. Intracellular Ca2+ increases the mitochondrial NADH concentration during elevated work in intact cardiac muscle. Circ Res. 1997;80:82–87. doi: 10.1161/01.res.80.1.82. [DOI] [PubMed] [Google Scholar]
- 16.Maack C, O'Rourke B. Excitation-contraction coupling and mitochondrial energetics. Basic Res Cardiol. 2007;102:369–392. doi: 10.1007/s00395-007-0666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O'Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006;99:172–182. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houser SR, Margulies KB. Is depressed myocyte contractility centrally involved in heart failure? Circ Res. 2003;92:350–358. doi: 10.1161/01.RES.0000060027.40275.A6. [DOI] [PubMed] [Google Scholar]
- 19.Bers DM. Altered cardiac myocyte ca regulation in heart failure. Physiology (Bethesda) 2006;21:380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- 20.Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na+ concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation. 2002;105:2543–2548. doi: 10.1161/01.cir.0000016701.85760.97. [DOI] [PubMed] [Google Scholar]
- 21.Pieske B, Maier LS, Piacentino V, III, Weisser J, Hasenfuss G, Houser S. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation. 2002;106:447–453. doi: 10.1161/01.cir.0000023042.50192.f4. [DOI] [PubMed] [Google Scholar]
- 22.Baartscheer A, Schumacher CA, Belterman CN, Coronel R, Fiolet JW. [Na+]i and the driving force of the Na+/Ca2+-exchanger in heart failure. Cardiovasc Res. 2003;57:986–995. doi: 10.1016/s0008-6363(02)00848-9. [DOI] [PubMed] [Google Scholar]
- 23.Murphy E, Eisner DA. Regulation of intracellular and mitochondrial sodium in health and disease. Circ Res. 2009;104:292–303. doi: 10.1161/CIRCRESAHA.108.189050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T, O'Rourke B. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ Res. 2008;103:279–288. doi: 10.1161/CIRCRESAHA.108.175919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 26.Aon MA, Cortassa S, Maack C, O'Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem. 2007;282:21889–21900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 28.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 29.Cox DA, Matlib MA. A role for the mitochondrial Na+-Ca2+ exchanger in the regulation of oxidative phosphorylation in isolated heart mitochondria. J Biol Chem. 1993;268:938–947. [PubMed] [Google Scholar]
- 30.Belch JJ, Bridges AB, Scott N, Chopra M. Oxygen free radicals and congestive heart failure. Br Heart J. 1991;65:245–248. doi: 10.1136/hrt.65.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maack C, Kartes T, Kilter H, Schäfers HJ, Nickenig G, Böhm M, Laufs U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation. 2003;108:1567–1574. doi: 10.1161/01.CIR.0000091084.46500.BB. [DOI] [PubMed] [Google Scholar]
- 32.Valgimigli M, Merli E, Malagutti P, Soukhomovskaia O, Cicchitelli G, Antelli A, Canistro D, Francolini G, Macri G, Mastrorilli F, Paolini M, Ferrari R. Hydroxyl radical generation, levels of tumor necrosis factor-alpha, and progression to heart failure after acute myocardial infarction. J Am Coll Cardiol. 2004;43:2000–2008. doi: 10.1016/j.jacc.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 33.Takemoto M, Node K, Nakagami H, Liao Y, Grimm M, Takemoto Y, Kitakaze M, Liao JK. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest. 2001;108:1429–1437. doi: 10.1172/JCI13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 35.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 37.Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem. 2003;86:1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- 38.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 39.Saks V, Dzeja P, Schlattner U, Vendelin M, Terzic A, Wallimann T. Cardiac system bioenergetics: metabolic basis of the Frank-Starling law. J Physiol. 2006;571:253–273. doi: 10.1113/jphysiol.2005.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heinzel FR, Luo Y, Dodoni G, Boengler K, Petrat F, Di Lisa F, de Groot H, Schulz R, Heusch G. Formation of reactive oxygen species at increased contraction frequency in rat cardiomyocytes. Cardiovasc Res. 2006;71:374–382. doi: 10.1016/j.cardiores.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Han D, Canali R, Rettori D, Kaplowitz N. Effect of glutathione depletion on sites and topology of superoxide and hydrogen peroxide production in mitochondria. Mol Pharmacol. 2003;64:1136–1144. doi: 10.1124/mol.64.5.1136. [DOI] [PubMed] [Google Scholar]
- 42.Michels G, Khan IF, Endres-Becker J, Rottlaender D, Herzig S, Ruhparwar A, Wahlers T, Hoppe UC. Regulation of the human cardiac mitochondrial Ca2+ uptake by 2 different voltage-gated Ca2+ channels. Circulation. 2009;119:2435–2443. doi: 10.1161/CIRCULATIONAHA.108.835389. [DOI] [PubMed] [Google Scholar]
- 43.Lin L, Sharma VK, Sheu SS. Mechanisms of reduced mitochondrial Ca2+ accumulation in failing hamster heart. Pflugers Arch. 2007;454:395–402. doi: 10.1007/s00424-007-0257-8. [DOI] [PubMed] [Google Scholar]
- 44.Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 45.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeitz O, Maass AE, Van Nguyen P, Hensmann G, Kögler H, Möller K, Hasenfuss G, Janssen PM. Hydroxyl radical-induced acute diastolic dysfunction is due to calcium overload via reverse-mode Na+-Ca2+ exchange. Circ Res. 2002;90:988–995. doi: 10.1161/01.res.0000018625.25212.1e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Methods
Cell isolation
Adult female guinea-pigs (Hartley; 200-250 g; Charles River, Germany) were anesthetized with pentobarbital (~350 mg/kg BW i.p.), and after thoracotomy, the heart was removed, the aorta cannulated and mounted on a Langendorff perfusion apparatus. Retrograde perfusion was started with a Ca2+-buffered Normal Tyrode's (NT) solution containing (in mmol/L) NaCl 130, KCl 5, MgCl2 1, Na-HEPES 10, glucose 10, Na-pyruvate 2, ascorbic acid 0.3 and Na-EGTA 0.05, pH 7.4., for 5 min at 37°C and a flow rate of 8.4 ml/min. For enzymatic digestion, the heart was perfused with the same NT solution lacking EGTA, but containing 100 μmol/L CaCl2, collagenase type II (136 U/ml; Worthington, USA) and protease (0.4 U/ml; Sigma, Germany) for 6 min. Finally, the heart was perfused with a “High [K+]” solution containing (in mmol/L) K-glutamate 120, KCl 25, MgCl2 1, HEPES 10, Na-EGTA 0.05, pyruvate 2 and ascorbic acid 0.3, pH 7.4, for 5 min. The ventricles were minced carefully in High [K+] solution and filtered into a Falcon tube. After 15 min, the pellet was resuspended and stored in Dulbecco's Modification of Eagle's Medium (DMEM; Invitrogen, Germany) supplemented with 5% fetal bovine serum (FBS; PAN, Germany), 1% penicillin-streptomycin (Invitrogen), and 15 mmol/L HEPES (Invitrogen), pH 7.4, in a 5% CO2 incubator at 37°C.
Whole-cell patch-clamp and fluorescence measurements in cardiac myocytes
Simultaneous measurements of [Ca2+]c and [Ca2+]m
To simultaneously measure [Ca2+]c and [Ca2+]m in cardiac myocytes, we combined the patch-clamp technique with dual (alternating) excitation of 2 different fluorescent probes as described previously.1 Myocytes were loaded with the cell-permeable Ca2+-indicator rhod-2 acetoxymethyl esther (rhod-2 AM, 3 μmol/L; Invitrogen; dissolved in DMSO +10% pluronic acid) in DMEM for 1h at 37°C, and then washed for 1h in rhod-2-free DMEM to allow deesterification. Due to its positive charge, rhod-2 accumulates primarily in the mitochondrial matrix.1-3 Rhod-2 was excited at λexc=540 nm, and fluorescence (F) recorded at λem=605 nm. Fluorescence was normalized to F at time=0 of the respective experiment (F/F0). To eliminate cytosolic traces of rhod-2 that may contaminate the mitochondrial signal, we patch-clamped myocytes in the whole-cell configuration (pipette resistances, 2-4 MΩ) and equilibrated the cytosol for 6-8 min with rhod-2-free pipette solution containing (in mmol/L) K-glutamate 130, KCl 19, MgCl2 0.5, Na-HEPES 5, HEPES 10, Mg-ATP 5, pH 7.2, resulting in [Na+]i = 5 mmol/L. In experiments with [Na+]i = 15 mmol/L, the pipette solution contained 15 mmol/L Na-HEPES and no HEPES free acid. To measure [Ca2+]c together with [Ca2+]m, pipette solution contained cell-impermeable indo-1 penta-K+ salt (75 μmol/L; Molecular Probes). Indo-1 was excited at λexc=360 nm, and emission collected at λem1= 405 nm and λem2= 485 nm. Cellular autofluorescence was recorded before rupturing the cell-attached patch and subtracted before determining R (ratio of emission at 405 over 485 nm). [Ca2+]c was calculated according to the equation [Ca2+]c = Kd×β×[(R−Rmin)/(Rmax − R)],4 using a Kd of 844 nmol/L,5 and experimentally determined Rmin=0.27, Rmax=2.02, and β=2.14. We have previously established this method and verified specific compartmentalization of rhod-2 and indo-1 to mitochondrial and cytosolic compartments, respectively, in various control experiments.1
Myocytes were placed on cover slips that were mounted in a heated recording chamber (37°C; Warner Instruments, USA) on the stage of an epifluorescence microscope (Nikon TE2000) and superfused with NT solution (composition see above, except lacking EGTA, but containing 2 mmol/L CaCl2). Currents were recorded in whole-cell voltage clamp mode (EPC-10 amplifier, Patchmaster software, HEKA Elektronik, Lambrecht/Pfalz, Germany) with 2-4 MΩ pipettes, to give typical total series resistances of <10 MΩ. Electrophysiological signals were acquired, stored and analyzed using Fitmaster software (HEKA, Germany).
After rupturing the cell-attached patch, myocytes were equilibrated with pipette solution for >6 min (Supplementary Figure S1C,D). Prior to the start of the experiment, the holding potential (EH) was polarized in 10 mV steps from 0 mV to -80 mV over ~1 min. For the actual protocol, cells were depolarized from -80 mV to +10 mV for 80 ms at a frequency of 3 Hz for 30 min. We determined the resulting currents at three time-points: before (Idias), 10-12 ms after depolarization (Iearly) and in the last 5 ms of the pulse (=75 to 80 ms after depolarization; Ilate; Supplementary Figure S2B). Since we used a protocol in which no currents were intentionally blocked (i.e., by inhibitors or voltage steps), and thus, the early inward current (Figure 1A, Supplementary Figure S2B) was mostly related to the current of voltage-gated Na+-channels (INa), we calculated the difference between Ilate and Iearly as a rough estimate of ICa,L, where Iearly was measured at a time-point when INa was mostly inactivated. The estimation of ICa,L served to control for isoproterenol-induced activation of ICa,L (especially in the CM-DCF/NAD(P)H protocol, where no [Ca2+]c was measured; see below) rather than to absolutely quantify ICa,L.
For fluorescence measurements, a mercury based lamp (EXFO X-CITE 120, Exfo Life Sciences, Mississauga, Ontario, Canada) was used as a light source. Excitation filters were rapidly switched by a filter wheel (LB-10-BS; Sutter Instruments, USA). Emitted fluorescence was recorded by a custom-built 3-fold photomultiplier system (3×PTI, Model 814; Photon Technology International, Birmingham, NJ, USA). Customized polychroic filters (Chroma Technology Corp., Rockingham, VT, USA) were used to separate the 2 excitation- (360 nm, 540 nm) from the 3 emission wavelengths (405, 485 and 605 nm).
Simultaneous measurements of NAD(P)H and reactive oxygen species (ROS)
To monitor NAD(P)H together with the formation of H2O2, cardiac myocytes were loaded with 5-(-6)-chloromethyl-2',7'-dichlorohydrofluorescein diacetate (CM-H2DCFDA). The acetate group of CM-H2DCFDA is hydrolyzed by esterases when it enters the cell and is trapped inside as the nonfluorescent 5-(-6)-chloromethyl-2',7'-dichlorohydrofluorescein (CM-H2DCFH). CM-H2DCFH is well retained in cells, where it locates primarily to the mitochondrial matrix.6 Oxidation of CM-H2DCFH particularly by H2O2, but also hydroxyl radicals, yields the fluorescent product CM-DCF.7 CM-DCF was excited at λexc=485 nm, and fluorescence was recorded at λem=525 nm. The fluorescence of CM-DCF was measured together with the autofluorescence of endogenous NAD(P)H, which derives primarily from mitochondria.6 NAD(P)H was excited at λexc=360 nm, and fluorescence was recorded at λem=450 nm.
We noted that the fluorescence of CM-DCF (FDCF) was variable at baseline (Supplementary Fig. S1A). This may be related to different loading efficiencies of the dye, but also to the fact that CM-H2DCFH is irreversibly oxidized by H2O2. Thus, since already before the onset of the experiment, endogenous H2O2 may be formed, CM-H2DCFH was already oxidized to CM-DCF by different degrees that should depend on i) the amount of basal endogenous ROS and ii) the time between completion of loading and the onset of the experiment. Therefore, we used cells within 2 hours of DCF-loading and carefully analyzed the baseline FDCF of every cell before attempting to patch it. We selected cells that were notably loaded with CM-DCF (usually with a minimum F>200 mV as the PMT output), but not oxidized to a higher degree yet (e.g., F<1000 mV), with an average FDCF of 604±85 mV (n=56), which resembled the mean F of all cells screened (n=854; Supplementary Fig. S1E). After rupturing the cell membrane and establishing a stable access with the pipette (indicated by stable currents induced by the test pulse; Supplementary Fig. S1C,D), the cytosol was equilibrated with dye-free pipette solution (composition as indicated above, but lacking indo-1) for 6 min. During this time, FDCF decayed by 11±2% over 6 min (Supplementary Fig. S1F). Since CM-H2DCFH locates primarily to the mitochondrial matrix,6 it can be assumed that by cell dialysis, cytosolic traces of CM-DCF were eliminated by this technique. It is of note that only in cells with considerable FDCF (on average, 692±100 mV), an exponential decay of FDCF was observed with a τ-value of 3.0±0.4 min, whereas in cells with lower FDCF (239±67 mV), no detectable decay was observed (Supplementary Fig. S1G, H).
Voltage clamping was performed as described above, except that during the CM-DCF/NAD(P)H protocol, in the initial 2 min of the protocol, the cells were held at -80 mV, and then depolarized to +10 mV for 80 ms at a frequency of 3 Hz for 15 min (Supplementary Fig. 2). After 3 min of pacing, the β-adrenergic agonist isoproterenol was washed-in (at 10 nmol/L for 2 min and 100 nmol/L for 10 min; Supplementary Fig. 2A). After a total time of 17 min, pacing was abruptly stopped and cells held at EH= -80 mV. After 45s, FCCP (5 μmol/L) was washed in for ~1 min to uncouple the ETC and thus, obtain complete oxidation of NAD(P)H to NAD(P)+ (Fmin; =0% reduced NAD(P)H; Supplementary Fig. S2C; see also ref1). Subsequently, Na-cyanide (4 mmol/L) was used to block complex IV of the ETC and thus, induce maximal reduction of NAD(P)H (Fmax; =100% reduced NAD(P)H). It is of note that during Na-cyanide, a large but reversible inward current occurred (at -80 mV; Supplementary Fig. S2A) which may be related to a shift of the ATP/ADP ratio and thus, opening of ATP-dependent K+-channels in the cell membrane (IKATP). All NAD(P)H fluorescence values during the experiment were related to their individual Fmax and Fmin values, and the NAD(P)H/NAD(P)+ redox state expressed as % reduced NAD(P)H. After wash-out of Na-cyanide, the NAD(P)H/NAD(P)+ redox state slightly oxidized towards its initial values. 1.5-2 min after Na-cyanide wash-out, H2O2 (10 mmol/L) was washed in to verify the specificity of FDCF. Only cells that showed a considerable increase of FDCF in response to exogenous H2O2 were used for analysis of FDCF. All cells were still intact and alive at the end of this protocol.
Heart failure model and functional evaluation
Adult male Hartley guinea-pigs (250-300 g) were anesthetized and intubated. Aortic banding was produced by tying a suture around the ascending aorta using an 18-gauge needle as a spacer, which was then removed. When animals were breathing spontaneously after the procedure, buprenex (0.05mg/kg) was administered for analgesia and animals were observed until full recovery.
Field stimulation in failing and nonfailing myocytes
To monitor the formation of H2O2 in failing myocytes during increased work, cells were loaded with CM-H2DCFDA and placed on cover slips that were mounted on a heated field-stimulation chamber (37°C; Warner Instruments, USA). CM-DCF fluorescence was recorded for 1 min without stimulation in NT solution (containing 2 mmol/L CaCl2), and then for 1 min in the presence of isoproterenol, with or without 1 μmol/L CGP-37157. After these first 2 min, cells were field-stimulated at 4 Hz for 3 min, and then monitored for 2 min after stopping stimulation. Cardiac myocytes from age and sex matched animals were used as control cells.
Figure S1: Experimental protocol for simultaneous determination of NAD(P)H and H2O2. Cardiac myocytes were loaded with the H2O2-sensitive fluorescent dye CM-H2DCFDA, and fluorescence of CM-DCF (FDCF) was monitored together with autofluorescence of NAD(P)H (FNAD(P)H) in the same cells, respectively. A total of n=854 cells was screened and their fluorescence collected. A, distribution of baseline FDCF and FNAD(P)H in these 854 cells, respectively. B, FDCF plotted against FNAD(P)H in all cells. Of n=854 cells screened, n=56 cells were patched (voltage-clamp) and equilibrated for 6 min with a dye-free pipette solution. During these 6 min, cells were held at 0 mV and depolarized to +10 mV at 0.25 Hz. Original test-pulse (C) and the averaged currents (n=56) at pre-pulse, peak and during the pulse (D), as indicated in C, over the 6 min equilibration phase. E, Averaged baseline FDCF of screened and patched cells, respectively. F, FDCF in all patched cells during the 6 min equilibration phase. G and H, FDCF at baseline (G) and during the 6 min equilibration phase in cells in which a decay of fluorescence was observed, compared to cells where no decay occurred.
Figure S2: Calibration of mitochondrial NAD(P)H and DCF fluorescence in guinea-pig cardiac myocytes. Cardiac myocytes were loaded with CM-H2DCFDA, and the fluorescence of CM-DCF was collected together with the autofluorescence of NAD(P)H as described in Figure S1. The cells were held at -80 mV for 2 min, and then depolarized in voltage-clamp mode from -80 to +10 mV for 80 ms (B) at 3 Hz for 15 min. After 3 min of pacing, isoproterenol was washed in at 10 nmol/L for 2 min, and then at 100 nmol/L for 10 min. After 15 min of pacing (at t=17 min), depolarization was stopped and cells held at -80 mV. After 45 s, FCCP (5 μmol/L) was washed in for ~1 min (in the absence of isoproterenol), and then Na-cyanide (CN, 4 mmol/L) for ~1.5 min. After this, cells were superfused with drug-free control solution for ~1.5-2 min, before H2O2 (10 mmol/L) was washed in. The given time points varied slightly depending on the individual response of the myocytes to FCCP and CN, respectively. A, Idias, ICa,L and Ilate, as indicated in the original trace in B, over the course of the experiment. ICa,L was estimated as the difference between Ilate and Iearly. Note the consistent, reversible inward current at -80 mV during the exposure to CN, and an outward current during the exposure to H2O2. C, Fluorescence of H2-DCF and autofluorescence of NAD(P)H during the experiments. Maximal oxidation of NAD(P)H by FCCP was considered as 0% reduced NAD(P)H, whereas maximal reduction of NAD(P)H by CN was defined as 100% reduced NAD(P)H in all individual cells. Since the time points at which these values were reached varied slightly between cells, the average data for NAD(P)H do not completely span from 0 to 100%.
Figure S3: Pronounced net formation of H2O2 during complete NAD(P)H oxidation. a, Net formation of H2O2 and the redox state of NAD(P)H/NADP+ in all myocytes taken from Figure S2 (n=16) during the application of FCCP (5 μmol/L) and Na-cyanide (CN, 4 mmol/L), respectively. During this time of the protocol, myocytes were held at -80 mV in voltage-clamp mode and were not depolarized. The arrow indicates the time at which depolarization to +10 mV at 3 Hz was abruptly stopped (note the typical overshoot of NAD(P)H after cessation of stimulation8, 9). b, Similar plot as in Figure 3G of the manuscript, except that the 2 data points for the conditions in the presence of FCCP or CN, respectively, were added. The values were calculated as the averages of values during the indicated time-frames for FCCP and CN, respectively. It can be observed that the rate of H2O2 formation increases when NAD(P)H is oxidized by FCCP. When NADH is completely consumed, H2O2 formation decreases again, since no more electrons are available for the ETC, substantially limiting electron flux. When NADH is reduced in the presence of cyanide, H2O2 formation slightly increases (despite the lack of electron flow along the ETC), potentially due to reduction of ETC complexes and thus, slippage of electrons to O2 (as observed in isolated mitochondria in state 4 conditions10-13).
Supplemental References
1. Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O'Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006;99(2):172-182.
2. Pacher P, Csordas P, Schneider T, Hajnoczky G. Quantification of calcium signal transmission from sarco-endoplasmic reticulum to the mitochondria. J Physiol. 2000;529 Pt 3:553-564.
3. Pacher P, Thomas AP, Hajnoczky G. Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc Natl Acad Sci U S A. 2002;99(4):2380-2385.
4. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260(6):3440-3450.
5. Bassani JW, Bassani RA, Bers DM. Calibration of indo-1 and resting intracellular [Ca]i in intact rabbit cardiac myocytes. Biophys J. 1995;68(4):1453-1460.
6. Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278(45):44735-44744.
7. Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol. 1997;29(9):2571-2583.
8. Brandes R, Bers DM. Intracellular Ca2+ increases the mitochondrial NADH concentration during elevated work in intact cardiac muscle. Circ Res. 1997;80(1):82-87.
9. Cortassa S, Aon MA, Marban E, Winslow RL, O'Rourke B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys J. 2003;84(4):2734-2755.
10. Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416(1):15-18.
11. Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem. 2003;86(5):1101-1107.
12. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(Pt 2):335-344.
13. Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483-495.