Abstract
Objectives
Rheumatoid arthritis (RA) is a chronic inflammatory disease resulting in substantial pain. The physical and emotional effects of RA are well known, but little attention has been given to the potential cognitive effects of RA pain, although intact executive functioning in patients with chronic illness is crucial for the successful completion of many daily activities. We examined the relationship between pain and executive functioning in patients with RA, and also considered the influence of positive and negative affect in the relationship between pain and executive functioning.
Methods
A sample of 157 adults with RA completed measures of pain and positive and negative affect and were tested for working memory and selective attention using the Letter Number Sequencing subtest from the WAIS-III and the Stroop Color Word Test tests, respectively. Results: Consistent with prior research, pain was inversely related to executive functioning, with higher pain levels associated with poorer performance on executive functioning tasks. This relationship was not moderated or mediated by negative affect; however, positive affect moderated the relationship between pain and executive functioning. For patients high in positive affect there was a significant inverse relationship between pain and executive functioning, whereas there was no such relationship for patients low in positive affect.
Discussion
These findings are discussed in the context of cognitive research on the effects of positive affect on executive functioning as well as functional neuroanatomical research suggesting neurocognitive mechanisms for such moderation.
Keywords: pain, executive functioning, negative affect, positive affect, rheumatoid arthritis
Introduction
The executive functions, which include working memory, inhibition, planning, decision-making, and cognitive control, are considered to be central to self-regulatory functions, such as emotional, cognitive, and behavioral control [1]. Furthermore, the executive functions have been shown to predict adaptation in a few patient groups [2, 3], but are likely to be important to functional outcomes in a wide range of patients. For people with chronic illnesses, intact cognitive functioning is crucial for performing many key daily activities, including adhering to medical regimens, planning activities based upon one’s current physical condition, and changing plans should pain worsen unexpectedly during daily activities. Rheumatoid arthritis (RA) is a chronic inflammatory disease that results in substantial pain. The physical and emotional effects of RA can be significant; however, little attention has been given to the potential cognitive effects of chronic pain in RA patients. In addition, as noted by Kreitler and Niv [4], psychological interventions that have been shown to be effective for RA [5] often depend on the intact cognitive functioning of patients to generate changes to thoughts and behaviors. Consequently, declines in executive functioning may exacerbate suffering and lead to poorer physical, social, and emotional health.
The relationship between pain and executive functioning has been studied correlationally in people with chronic pain and experimentally in healthy people exposed to induced laboratory pain. A comprehensive review of pain and executive functioning in chronic pain patients by Hart, Martelli, and Zasler [6] showed that chronic pain disrupts attention, processing speed, and some executive functions, including multi-tasking ability [7], sustained attention and working memory [8, 9, 10, 11, 12, 13], and verbal fluency [11]. Other aspects of executive functioning, such as mental flexibility and selective attention, have shown less consistent relationships with chronic pain [8, 12, 14]. Experimental studies of laboratory pain have shown less reliable effects on cognition than have studies of chronic pain, although this may be due to the differences in pain intensity or duration between naturalistic chronic pain and the experimental acute pain. Nonetheless, the disruptive effect of pain on cognitive functioning makes evolutionary sense, because pain typically signals the need for action, which demands cognitive resources [15]. Furthermore, there are overlapping neuroanatomical pathways between nociceptive and cognitive systems, particularly executive functioning systems, providing a pathway for the two systems to interact. The areas that are most relevant because of their overlap with cognitive functions are the parietal cortex, for orienting or directing of attention [16, 17], dorsolateral prefrontal cortex, for working memory, selective attention, inhibition, and problem-solving [18, 19], and anterior cingulate cortex, for nociception, cognition and affect.
Pain and negative affect (NA) are related constructs in that a central feature of the definition of pain is that it is both a sensory and an emotional experience. Experimental and clinical studies demonstrate the close relationship between emotion and pain. For instance, experimental induction of NA is associated with increased pain intensity and decreased pain tolerance, whereas the induction of positive affect (PA) decreases pain intensity and increases pain tolerance [20, 21, 22, 23]. Furthermore, the relationship between pain and affect in RA patients is consistent with the experimental literature [24]. For example, Connelly et al. [25] had RA patients record positive and negative affect and pain daily over the course of 30 days. Increased NA one day was accompanied by increased pain ratings the following day, whereas increased PA was followed by decreased pain ratings.
Although NA has not been shown to consistently influence cognition, PA has been shown to influence a number of executive functions [26, 27]. Positive affect improves performance on novel solution or creative tasks such as verbal fluency [28], increases mental flexibility [29, 30] and breadth of attentional focus [31], and increases distractibility, although it reduces perseverations [32]. In general, PA appears to loosen cognitive control in order to allow for greater mental flexibility and divided attention.
The relationship between pain, general cognitive functioning and depression has been examined in RA patients, particularly with the goal of examining the potential mediating role of depression in the relationship between pain and cognitive functioning. Brown, Glass, and Park [33] created a cognitive functioning composite by combining tests across multiple domains, including processing speed, inductive reasoning, working memory and long-term episodic memory. They found that pain was positively related to depression and negatively related to cognitive functioning, and that depression mediated the relationship between pain and cognitive functioning, suggesting that pain leads to depression which leads to poorer cognitive functioning. This important study, however, was limited by a few factors: 1) it combined many different cognitive domains, which obviated understanding of how pain and depression were related to specific domains, such as executive functioning, 2) they did not consider the effects of positive mood, which appears to influence executive functioning, and 3) they examined depression, a clinical mood disorder with many complex and confounding symptoms (e.g. sleep and eating disturbances) that may also influence executive functioning independently of the effects of mood, rather than NA and PA, which are theoretically-derived constructs.
Further, researchers agree that it is important to control for NA in studies of self-reported variables such as pain, because NA often confounds subjective pain assessments. Prior studies of pain and executive functioning have not done this. Consistent with the differential predictions for NA and PA, Zautra and colleagues [34] have proposed the Dynamic Model of Affect, which highlights the importance of both PA and NA and proposes that stressors such as pain moderate how PA and NA are related to each other and to other variables.
The primary goal of this study was to examine the relationship between pain and executive functioning in RA patients. Whereas Brown et al. [33] examined pain and general cognition in RA patients, executive functioning was specifically examined in the current study using two widely used measures, a measure of inhibition and selective attention as well as a measure of working memory, due to the importance of the executive functions for self-regulatory behaviors. We hypothesized that pain would have an inverse relationship with executive functioning in RA patients. We also tested whether the relationship between pain and executive functioning is mediated or moderated by affect. In particular, we tested whether NA confounds or potentially mediates the pain/executive functioning relationship, and whether PA moderates the pain/executive functioning relationship. Because the measure of NA and PA that we used is biased toward high activation states, we also explored the roles played by low activation NA and PA states [35]. Finally, we controlled for other potentially confounding variables, including fatigue and depressed mood, as these variables may have effects on executive functioning, independent of pain.
Materials and Methods
Participants
Participants were 157 patients (140, 89.2% women) with a rheumatologist-provided diagnosis of RA based on American College of Rheumatology criteria. Patients’ mean age was 54 (range = 20 – 74), and mean age at diagnosis with RA was 42.5 (range = 3 – 71). Participants had a mean of 13.5 (range = 1 – 21) years of education. The sample was composed of 59.4% Caucasian and 40.6% African-American patients. Participants were recruited from one of five rheumatology clinics to participate in a randomized clinical trial of written or verbal emotional disclosure. Patients were excluded by their rheumatologists if they had dementia or a psychotic disorder, and only participants who could read and write in English were enrolled. This study was approved by the appropriate institutional review boards, and after participants gave written informed consent, they were administered a battery of tests and questionnaires by trained research staff. Data for this report were taken from the baseline (pre- randomization and intervention) assessment, which occurred at a private room at the medical clinic.
Measures
The assessment of executive functions included a selective attention/inhibition task, the Stroop interference task [36], and a working memory task, the Letter-Number Sequencing subtest from the Wechsler Adult Intelligence Scale – Third Edition [WAIS-III; 37], as they are executive functioning measures that are largely mediated by brain regions that are also thought to be involved in nociception (dorsolateral prefrontal cortex and anterior cingulate cortex) and may, therefore, be influenced by pain.
The Stroop Neuropsychological Screening Test [SNST; 36]
This is a measure of inhibitory control or selective attention in which two conditions presented in this order: a color naming condition and an incongruent “color-word” condition, in which participants are instructed to name the color of the ink that an incongruent color word is written in; for example, the word “BLUE” is written in red ink. The SNST measures the ability to inhibit one’s prepotent tendency to read the word rather than name the color of the ink. The color-word incongruent condition of the SNST is the key scored condition, in which the number of correct answers (colors named) in 120 seconds is recorded. Each SNST raw score was converted first to a percentile, based upon age-corrected normative data in the manual [36] and the percentiles were then converted to standard scores (M = 100, SD = 15). Performance on Stroop tasks has been shown to correlate with activity in the lateral prefrontal cortex as well as the anterior cingulate cortex [19], both of which are regions that have been implicated in nociception and executive functioning, in addition to affect processing.
The Letter-Number Sequence (LNS) subtest of the WAIS-III [37]
This is a measure of working memory, in which participants are verbally presented with a sequence of letters and numbers and asked to reorganize the sequence such that the letters are reported back first in alphabetical order followed by the numbers in numerical order. This task requires the participant to be able to hold all of the digits in memory while manipulating the order according to the rules. The task is discontinued when the examinee fails all three trials for sequences of the same length. The raw score is computed by adding the number of correct trials. Raw scores were first converted to scaled scores (M = 10, SD = 3) based on the age-corrected norms in the manual [37], and the scaled scores were converted to standard scores. Performance on similar working memory tasks is associated with increased activity in the dorsolateral prefrontal cortex, an area that has been implicated in nociception as well.
Pain
This was assessed with a 100 mm Visual Analogue Scale (VAS). Participants indicated their current level of pain by placing a mark on line with anchors of “no pain sensation” and “pain as bad as could be.” The distance to their mark, in millimeters, was used as the index of current pain.
Positive and Negative Affect Schedule-Expanded Form [PANAS-X; 38]
This measure yields two 10-item subscales, one for negative affect and one for positive affect, as well as 11 specific affect subscales including: Fear, Hostility, Guilt, Sadness, Joviality, Self-Assurance, Attentiveness, Shyness, Fatigue, Serenity, and Surprise. Participants rated the degree to which they recently experienced each adjective on a 1 (not at all) to 5 (extremely) scale. Items were averaged within subscales. This measure has excellent reliability and a wide range of validity evidence [39] and has been used in chronic pain populations [40].
Fatigue Severity Scale [41]
This 9- item scale measures the severity of functional consequences due to fatigue. It has been shown to have excellent reliability in various clinical samples and has range of validity evidence.
Depressed mood
We used the mood subscale of the Arthritis Impact Measurement Scales–2 [42], which is a questionnaire that surveys the effects of arthritis during the previous month. Items were rated on a scale of 1 to 5 with respect to the frequency (number of days in a week) that a particular behavior or symptom was experienced. The AIMS-2 scales have excellent internal consistency, have been widely used and validated, and are recommended by the American College of Rheumatology for clinical trials.
Erythrocyte sedimentation rate (SED rate)
This measure of inflammation in the body is commonly used to track disease activity in RA.
Results
Descriptive data for variables are given in Table 1. The Stroop and Letter-Number Sequencing scores were correlated r =.40, p < .001; therefore, in order to improve stability of the measurement of executive functioning, reduce the number of analyses, and facilitate interpretation of executive functioning, a composite was created by converting the Stroop and Letter-Number Sequencing scores into standard scores (M = 100, SD = 15) and taking the mean of the two scores for each participant. Correlational analyses showed that, as hypothesized, the patients’ current pain level was inversely related to the score on the executive functioning composite (r = −.24, p = .003). (Note that pain was also inversely correlated with each of the two executive functioning measures separately: Stroop, r = −.17, p = .028; and LNS, r = −.24, p = .002). The relationship between pain and executive functioning was further examined through regressions, controlling for the potentially confounding effects of age, education, duration of RA, and erythrocyte sedimentation rate. Controlling for all of these variables simultaneously did not eliminate the significant relationship between pain and executive functioning (β = −.26, p = .004).
Table 1.
Descriptive Data for Study Measures (N = 157)
| Measure | Mean | SD |
|---|---|---|
| Positive Affect | 2.96 | 0.76 |
| Negative Affect | 1.91 | 0.74 |
| LNS (SS) | 98.1 | 18.7 |
| Stroop Color-Word (SS) | 96.2 | 21.6 |
| Executive Function (SS) | 97.2 | 16.9 |
| Current Level Of Pain | 36.4 | 21 |
SS = Standard score (M = 100, SD = 15)
The correlation between PA and NA in the sample was r = −.45 (p <.001). This expected inverse relationship did not differ significantly (p = .22) between those with high and low levels of pain, based on a non-significant interaction between pain and NA in predicting PA. Consistent with prior research, pain was positively related to NA (r = .34, p < .001), but NA was unrelated to executive functioning (r = .05, p = .53). Although it has been found that depression mediates (or confounds) the relationship between pain and executive functioning, we found that controlling for NA actually slightly (albeit non-significantly) strengthened the relationship between pain and executive functioning (β increased from −.25 to −.29). Thus, NA did not mediate or confound the relationship between pain and executive functioning in our sample. The same pattern of results was found when controlling for fatigue and depressed mood. Neither fatigue (β increased from −.25 to −.27) nor depressed mood (β remained −.25) mediated or confounded the relationship between pain and executive functioning. Additionally, NA did not moderate the relationship between pain and executive functioning, as indicated by a non-significant pain x NA interaction (β = .06, p = .46) in a model with pain and NA entered first as main effects after centering them.
Positive affect was not significantly related to either pain (r = −.13, p = .10) or executive functioning (r = −.03, p = .75). As a result, controlling for PA did not change the relationship of pain to executive functioning (the β remained unchanged at −.25). As shown in the regression analysis in Table 2, however, PA significantly moderated the relationship between pain and executive functioning. Figure 1 presents the relationship graphically, in which both pain and PA were left continuous and, per Aiken and West [43], the mean of each variable as well as values that were 1 SD above and below the mean were used to plot regression lines. The inverse relationship between pain and executive functioning was stronger for those with relatively high levels of PA than it was for those with relatively low levels of PA. Stated alternatively, executive functioning was poorer for those with high PA than for those with low PA when experiencing high pain, but was slightly better for those with high PA than for those with low PA when experiencing low pain.
Table 2.
Hierarchical Regression Analyses Demonstrating that Positive Affect Moderates the Relationship Pains Relationship to Executive Functioning
| Variable | Step 1 | Step 2 | ||||
|---|---|---|---|---|---|---|
| B | SE | β | B | SE | β | |
| Step 1 | ||||||
| Pain | −.20 | .06 | −.25** | −.19 | .06 | −.23** |
| PA | −.63 | 1.77 | −.03 | −1.30 | 1.78 | −.06 |
| Step 2 | ||||||
| Pain x PA | −.17 | .08 | −.16* | |||
| Model F (df) | 4.96** (2, 154) | 4.73** (3, 153) | ||||
| Overall R2 | .061 | .085 | ||||
| ΔR2 | .024* | |||||
p<.05;
p<.01
Figure 1.
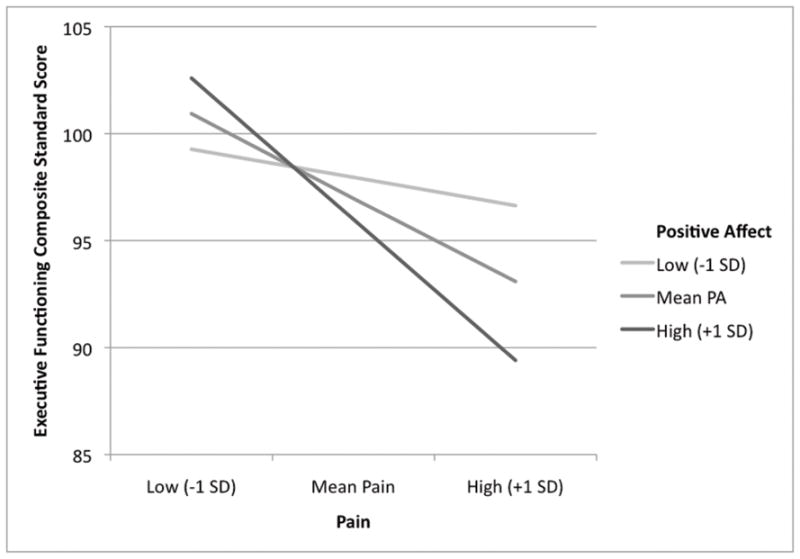
Positive affect moderates the inverse relationship between pain and executive functioning. Variables were left continuous and plotted at the mean and plus/minus 1 SD for both variables [43].
To better understand and determine the specificity of the finding that PA moderated the pain/executive functioning relationship, we conducted several additional analyses. First, we examined subscales for specific affects from the PANAS-X to explore whether global PA was responsible for the moderation, or more specifically high or low activation PA. We conducted moderation analyses on the specific affect scales, in the same fashion as the PA moderator analyses were conducted. We found that the Joviality (β = −.192, p = .013) and Surprise (β = −.157, p = .045) subscales moderated the relationship between pain and executive functioning. These are arguably the two subscales that measure positive affect with the greatest activation. Serenity, a low activation positive affect, did not moderate the relationship between pain and executive functioning (β = −.09, p = .25). None of the negative affect subscales moderated the relationship between pain and executive functioning, regardless of activation level. We also sought to determine whether broader clinical states, such as depressed mood and fatigue might have accounted for the PA moderation effect, because they are related to low PA, or because they can influence executive functioning. Unlike PA, neither of these variables moderated the relationship between pain and executive functioning. Furthermore, controlling for either fatigue or depressed mood in the moderated regression equation did not change the significant moderation of PA. Thus, the moderating effects of PA on the relationship between pain and executive functioning cannot be accounted for by fatigue or depressed mood.
Discussion
The current findings provide a more complex perspective on the relationship between pain, affect, and executive functioning in patients with chronic pain than has been offered previously. We found that pain was inversely related to executive functioning, such that patients with higher levels of pain had poorer executive functioning. Although a prior study showed that pain is related to poorer cognition generally in RA, this is the first study to show that pain is related to poorer executive functioning in this population. This relationship was not due to potential confounds such as age, education, duration of RA, depressed mood, fatigue or even the severity of the disease.
Although, as expected, pain was positively related to NA, executive functioning was unrelated to NA, and so NA neither mediated nor confounded the relationship between pain and executive functioning. This finding appears to contrast with that of Brown et al. [33], who found that depression mediated the relationship between pain and general cognitive functioning (processing speed, reasoning, working memory, and long-term memory), but the studies differed in that we measured NA rather than depression, and aspects of depression other than NA (e.g., sleep disturbance, psychomotor slowing, anhedonia) might have accounted for the relationship between pain and cognitive functioning in the Brown et al. study. It is also possible that different cognitive functions are linked to pain and NA in different ways and to differing degrees. For instance, pain may influence some aspects of cognitive functioning through pain’s effects on emotional functioning, whereas it may have more direct effects on other aspects of cognitive functioning. Regardless, our study indicates that patients with RA who have greater pain have poorer executive functioning, and that NA neither confounds nor mediates this relationship.
An even more novel finding is that PA moderated the relationship between pain and executive functioning, such that for patients with higher levels of PA, there was a moderately strong inverse relationship between pain and EF, but there was no relationship between pain and executive functioning for patients with lower PA. This moderation effect was not reduced after controlling for potential confounds, such as fatigue and depressed mood. There are several possible explanations for this finding. First, the moderation of PA may be related to the increased demand for cognitive resources when in pain. To the extent that those who have high PA try to maintain the high PA when challenged by pain, such people may engage cognitive strategies that both maintain affect but simultaneously require attentional and executive resources, thus taxing those resources available for external tasks. In contrast, people who report lower PA are accustomed to the absence of positive feelings and, therefore, do not exert effort and cognitive resources to regulate their affect when in pain, leaving reserve for executive functions. This interpretation accords with the Dynamic Integration Theory, which posits tradeoffs between affective balance and cognitive complexity; when people try to maintain PA in the face of threat (such as pain), they sacrifice cognitive resources and complexity [44]. A similar view stems from the literature on repressive coping, which finds physiological and cognitive costs to maintaining PA and denying NA when under stress or threat [45].
Another possible explanation for the inverse relationship between pain and executive functioning for those with high PA may be related to the neuromodulatory effects of increased dopamine, under high PA conditions. Ashby et al. [26] suggest that PA is associated with increased dopamine in pathways innervating the anterior cingulate and prefrontal cortices. Increased activity in this pathway under high PA conditions may translate into improvements in attentional switching, as has been shown [46]. Although improved attentional switching is beneficial under certain circumstances, it increases distractibility [32] and may be detrimental to individuals who are in great pain as the pain stimulus is able to more effectively recruit cognitive resources thereby minimizing the ability to selectively attend to the non-pain stimuli for extended periods of time.
It is also possible that the moderating effects of PA are related to the effects of PA on pain perception. Positive affect is typically associated with lower pain ratings in chronic pain patients, and PA has been shown to decrease pain ratings in experimentally induced pain [20, 21, 22, 23]. Given that higher PA is associated with lower levels of reported pain, those with higher PA may endure a more intense bodily pain stimulus than those with lower PA, despite providing the same subjective pain ratings. A greater pain stimulus paired with the greater distractibility associated with high PA may create the perfect combination of greater susceptibility to distraction along with greater pain stimulus intensity (as compared to the same pain ratings in those with low PA).
Analyses of various positive affects found that it was specifically high activation PA states, such as joviality and surprise, that moderated the pain/executive functioning relationships, and not low activation PA status, such as serenity. It is possible, therefore, that generally elevated emotional stimulation from high activation affects combined with the heightened activation from elevated pain overwhelms the information processing capacity in brain areas responsible for executive functions. However, this was found only for highly activating positive affects and not for highly activating negative affects, which does not support the idea that affective intensity is the primary factor of interest. Rather, it appears that high activation PA, in particular, plays an important role in the relationship between pain and executive functioning.
The finding that PA is not beneficial under some circumstances appears to be contrary to the view that PA functions as a resiliency or buffering factor [47]. However, it may simply be the other side of the resiliency coin. That is, that the maintenance of PA under difficult conditions, such as pain, may be beneficial to emotional functioning, but may come at a cost to the neurocognitive systems that subserve, or share resources with, such functions.
Future research is needed to provide a better understanding of the causal relationships among chronic pain, affect, and executive functioning than these cross-sectional data provide. It is possible, for example, that pain reports are influenced by executive functioning. Longitudinal studies and experimental manipulations of both pain and affect would illuminate these relationships. It also is important to test these relationships on other chronic pain samples. We were not able to assess other cognitive functions, but future research would benefit from the assessment of cognitive functions such as processing speed and memory in addition to executive functioning, to determine which other functions are diminished in relation to elevated pain. Our measure of pain was quite simple—a multidimensional assessment of pain would be beneficial. Also, we measured pain “currently,” but affect “recently,” and even though we found some hypothesized relationships, it would be ideal to use the same time window for both measures, and “recently” is too vague. The use of the term “recently” creates a degree of uncertainty as it does not allow for interpretation as state or trait affect. Finally, given that the more activating components of PA seem to be driving the PA moderation effect, the role of affect intensity, as an individual difference variable, should be examined in future studies.
The current findings have implications for the assessment and treatment of patients with RA and possibly chronic pain patients more broadly. Clinicians are advised to assess current pain levels during neuropsychological assessments, given that pain levels influence executive functioning and NA. In addition, if this finding replicates, affect should be measured, in addition to the evaluation of depression, with attention paid to PA as well as NA; patients with both high PA and high pain should be carefully evaluated for disturbance in executive functioning, which may be predictive of poorer treatment compliance or other treatment complications [48]. In addition, many types of therapy rely upon intact cognitive abilities; thus, for patients with more intense chronic pain, especially if trying to maintain their PA, the potential decline in executive functioning should be factored into treatment planning, which may necessitate the use of less cognitively demanding interventions. Psychological treatments that reduce the negative emotional aspects of chronic pain might particularly benefit high PA individuals.
Acknowledgments
Author Notes
This research was funded by a Clinical Science Award from the Arthritis Foundation (Atlanta, Georgia, USA) and grant R01 AR049059 from the National Institutes of Health (Bethesda, Maryland, USA), awarded to the final author.
We thank Jose Granda, MD (Division of Rheumatology, Department of Medicine, Wayne State University, Detroit, Michigan, USA), Lydia Lasichak, MD (Kamil Orthopedic Group, West Bloomfield, Michigan, USA), and Michael Lubetsky, MD (Division of Rheumatology, Department of Medicine, Henry Ford Health System, Detroit, Michigan, USA) for their assistance in recruiting patients. We thank Debra Macklem, PhD, Tina Meyer, PhD, Linda Naoum, MSW, and Alison Radcliffe, PhD, (Department of Psychology, Wayne State University, Detroit, Michigan, USA) for assistance in data collection.
References
- 1.Solberg Nes L, Roach AR, Segerstrom SC. Executive functions, self-regulation, and chronic pain: a review. Ann Behav Med. 2009;37:173–83. doi: 10.1007/s12160-009-9096-5. [DOI] [PubMed] [Google Scholar]
- 2.Hanks R, Rapport L, Millis S, et al. Measures of executive functioning as predictors of functional ability and social integration in a rehabilitation sample. Arch Phys Med Rehabil. 1999;80:1030–37. doi: 10.1016/s0003-9993(99)90056-4. [DOI] [PubMed] [Google Scholar]
- 3.Struchen MA, Clark AN, Sander AM, et al. Relation of executive functioning and social communication measures to functional outcomes following traumatic brain injury. NeuroRehabilitation. 2008;23:185–98. [PubMed] [Google Scholar]
- 4.Kreitler S, Niv D. Cognitive impairment in chronic pain. Pain Clin Updates. 2007;15:1–4. [Google Scholar]
- 5.Dixon KE, Keefe FJ, Scipio CD, et al. Psychological interventions for arthritis pain management in adults: a meta-analysis. Health Psychol. 2007;26:241–50. doi: 10.1037/0278-6133.26.3.241. [DOI] [PubMed] [Google Scholar]
- 6.Hart RP, Martelli MF, Zasler ND. Chronic pain and neuropsychological functioning. Neuropsychol Rev. 2000;10:131–49. doi: 10.1023/a:1009020914358. [DOI] [PubMed] [Google Scholar]
- 7.Cote KA, Moldofsky H. Sleep, daytime symptoms, and cognitive performance in patients with fibromyalgia. J Rheumatol. 1997;24:14–23. [PubMed] [Google Scholar]
- 8.Gimse R, Bjorgen IA, Tjell C, et al. Reduced cognitive functions in a group of whiplash patients with demonstrated disturbance in the posture control system. J Clin Exp Neuropsychol. 1997;19:838–49. doi: 10.1080/01688639708403764. [DOI] [PubMed] [Google Scholar]
- 9.Grace GM, Nielson WR, Hopkins M, et al. Concentration and memory deficits in patients with fibromyalgia syndrome. J Clin Exp Neuropsychol. 1999;21:477–87. doi: 10.1076/jcen.21.4.477.876. [DOI] [PubMed] [Google Scholar]
- 10.Radanov BP, Hirlinger I, di Stefano G, et al. Attentional processing in cervical spine syndromes. Acta Neurol Scand. 1992;85:358–62. doi: 10.1111/j.1600-0404.1992.tb04060.x. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz DP, Barth JT, Dane JR, et al. Cognitive deficits in chronic pain patients with and without a history of head/neck injury: development of a brief screening battery. Clin J Pain. 1987;3:94–101. [Google Scholar]
- 12.Sletvold H, Stiles TC, Landro NI. Information processing in primary fibromyalgia, major depression and healthy controls. J Rheumatol. 1995;22:137–42. [PubMed] [Google Scholar]
- 13.Taylor AE, Cox CA, Mailis A. Persistent neuropsychological deficits following whiplash: evidence for chronic mild traumatic brain injury? Arch Phys Med Rehabil. 1996;77:529–35. doi: 10.1016/s0003-9993(96)90290-7. [DOI] [PubMed] [Google Scholar]
- 14.Pincus T, Fraser L, Pearce S. Do chronic pain patients “stroop” on pain stimuli? Br J Clin Psychol. 1998;37:49–58. doi: 10.1111/j.2044-8260.1998.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 15.Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psych Bull. 1999;125:356–66. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- 16.Peyron R, García-Larrea L, Grégoire M, et al. Hemodynamic brain responses to acute pain in humans: Sensory and attentional networks. Brain. 1999;122:1765–79. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- 17.Posner M, Petersen S. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 18.Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006;16:1679–89. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell RLC. The BOLD response during Stroop task-like inhibition paradigms: effects of task difficulty and task-relevant modality. Brain Cogn. 2005;59:23–37. doi: 10.1016/j.bandc.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Weisenberg M, Raz T, Hener T. The influence of film-induced mood on pain perception. Pain. 1998;76:365–75. doi: 10.1016/S0304-3959(98)00069-4. [DOI] [PubMed] [Google Scholar]
- 21.de Wied M, Verbaten MN. Affective pictures processing, attention, and pain tolerance. Pain. 2001;90:163–72. doi: 10.1016/s0304-3959(00)00400-0. [DOI] [PubMed] [Google Scholar]
- 22.Meagher MW, Arnau RC, Rhudy JL. Pain and emotion: effects of affective picture modulation. Psychosom Med. 2001;63:79–90. doi: 10.1097/00006842-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Zelman DC, Howland EW, Nichols SN, et al. The effects of induced mood on laboratory pain. Pain. 1991;46:105–11. doi: 10.1016/0304-3959(91)90040-5. [DOI] [PubMed] [Google Scholar]
- 24.Strand EB, Kerns RD, Christie A, et al. Higher levels of pain readiness to change and more positive affect reduce pain reports - a weekly assessment study on arthritis patients. Pain. 2007;127:204–13. doi: 10.1016/j.pain.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Connelly M, Keefe FJ, Affleck G, et al. Effects of day-to-day affect regulation on the pain experience of patients with rheumatoid arthritis. Pain. 2007;131:162–70. doi: 10.1016/j.pain.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashby FG, Isen AM, Turken AU. A neuropsychological theory of positive affect and its influence on cognition. Psychol Rev. 1999;106:529–50. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell RLC, Phillips LH. The psychological, neurochemical and functional neuroanatomical mediators of the effects of positive and negative mood on executive functions. Neuropsychologia. 2007;45:617–29. doi: 10.1016/j.neuropsychologia.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 28.Phillips LH, Bull R, Adams E, et al. Positive mood and executive function: evidence from Stroop and fluency tasks. Emotion. 2002;2:12–22. doi: 10.1037/1528-3542.2.1.12. [DOI] [PubMed] [Google Scholar]
- 29.Isen AM, Daubman KA. The influence of affect on categorization. J Pers Soc Psychol. 1984;47:1206–17. [Google Scholar]
- 30.Isen AM, Niedenthal PM, Cantor N. An influence of positive affect on social categorization. Motiv Emot. 1992;16:65–78. [Google Scholar]
- 31.Rowe G, Hirsh JB, Anderson AK, et al. Positive affect increases the breadth of attentional selection. Proc Natl Acad Sci U S A. 2007;104:383–8. doi: 10.1073/pnas.0605198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dreisbach G, Goschke T. How positive affect modulates cognitive control: reduced perseveration at the cost of increased distractibility. J Exp Psychol Learn Mem Cogn. 2004;30:343–53. doi: 10.1037/0278-7393.30.2.343. [DOI] [PubMed] [Google Scholar]
- 33.Brown SC, Glass JM, Park DC. The relationship of pain and depression to cognitive function in rheumatoid arthritis patients. Pain. 2002;96:279–84. doi: 10.1016/S0304-3959(01)00457-2. [DOI] [PubMed] [Google Scholar]
- 34.Zautra A, Smith B, Affleck G, et al. Examinations of chronic pain and affect relationships: applications of a dynamic model of affect. J Consult Clin Psychol. 2001;69:786–95. doi: 10.1037//0022-006x.69.5.786. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton NA, Zautra AJ, Reich JW. Affect and pain in rheumatoid arthritis: Do individual differences in affective regulation and affective intensity predict emotional recovery from pain? Ann Behav Med. 2005;29:216–24. doi: 10.1207/s15324796abm2903_8. [DOI] [PubMed] [Google Scholar]
- 36.Trennery MR, Crosson B, DeBoe J, et al. Stroop Neuropsychological Screening Test manual. Odessa, FL: Psychological Assessment Resources; 1989. [Google Scholar]
- 37.Wechsler D. Wechsler Adult Intelligence Scale. 3. New York: Psychological Corporation; 1997. [Google Scholar]
- 38.Watson D, Clark LA. The PANAS-X. Manual for the Positive and Negative Affect Schedule-Expanded Form. Iowa City, IA: The University of Iowa; 1994. [Google Scholar]
- 39.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 40.Finan PH, Zautra AJ, Davis MC. Daily affect relations in fibromyalgia patients reveal positive affective disturbance. Psychosom Med. 2009;71:474–82. doi: 10.1097/PSY.0b013e31819e0a8b. [DOI] [PubMed] [Google Scholar]
- 41.Lauren B, Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–23. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 42.Meenan RF, Mason JH, Anderson JJ, Guccione AA, Kazis LE. AIMS2. The content and properties of a revised and expanded Arthritis Impact Measurement Scales Health Status Questionnaire. Arthr Rheum. 2005;35:1–10. doi: 10.1002/art.1780350102. [DOI] [PubMed] [Google Scholar]
- 43.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA, US: Sage Publications, Inc; 1991. [Google Scholar]
- 44.Labouvie-Vief G. Dynamic integration: Affect, cognition, and the self in adulthood. Curr Dir Psychol Sci. 2003;12:201–6. [Google Scholar]
- 45.Weinberger DA. The construct validity of the repressive coping style. In: Singer JL, editor. Repression and dissociation: implications for personality theory, psychopathology, and health. Chicago, IL: University of Chicago Press; 1990. pp. 337–86. [Google Scholar]
- 46.Coull JT, Nobre AC. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci. 1998;18:7426–35. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis MC, Zautra AJ, Smith BW. Chronic pain, stress, and the dynamics of affective differentiation. J Pers. 2004;72:1133–59. doi: 10.1111/j.1467-6494.2004.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kreitler S, Kreitler M. Psychological approaches to treatment of pain. In: Kreitler S, et al., editors. Handbook of chronic pain. New York: Nova Science; 2007. pp. 299–321. [Google Scholar]


