Abstract
The Drosophila standard brain has been a useful tool that provides information about position and size of different brain structures within a wild-type brain and allows the comparison of imaging data that were collected from individual preparations. Therefore the standard can be used to reveal and visualize differences of brain regions between wild-type and mutant brains and can provide spatial description of single neurons within the nervous system. Recently the standard brain was complemented by the generation of a ventral nerve cord (VNC) standard. Here the authors have registered the major components of a simple neuronal circuit, the Giant Fiber System (GFS), into this standard. The authors show that they can also virtually reconstruct the well-characterized synaptic contact of the Giant Fiber with its motorneuronal target when they register the individual neurons from different preparations into the VNC standard. In addition to the potential application for the standard thorax in neuronal circuit reconstruction, the authors show that it is a useful tool for in-depth analysis of mutant morphology of single neurons. The authors find quantitative and qualitative differences when they compared the Giant Fibers of two different neuroglian alleles, nrg849 and nrgG00305, using the averaged wild-type GFS in the standard VNC as a reference.
Keywords: Giant Fiber, neuronal circuit, Neuroglian, Drosophila, standard brain, ventral nerve cord
Introduction
We are excited to contribute to the special issue of the Journal of Neurogenetics to honor the lifelong contributions of Erich Buchner to Drosophila Neurogenetics. One of the more recent discoveries of the Buchner lab was that the monoclonal antibody nc82 (MAB nc82, now also called anti-Brp), which was widely used among the Drosophila community as a reliable label for neuropil, binds to an epitope of the Bruchpilot protein (Kittel et al., 2006; Wagh et al., 2006). The Bruchpilot protein has been shown to be a component of the T-bar structure, which is a crucial part of the synaptic active zones important for evoked synaptic vesicle release in Drosophila (Fouquet et al., 2009). There it is important for the clustering of Ca2+ channels at the active zone (Fouquet et al., 2009; Kittel et al., 2006; Wagh et al., 2006).
The MAB nc82 was initially isolated in antibody screens against the Drosophila nervous system tissue to identify and characterize Drosophila neuronal proteins (Hofbauer, 1991; Hofbauer et al., 2009). Immunohistological labeling of Drosophila brains with the MAB nc82 resulted in strong staining of all brain neuropil, making this antibody a valuable tool as a neuropil marker. Fluorescence staining of the MAB nc82 in high resolution confocal microscopy allows for three-dimensional (3D) visualization of specific neuropil regions in the brain that has been used for reference models (Jefferis et al., 2007; Laissue et al., 1999; Rein, Zockler, & Heisenberg, 1999; Rein, Zockler, Mader, Grubel, & Heisenberg, 2002). The Drosophila standard brain comprises the average of multiple wild type Drosophila brains and includes different neuropil regions covering 60% of the total brain neuropil. Information about position and size of the wild type brain and its anatomical parts is represented by the standard. The standard allows for comparison of imaging data that were collected from individual preparations and can help to identify group specific variations in brain neuropil anatomy (Rein et al., 2002). In addition, using double staining of single neuronal structures together with the MAB nc82 marker in one preparation enables the spatial description of neurons within the reference neuropil of the nervous system (Jefferis et al., 2004; Riemensperger, Voller, Stock, Buchner, & Fiala, 2005; Wong, Wang, & Axel, 2002).
Anatomical studies of the Drosophila brain focus on the mapping of neuronal circuits that relay the sensory inputs to higher brain centers and on processing of the information (Olsen & Wilson, 2008). The ventral nerve cord (VNC) houses neuronal circuits for motor behaviors such as walking (Burrows, Laurent, & Field, 1988; Laurent & Burrows, 1988; Yellman, Tao, He, & Hirsh, 1997)and flying (Burrows, 1975; Peters, Altman, & Tyrer, 1985; Reye & Pearson, 1987). With the generation of the VNC standard, circuits in the ventral nerve cord can now be studied in a common reference system as well. Similar to the standard brain, the recently generated VNC standard is based on neuropil structure labeled with the MAB nc82 (Boerner & Duch, 2010). However, the more homogenous neuropil staining in the VNC required a slightly different method for standardization. In the VNC standard the entire neuropil is regarded as one structure that is not divided into sub-neuropil regions like in the standard brain. This simplifies the application of the standard for integration of VNC components for the users. The neuropil staining can be segmented semi automatically in a standardized procedure allowing for fast data registration (Boerner & Duch, 2010).
In insect standard brains only single neurons have been integrated exemplarily into the reference systems so far (Brandt et al., 2005; Kurylas, Rohlfing, Krofczik, Jenett, & Homberg, 2008; Kvello, Lofaldli, Rybak, Menzel, & Mustaparta, 2009). Although neuronal projections and branching patterns from identified neurons in insects are similar overall between animals, many neurons show variability in their fine structures that cause deviations of the processes between the individuals after standard registration (Jefferis et al., 2007). Different approaches can be used to create a reference map for neurons inside the standards taking in account individual differences. In quantitative maps the projections of single neurons can be summarized for fine projections (Jefferis et al., 2007). Big neuronal structures like thick primary neurites match well between different preparations (Boerner & Duch, 2010). Finally, neurons of the VNC that show prominent structures projecting in a very stereotypic manner with little variability can be integrated in the reference space as ‘single units’.
Here, we integrated the major components of a simple neuronal circuit, the Giant Fiber System (GFS), into the VNC standard to show the applicability of the VNC standard for neuronal network reconstruction. The Giant Fibers (GF) are a pair of command neurons that can in response to a “light off” stimulus elicit the escape response of the fly (Allen, Godenschwege, Tanouye, & Phelan, 2006). They have their somata in the brain and send their single axons posteriorly through the cervical connective (CvC) into the thorax (Figure 1 and 2,). In the mesothoracic neuromere the GFs (Figure 1A, red) bend laterally and form a large mixed electrical and chemical synapse with the medial dendrite of the Tergotrochanteral Motorneurons (TTMn, Figure 1A, blue). Anterior to the GF-TTMn synaptic terminals, the GFs also connect electrically and chemically with the Peripherally Synapsing Interneurons (PSI, Figure 1A, yellow), which in turn synapse with the Dorsal Longitudinal Motorneurons (DLMn, Figure 1A, green) (Allen et al., 2006).
Figure 1.
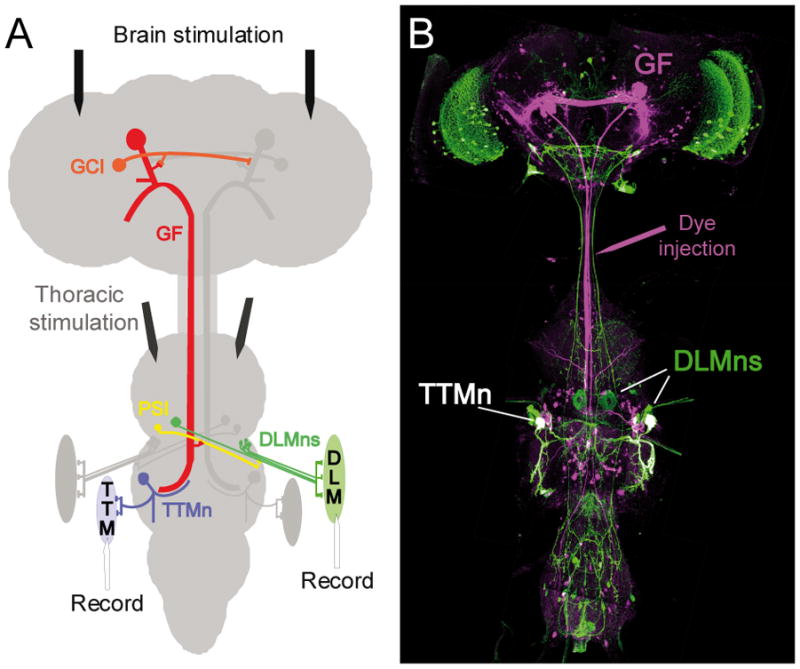
A) Schematic of the Giant Fiber System (adapted and modified from Godenschwege et al, 2006 and Allen et al, 2006). For simplicity only half of the circuit is displayed in color. The Giant Fibers (GF, red) are electrically coupled to each other via the Commissural Giant Interneurons (CGI) in the brain. They also make mixed electrical and chemical synapses with the Peripherally Synapsing Interneurons (PSI, yellow) and the medial dendrite of the Tergotrochanteral Motorneurons (TTMn, blue) in the second thoracic neuromere. The PSIs synapse with the Dorsal Longitudinal Motorneurons (DLMn, green), which innervate the flight muscles (DLM), while the TTMn have their output to the jump muscles (TTM). Stimulating and recording electrodes of the electrophysiological setup are indicated. Brain stimulation tested for the functional output of the GF to the TTM and DLM, while thoracic stimulation bypasses the GF and allows testing for the function of the neuromuscular junctions directly. B) High resolution confocal image of the Drosophila central nervous system with the labeled GFS. In flies expressing Green Fluorescent Protein under the shakB-Gal4 line, the GFs (magenta) were injected with Neurobiotin in the cervical connective.
Figure 2.
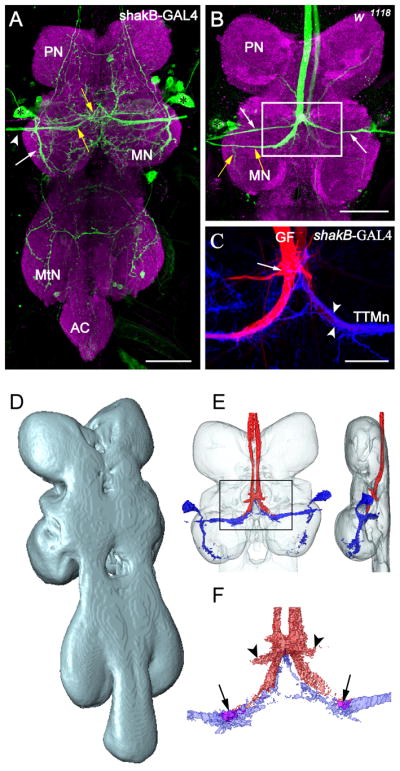
Registration of GFS components into the ventral nerve cord neuropil standard. (A) Projection view from a confocal image stack of the ventral nerve cord (VNC) showing the GFP expression pattern under the control of shakB-GAL4 (green). The neuropil was counterstained with anti-Brp (magenta). The positions of TTMn (Tergotrochanteral Motorneuron) somata are marked with asterisks. Fine processes and small somata were visible in the pro- and metathoracic neuromere (PN, MtN) and the abdominal center (AC). The TTMn extended its medial dendrite (yellow arrows) to the midline of the VNC. The posterior dendrite (white arrow) projected more ventrally in the MN. The TTMn axon (arrowhead) left the MN via the posterior dorsal mesothoracic nerve. Scale bar=50μm. (B) Projection view through the PN and MN of a wild type animal. The left Giant Fiber was dye injected with Neurobiotin (green). Dendrites and axons of the Peripherally Synapsing Interneurons (PSI, white arrows) and the TTMn (yellow arrows), and the TTMn soma (asterisk) were dye coupled to the GF. The left GF was stained due to electrical coupling of both GFs via the giant commissural interneurons in the brain. Scale bar=50μm. (C) Double staining of the GF-TTMn synaptic contact area. GFP expression under the control of shakB-GAL4 labeled the postsynaptic TTMn terminal (blue). The left GF was stained with Neurobiotin in the same preparation (red). The TTMn dendrite (arrow heads) enwrapped the GF terminal partially. The arrow marks the contact area to the PSIs. Scale bar=20μm. (D) Dorsolateral view on the surface reconstruction of the ventral nerve cord neuropil standard. (E) The projections of six reconstructed GF staining and six TTMn labels were transformed into the VNC standard and subsequently averaged. The averaged GF (red) and the averaged TTMn (blue) were displayed together as a surface reconstruction within the neuropil standard (transparent) from dorsal (left) and lateral (right). (F) Close up from the averaged GF terminal. The averaged pre- and postsynaptic terminals were aligned to each other as seen for double staining (see C). The terminals overlapped in a small area in the neuropil standard (arrows). The connection from the GF to the PSI was visible after averaging (arrowheads).
Here we test whether we are able to virtually reconstruct the GF-TTMn synapse reliably if we register individual components of the GFS from different preparations into the VNC standard. We have previously shown that Neuroglian is crucial in the assembly of the GF circuit (Godenschwege, Kristiansen, Uthaman, Hortsch, & Murphey, 2006; Godenschwege & Murphey, 2009). We also use the VNC standard containing the previously integrated averaged GFS to analyze the GFs of two different neuroglian mutant alleles with regard to quantitative and qualitative morphological differences.
Results
Registration of the GFS in the standard VNC and reconstruction of the GF-TTMn synapse
We used different labeling techniques, transgenic expression and dye-injections, to stain multiple or individual neurons of the Giant Fiber System in the ventral nerve cord (VNC) of Drosophila (Figure 1B, (Brand & Perrimon, 1993; Phelan et al., 1996)). We expressed Green Fluorescent Protein (GFP) as a reporter under the control of shakB-GAL4 to visualize the postsynaptic targets of the Giant Fiber in the VNC (Jacobs, Todman, Allen, Davies, & Bacon, 2000). The expression of shakB-GAL4 is restricted to a few neurons in the VNC, which includes the TTMn somata (Figure 2A, asterisk), its dendrites (Figure 2A, arrows), and its axon (Figure 2A, arrow head). The Giant Fiber is accessible to injection of dyes with glass microelectrodes at the dorsal surface in the cervical connective (CvC, Figure 1B). While large dyes like Rhodamine-dextran and Alexa555 stain the GF individually, Neurobiotin molecules are small enough to diffuse through the gap junctions and therefore label the postsynaptic neurons, PSI and TTMn, in addition to the GF (Figure 2B, TTMn yellow arrows, PSIs white arrows). Using double staining, the GF and the TTMn can also be displayed in different colors in one animal to better visualize the morphology of the synaptic connection (Figure 2C).
However, in some experimental situations labeling of two or multiple neurons individually in one preparation is not feasible. Therefore we intended to test whether the VNC standard could be used to virtually reconstruct a circuit from different preparations. We choose the well-characterized GF-TTMn synapse to test this possibility and employed the VNC standard to generate reference GFs and TTMns in the common standard reference space.
In order to be able to integrate both neurons separately into the neuropil standard (Figure 2D), the preparations with the GF and/or TTMn labeled were also counterstained with the Bruchpilot antibody (Wagh et al., 2006). Anti-Brp marking the entire VNC neuropil (Figure 2A, B, magenta) served as reference for the transformation of the GF and the TTMn into the VNC standard. The GFP expression patterns of six shakB-GAL4 animals were manually reconstructed in three dimensions using Amira software. In a standardized registration procedure the TTMn labels were transformed onto the VNC standard (Boerner & Duch, 2010). By transformation of each individual TTMn into the standard the projection, the areas of the TTMn dendrites and the somata could be aligned to each other. Areas that were occupied by the transformed TTMns in three or more individual preparations were counted as an averaged TTMn in the standard (Figure 2E, blue). The same transformation procedure was performed for six preparations with the GFs labeled and averaging of all transformed GF labels resulted in the averaged GFs (Figure 2E, red). Thus the averaged structures represent areas which contain the GF or TTMn processes with the highest likelihood. The surface reconstruction of both averaged GFS components in the VNC standard showed that the GF terminals are in close proximity to the TTMn dendrites. In fact, even an overlap of both neurons could be observed in small areas (Figure 2F, arrows) that have been shown to house the GF-TTMn synapses in various ultra structural studies (Blagburn, Alexopoulos, Davies, & Bacon, 1999; Godenschwege et al., 2006; King & Wyman, 1980). In addition, the contact area of the GF with the PSI was conserved through the averaging procedure as well (Figure 2F, arrow heads). This result demonstrates that using the VNC standard, the GF-TTMn synaptic contact could be reconstructed reliably from different preparations labeling either neuron individually.
Anatomical and functional comparison of the nrg849 and nrgG00305 mutant alleles
We compared two neuroglian (nrg) mutant alleles, nrg849 and nrgG00305, with respect to the anatomical and functional coupling of their GFs to the target neurons, the TTMns and the PSIs. We have previously shown that nrg849 has anatomical and physiological defects in the GFS and revealed distinct roles for Neuroglian in axon guidance and synapse formation (Godenschwege et al., 2006; Godenschwege & Murphey, 2009). The nrg849 allele carries a missense mutation in the extracellular domain which does not affect the expression of Nrg but the phosphorylation of its intracellular ankyrin-binding motif (Godenschwege et al., 2006). A transposon in an intron in the nrgG00305 allele results in the insertion of GFP into the intracellular domain of Nrg close to the ankyrin binding motif (Morin, Daneman, Zavortink, & Chia, 2001; Yamamoto, Ueda, Takahashi, Saigo, & Uemura, 2006). For this allele the expression of the neuronal but not the non-neuronal Neuroglian isoform is dramatically reduced. However, the localization the Nrg-GFP fusion protein and the phosphorylation of the ankyrin binding motif appear to be normal (Williams, 2009; Yamamoto et al., 2006). Here we describe the phenotypes of the nrgG00305 allele in the GFS for the first time.
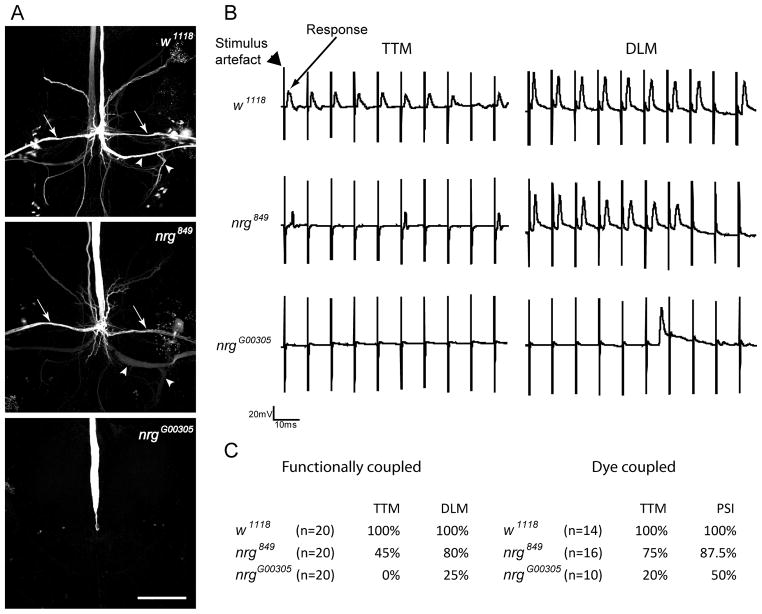
In order to look for qualitative and quantitative differences between the two neuroglian alleles, we used Neurobiotin as a marker to trace anatomical coupling of the GF to the PSI and the TTM as well as recordings from the TTM and DLM muscle upon stimulation of the GF to test for the function of the outputs of the circuit. In all wild type animals, strong dye coupling of the GF to the PSI and the TTM was observed (Figure 3A) and TTM as well as DLM responses could be recorded when the GFs were activated with stimulation electrodes in the brain (Figure 1A and 3C). In contrast to wild type, the GF terminals of nrg849 mutants showed a variety of different morphologies (Figure 3 and 4). As previously described, in most nrg849 preparations the characteristic bend of the GF could be observed, but often the synaptic terminal appeared to be thinner and/or shorter (Figure 3 and 4) (Godenschwege et al., 2006). When the GFs of nrg849 mutants were injected with Neurobiotin, only in 75% and 87.5% of the cases the GFs dye coupled to the TTM and the PSI, respectively (Figure 3C). In addition the trans-synaptic fills of the TTMn but not the PSI were usually much weaker in the nrg849 mutants when compared to the wild type (Figure 3A). Fewer GFs were connected to the TTM functionally (45% versus 74%, Figure 3C) than with dye-coupling. The impendence mismatch of the small GF terminal and the large postsynaptic dendrite is a likely reason that often the mutant GF synapse was not strong enough to evoke an action potential in the TTMn at all or was less reliable. This was reflected in the inability to follow multiple stimuli given at 100Hz (Figure 3B and 3C). Finally, it is worth mentioning that in about 10% of investigated preparations, the GF established a second terminal to the contra-lateral side (Figure 4A), which often dye-coupled to the contra-lateral TTMn as well.
Figure 3.
Synaptic connectivity of the GFS components. (A) Projection views of confocal image stacks from Neurobiotin staining in wild type animals and nrg mutants. PSI (arrows) and TTMn (arrow heads) dendrites and axons were stained trans-synaptically via gap junctions to the GF in w1118 and nrg849. The dye coupling of the GF to the TTMn was weak in nrg849 mutants when compared to w1118 controls. In contrast, nrgG00305 flies often showed no dye coupling to the postsynaptic neurons and lacked the characteristic bended GF terminal. (B) Muscle recordings from wild type and nrg mutant flies. Shown are traces of recordings from the dorsal longitudinal muscle (DLM) and the tergotrochanteral muscle (TTM) while the GFs were stimulated in the brain 10 times at 100 Hz. In wild type but not in the nrg mutants the TTMn responses (arrow) could be seen after each stimulus (arrowhead). (C) Muscle recordings and Neurobiotin staining from wild type and nrg mutants charted for functional coupling and dye coupling of the GF to the postsynaptic targets. The w1118 control flies but not the nrg mutant flies were physiologically and anatomically coupled to the TTM and the DLM in all recorded animals.
Figure 4.
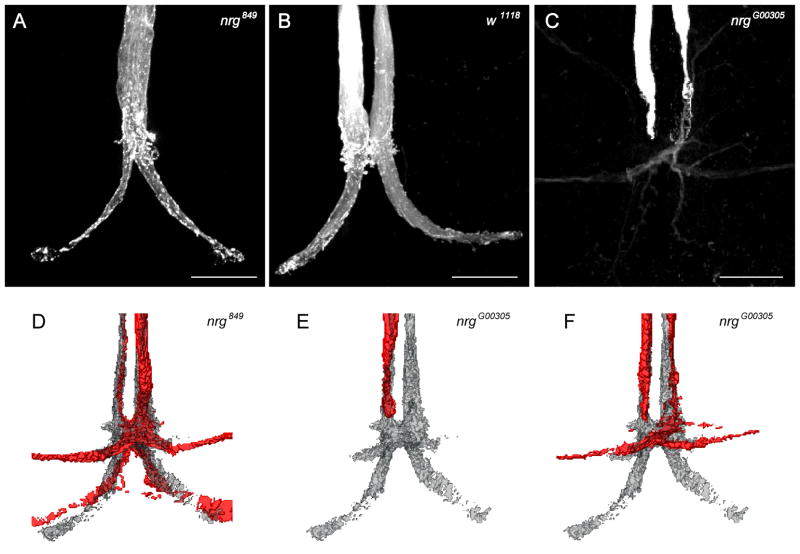
Analyses of mutant morphologies in the standard reference space. (A–C) Different GF morphological phenotypes were displayed as projection views from confocal image stacks. Scale bars=20μm. (A) Example of the right GF in a nrg849 mutant injected with Alexa555 dye. The terminal shows an additional branch projecting to the contra-lateral side, which is thinner when compared to the wild type GF terminals. (B) Example of wild type GF terminals stained with Alexa555 dye. (C) Dye coupling as observed with Neurobiotin in nrgG00305 mutants. Both GFs failed to form the large synaptic GF-TTMn terminal. The right GF was weakly dye coupled to the PSI. (D) Transformation of one nrg849 phenotype (red) onto the averaged wild type GF (gray). (E, F) Comparison of two transformed nrgG00305 staining (red) to the standard GF (gray).
The nrgG00305 allele showed much more severe GF terminal defects. In more than 50% of GF staining, the characteristic bend of the large GF terminal was missing (Figure 3A) but when present they were always thinner when compared to wild type terminals. Although a dye coupling of the GF to the TTM was observed in 20% of the preparations (Figure 3C), the staining of the TTM was very weak in all samples. In addition, dye coupling of the GFs to the PSI in nrgG00305 mutants was only found in 50% of the GFs injected with Neurobiotin (Figure 3C). Electrophysiological recordings from the GFS confirmed the severity of the phenotypes. Only 25% of the tested DLM muscles, but none of the TTM muscles showed a response, when the GFs were stimulated in the brain (Figure 3C). However, in both muscles, DLM and TTM, responses were seen when the motorneurons were stimulated directly, demonstrating that lack of output was not due to defects at the neuromuscular junction (data not shown).
In-depth morphological analysis of the nrg849 and nrgG00305 mutant alleles using the VNC standard
Most GF terminals of nrg mutants were absent or abnormal. In addition, due to the lack of any dye-coupling in several preparations, it was not clear if some of the GF terminated outside of the target area or terminated at the target but did not develop a synaptic connection at all. Occasionally, it was also not absolutely clear if the GF connected to the correct targets. Here we tested whether we could use the VNC standard to address these questions.
In order to do so, dye injections were therefore combined with anti-Brp staining and the GF anatomy of nrg849 or nrgG00305 mutants were compared to the averaged GF morphology of the VNC neuropil standard. Individual staining of GFs injected with Neurobiotin or Alexa555 (Figure 3 and 4) for both nrg mutant alleles were processed as described above. Registration of the reconstructed mutant GFs allowed displaying their morphology together with the averaged GF terminal projections (Figure 4D-F).
As previously mentioned, in nrg849 mutants a few GFs split and made an additional terminal at the contra-lateral side, a phenomenon never seen in wild type (Figure 4A). In another example, in which the right GF was injected, we could observe an overlap of the right GF-TTM terminal area (red, Figure 4D) with the standard GF terminal (gray, Figure 4D). However, after standard registration the additional left GF terminal was thinner in this sample, and the left GF-TTM connection was slightly deviated from the projection of the left average GF terminal (Figure 4D). However, the connection to the PSI was clearly visible and matched to a high degree the standard GF-PSI connection (Figure 4D).
In two examples, the GFs of nrgG00305 mutants failed to make the characteristic bend (Figure 3A and 4C). The reconstructions of these terminals after registration into the reference space revealed that in both animals the GF terminated anterior to the GF-PSI contact zone (Figure 4E and 4F), explaining why no dye coupling to the PSIs was observed in these cases. Only the right GF of a nrgG00305 mutant showed weak dye coupling (Figure 4C and 4F) and the standard registration allowed to verify that this GF projected to the correct GF-PSI contact zone enabling it to dye coupling to the PSI. Our results demonstrate that registered neuronal circuits in the VNC standard are a very useful tool, allowing a more in depth analysis and comparison of morphological phenotypes.
Discussion
In theory any labeled structure that is counterstained with the MAB nc82 can be integrated into the Drosophila neuropil standards. Integration of anatomical or functional units into one common reference space may give us new hints towards possible interactions of nervous system components that are not noticeable in an image side by side analysis. Further, integration of wild type structures into one framework will facilitate comparison of wild type to mutant phenotypes, offering ways to analyze and describe even small anatomical differences. Here, we show examples that demonstrate the feasibility of these applications of the VNC standard.
Stereotypical pattern of GFS synaptic contacts in the VNC standard
We used the Drosophila ventral nerve cord standard to describe the major components of the Giant Fiber System in a common reference system. We showed that after standard registration of labeled neurons from individual preparations and subsequently averaging, the neuronal structure can be integrated into the neuropil standard as reference structure.
With averaging a sample size of only 6 using Neurobiotin as a label, the GFs with their electrically coupled postsynaptic target cells were conserved within the standard; in particular the GF to TTMn synapse stood out as a prominent structure. This demonstrates that both, pre- and postsynaptic structure of the GF to TTMn terminal, have very little projection variability, resulting in a high probability of overlay after standard registration. The same could be observed for the GF-PSI contact through standard registration. The precise location of the contact area between these two neurons seemed to be highly conserved between samples. However, axonal and dendritic projections of the PSI were lost through the averaging procedure. Similarly, the part of the medial TTMn dendrite connecting to its posterior branch, the posterior dendrite as well as the soma showed a much higher variability between all 6 transformed samples resulting in a less precise overlay within the VNC standard.
In summary, despite some variability overall the GFS has a very stereotypical projection pattern indicated by the fact that there is little variation between individuals with regard to the synaptic contact zones of the GF to its postsynaptic partners within the nervous system. This is supported by the finding that the separate integration of pre- and postsynaptic neurons collected from individual animals resulted in the close proximity or overlap of the pre- and postsynaptic structures similar to double staining of both neurons in the same preparation. This shows that the VNC standard can be used to virtually reconstruct synaptic contact zones of individual neurons, here between the GF and the TTMn, and therefore may also be a useful tool to provide evidence for a potential yet unidentified synaptic contact between any neurons that are registered into the VNC standard.
Differences between nrg849 and nrgG00305 mutants revealed by standard registration
The integration of reference GFS components allowed us to compare wild type neuron projections to single neuron staining collected from mutant preparations. With labeling of only one reference structure, the neuropil with the commonly available antibody MAB nc82, we could transform mutant GF axon projections onto the standard. Registration into the reference system enabled us to analyze mutant morphology and to compare mutant phenotypes in relation to the previously registered wild type GFS reference structures.
We observed anatomical differences of GF phenotypes for both mutant nrg849 and nrgG00305 alleles when compared to wild type as well as to each other. Previously the characterization of mutant GF morphology was limited to the crude description of only severe GF terminal abnormalities such as the lack of the characteristic bended GF terminal. However, now with the registration of the GFS into the VNC standard, we can additionally describe and analyze in a standardized way the morphological phenotypes in detail by comparison with the projections of the standardized wild type GFS.
We found quantitative as well as qualitative differences between mutant nrg849 and nrgG00305 alleles. The nrgG00305 mutants exhibited much more severe phenotypes than nrg849 mutants as nrgG00305 GFs were less frequently connected anatomically as well as functionally with their postsynaptic targets (Figure 3). In addition, in nrgG00305 mutants but not in nrg849 mutants we found GFs that terminated in the VNC outside of the target area just anterior of the contact region between the GFs and the PSIs as revealed by the standard registration of the mutant GF anatomy (Figure 4). This indicates that the PSI and the TTMn do not extend outside their anterior-posterior projection zone to contact the GF stumps. Although nrg849 mutants occasionally do exhibit guidance defects because a few GFs do not leave the brain (Godenschwege et al., 2006), once they do leave the brain, so far we have never observed a GF that does not reach the GF-PSI target region but stops anterior as seen in nrgG00305 mutants. Finally, we have not observed in either mutant background that the GFs dye-coupled to an inappropriate target.
In wild type the GF always synapses only with the ipsi-lateral TTMn but never with the contra-lateral TTMn or both TTMns. This raises the question of whether the GF is not capable to make synaptic contacts to two TTMns or whether mechanisms such as the expression of GF repellent molecules at the midline or synaptic competition are the cause. Synaptic competition is that the postsynaptic cell is only innervated by one neuron despite the presence of multiple functional homolog neurons that are able to make a synapse with the target cell (Van Essen, Gordon, Soha, & Fraser, 1990; van Ooyen, 2001). However, the molecular mechanisms of this phenomenon are not well understood yet. Interestingly, though only seen occasionally in nrg849 mutants we did observe some GFs that made synaptic terminals to both TTMns as indicated by dye-coupling, suggesting that the GF has the intrinsic ability to cross the midline and innervate two TTMns simultaneously. This indicates that in a wild type situation each GF only innervates the ipsi-lateral TTMn because synaptic competition may be at play. Further studies that test this hypothesis and characterize the mechanisms involved are underway (Hill, Keshishian, & Murphey, 2008).
Material and Methods
Drosophila Stocks
All stocks were grown at 25oC on standard medium. The neuroglian nrg849, (originally named central body deranged 849) and nrgG00305 fly stocks as well as the shakB-GAL4 line have been described previously (Godenschwege et al., 2006; Jacobs et al., 2000; Morin et al., 2001; Strauss & Heisenberg, 1993; Williams, 2009; Yamamoto et al., 2006). The w1118 control strain was obtained from the Bloomington stock center.
Dissection
For all experiments we used 2–5 days old female flies. Prior to tissue preparation for dye filling the flies were chilled on ice. After the removal of legs and wings, flies were pinned in a sylgard-coated (Dow Corning Co., Midland, MI) petri dish dorsal side up and submerged in Drosophila saline. Two insect pins were placed on the posterior side of the head to slightly stretch the neck. An incision was made along the dorsal midline of abdomen and thorax, and the thorax was opened by placing two pins through the flight muscles. Gut and glands were removed to expose the ventral nerve cord. Tissue lying beneath the cervical connective was carefully removed with forceps.
GF dye filling
Dissected Drosophila were dye filled under a microscope with fluorescence light source. Dye solution of 10% w/v Neurobiotin (Vector labs) with tetramethylrhodamine-labeled dextran (Invitrogen), to visualize dye diffusion, was prepared in 2M potassium acetate. The Neurobiotin solution was injected with a depolarizing current for 10 minutes into the axon of the GF at the cervical connective. A 10 mM Alexa Fluor 555 Hydrazide (Molecular Probes) dilution was prepared with 200mM KCl. Alexa Fluor hydrazide was injected with a hyperpolarizing current through a glass microelectrode (100 MΩ resistance).
Immunohistochemistry
Dye filled samples were immediately fixed in 4% paraformaldehyde (Fisher Scientific) in phosphate-buffered saline (PBS, Calbiochem). Fixative was removed and samples were rinsed in PBS. Prior to antibody incubation the samples were washed 6×30 minutes in PBS containing 0.5% TritonX-100 (Sigma). Primary antibodies were diluted in PBS containing 0.3% TritonX-100 and 2% bovine serum albumin (BSA, Sigma). The monoclonal mouse antibody nc82 (kindly provided by Erich Buchner) was used at a concentration of 1:100 and the monoclonal rabbit anti-GFP antibody (Sigma) was used at 1:400. After antibody incubation for 2 nights at 4°C samples were rinsed in PBS. For the detection of MAB nc82 we used a goat anti-mouse secondary antibody coupled to the Cy2 or Cy3 fluorophore. Anti-GFP signal was detected with a goat anti-rabbit-Cy2 antibody (Jackson ImmunoResearch). To visualize the Neurobiotin dye fills a Streptavidine-Cy3 conjugate (Jackson; 1:750) was applied together with the secondary antibodies. Incubation with the secondary antibodies was preformed over night in PBS at 4°C. Finally, samples were rinsed with PBS.
Confocal microscopy
Samples were dehydrated in an ascending ethanol series and embedded in methyl salicylate (MP Biomedicals). The fluorescence signals were scanned with a Nikon C1si Fast Spectral Confocal system with AOTF laser unit (Nikon) using a 40× oil immersion objective (numerical aperture 1.3). Each sample was scanned at an image resolution of 1024 times 1024 pixels, a 1.5 × zoom, and a z-step size of 0.7 μm.
Image preparation and standard registration
Image preparation and neuropil reconstruction was performed in Amira 4.1.1 (Mercury Computer Systems) as described in Boerner & Duch (2010). The projections of the GF and the TTMn were labeled manually with the magic wand tool in the segmentation editor in Amira. Registration of the labeled structures into the ventral nerve cord standard was performed in Amira 3.1, following the protocol for standard registration (Boerner & Duch, 2010).
Electrophysiological recordings from the Giant Fiber System
We used standard methods to obtain recordings from the TTM and the DLM muscle that we have previously described in detail elsewhere (Godenschwege et al., 2002). We gave single pulses of 40–60 V for 0.03 msec with tungsten electrodes placed in the brain to activate the GF and use saline-filled glass electrodes with a resistance of 40–60MΩ for recordings from the TTM and DLM muscle. While responses to single pulse were used to determine whether the output of the GF via the TTM or the DLM is functionally coupled (Figure 3C), 10 stimuli given at 100Hz were used to further test for the reliability of this coupling (Figure 3B).
Acknowledgments
The project described was supported by Grant Number R01HD050725 from the National Institute Of Child Health And Human Development to T.A.G. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Child Health And Human Development or the National Institutes of Health. We are grateful to the C. Duch and M. Heisenberg labs and to the Bloomington Stock Center for providing fly stocks and other resources. We also thank C. Trivigno and R. K. Murphey for their comments on the ms.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Allen MJ, Godenschwege TA, Tanouye MA, Phelan P. Making an escape: development and function of the Drosophila giant fibre system. Semin Cell Dev Biol. 2006;17(1):31–41. doi: 10.1016/j.semcdb.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Blagburn JM, Alexopoulos H, Davies JA, Bacon JP. Null mutation in shaking-B eliminates electrical, but not chemical, synapses in the Drosophila giant fiber system: a structural study. J Comp Neurol. 1999;404(4):449–458. [PubMed] [Google Scholar]
- Boerner J, Duch C. An average shape standard atlas for the adult Drosophila ventral nerve cord. J Comp Neurol. 2010;518(13):2437–2455. doi: 10.1002/cne.22346. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brandt R, Rohlfing T, Rybak J, Krofczik S, Maye A, Westerhoff M, et al. Three-dimensional average-shape atlas of the honeybee brain and its applications. J Comp Neurol. 2005;492(1):1–19. doi: 10.1002/cne.20644. [DOI] [PubMed] [Google Scholar]
- Burrows M. Monosynaptic connexions between wing stretch receptors and flight motoneurones of the locust. J Exp Biol. 1975;62(1):189–219. doi: 10.1242/jeb.62.1.189. [DOI] [PubMed] [Google Scholar]
- Burrows M, Laurent GJ, Field LH. Proprioceptive inputs to nonspiking local interneurons contribute to local reflexes of a locust hindleg. J Neurosci. 1988;8(8):3085–3093. doi: 10.1523/JNEUROSCI.08-08-03085.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquet W, Owald D, Wichmann C, Mertel S, Depner H, Dyba M, et al. Maturation of active zone assembly by Drosophila Bruchpilot. J Cell Biol. 2009;186(1):129–145. doi: 10.1083/jcb.200812150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godenschwege TA, Kristiansen LV, Uthaman SB, Hortsch M, Murphey RK. A conserved role for Drosophila Neuroglian and human L1-CAM in central-synapse formation. Curr Biol. 2006;16(1):12–23. doi: 10.1016/j.cub.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Murphey RK. Genetic interaction of Neuroglian and Semaphorin1a during guidance and synapse formation. J Neurogenet. 2009;23(1–2):147–155. doi: 10.1080/01677060802441380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godenschwege TA, Simpson JH, Shan X, Bashaw GJ, Goodman CS, Murphey RK. Ectopic expression in the giant fiber system of Drosophila reveals distinct roles for roundabout (Robo), Robo2, and Robo3 in dendritic guidance and synaptic connectivity. J Neurosci. 2002;22(8):3117–3129. doi: 10.1523/JNEUROSCI.22-08-03117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JJ, Keshishian HS, Murphey RK. Target Recognition in the Drosophila giant fiber system: a new take on old signals. 38th Society for Neuroscience meeting; Nov 15–19th; Washington DC. 2008. [Google Scholar]
- Hofbauer A. Unpublished Professorial dissertation. University of Wurzburg; Germany: 1991. A library of monoclonal antibodies against the brain of Drosophila melanogaster. [Google Scholar]
- Hofbauer A, Ebel T, Waltenspiel B, Oswald P, Chen YC, Halder P, et al. The Wuerzburg hybridoma library against Drosophila brain. J Neurogenet. 2009;23(1–2):78–91. doi: 10.1080/01677060802471627. [DOI] [PubMed] [Google Scholar]
- Jacobs K, Todman MG, Allen MJ, Davies JA, Bacon JP. Synaptogenesis in the giant-fibre system of Drosophila: interaction of the giant fibre and its major motorneuronal target. Development. 2000;127(23):5203–5212. doi: 10.1242/dev.127.23.5203. [DOI] [PubMed] [Google Scholar]
- Jefferis GS, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR, Jr, et al. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128(6):1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis GS, Vyas RM, Berdnik D, Ramaekers A, Stocker RF, Tanaka NK, et al. Developmental origin of wiring specificity in the olfactory system of Drosophila. Development. 2004;131(1):117–130. doi: 10.1242/dev.00896. [DOI] [PubMed] [Google Scholar]
- King DG, Wyman RJ. Anatomy of the giant fibre pathway in Drosophila. I. Three thoracic components of the pathway. J Neurocytol. 1980;9(6):753–770. doi: 10.1007/BF01205017. [DOI] [PubMed] [Google Scholar]
- Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, et al. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312(5776):1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- Kurylas AE, Rohlfing T, Krofczik S, Jenett A, Homberg U. Standardized atlas of the brain of the desert locust, Schistocerca gregaria. Cell Tissue Res. 2008;333(1):125–145. doi: 10.1007/s00441-008-0620-x. [DOI] [PubMed] [Google Scholar]
- Kvello P, Lofaldli BB, Rybak J, Menzel R, Mustaparta H. Digital, Three-dimensional Average Shaped Atlas of the Heliothis Virescens Brain with Integrated Gustatory and Olfactory Neurons. Front Syst Neurosci. 2009;3:14. doi: 10.3389/neuro.06.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laissue PP, Reiter C, Hiesinger PR, Halter S, Fischbach KF, Stocker RF. Three-dimensional reconstruction of the antennal lobe in Drosophila melanogaster. J Comp Neurol. 1999;405(4):543–552. [PubMed] [Google Scholar]
- Laurent G, Burrows M. A population of ascending intersegmental interneurones in the locust with mechanosensory inputs from a hind leg. J Comp Neurol. 1988;275(1):1–12. doi: 10.1002/cne.902750102. [DOI] [PubMed] [Google Scholar]
- Morin X, Daneman R, Zavortink M, Chia W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc Natl Acad Sci U S A. 2001;98(26):15050–15055. doi: 10.1073/pnas.261408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Cracking neural circuits in a tiny brain: new approaches for understanding the neural circuitry of Drosophila. Trends Neurosci. 2008;31(10):512–520. doi: 10.1016/j.tins.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BH, Altman JS, Tyrer NM. Synaptic connections between the hindwing stretch receptor and flight motor neurones in the locust revealed by double cobalt labelling for electron microscopy. J Comp Neurol. 1985;233(2):269–284. doi: 10.1002/cne.902330208. [DOI] [PubMed] [Google Scholar]
- Phelan P, Nakagawa M, Wilkin MB, Moffat KG, O’Kane CJ, Davies JA, et al. Mutations in shaking-B prevent electrical synapse formation in the Drosophila giant fiber system. J Neurosci. 1996;16(3):1101–1113. doi: 10.1523/JNEUROSCI.16-03-01101.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein K, Zockler M, Heisenberg M. A quantitative three-dimensional model of the Drosophila optic lobes. Curr Biol. 1999;9(2):93–96. doi: 10.1016/s0960-9822(99)80021-9. [DOI] [PubMed] [Google Scholar]
- Rein K, Zockler M, Mader MT, Grubel C, Heisenberg M. The Drosophila standard brain. Curr Biol. 2002;12(3):227–231. doi: 10.1016/s0960-9822(02)00656-5. [DOI] [PubMed] [Google Scholar]
- Reye DN, Pearson KG. Projections of the wing stretch receptors to central flight neurons in the locust. J Neurosci. 1987;7(8):2476–2487. [PMC free article] [PubMed] [Google Scholar]
- Riemensperger T, Voller T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol. 2005;15(21):1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993;13(5):1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Gordon H, Soha JM, Fraser SE. Synaptic dynamics at the neuromuscular junction: mechanisms and models. J Neurobiol. 1990;21(1):223–249. doi: 10.1002/neu.480210115. [DOI] [PubMed] [Google Scholar]
- van Ooyen A. Competition in the development of nerve connections: a review of models. Network. 2001;12(1):R1–47. [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49(6):833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Williams MJ. The Drosophila cell adhesion molecule Neuroglian regulates Lissencephaly-1 localisation in circulating immunosurveillance cells. BMC Immunol. 2009;10:17. doi: 10.1186/1471-2172-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109(2):229–241. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Ueda R, Takahashi K, Saigo K, Uemura T. Control of axonal sprouting and dendrite branching by the Nrg-Ank complex at the neuron-glia interface. Curr Biol. 2006;16(16):1678–1683. doi: 10.1016/j.cub.2006.06.061. [DOI] [PubMed] [Google Scholar]
- Yellman C, Tao H, He B, Hirsh J. Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc Natl Acad Sci U S A. 1997;94(8):4131–4136. doi: 10.1073/pnas.94.8.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]