Abstract
BACKGROUND
Children with cerebral palsy (CP) have decreased strength, low bone mass, and an increased propensity to fracture. High frequency, low magnitude vibration might provide a non-invasive, non-pharmacological, home-based treatment for these musculoskeletal deficits. The purpose of this study was to examine the effects of this intervention on bone and muscle in children with CP.
METHODS
Thirty-one children with CP ages 6-12 years (mean 9.4, SD 1.4) stood on a vibrating platform (30 Hz, 0.3 g peak acceleration) at home for 10 min/day for 6 months and on the floor without the platform for another 6 months. The order of vibration and standing was randomized, and outcomes were measured at 0, 6, and 12 months. The outcome measures included computed tomography measurements of vertebral cancellous bone density (CBD) and cross-sectional area, CBD of the proximal tibia, geometric properties of the tibial diaphysis, and dynamometer measurements of plantarflexor strength. Outcomes were assessed using mixed model linear regression and Pearson's correlation.
RESULTS
The main difference between vibration and standing was greater increases in the cortical bone properties (cortical bone area and moments of inertia) during the vibration period (all p's ≤ 0.03). There was no difference in cancellous bone or muscle between vibration and standing (all p's > 0.10) and no correlation between compliance and outcome (all r's < 0.27; all p's > 0.15). The results did not depend on the order of treatment (p > 0.43) and was similar for children in GMFCS 1-2 and GMFCS 3-4.
CONCLUSIONS
The primary benefit of the vibration intervention in children with CP was to cortical bone in the appendicular skeleton. Increased cortical bone area and structural (strength) properties could translate into a decreased risk of long bone fractures for some patients. More research is needed to corroborate these findings, to elucidate the mechanisms of the intervention, and to determine the most effective age and duration for the treatment.
LEVEL OF EVIDENCE
Level II, prospective randomized cross-over study.
INTRODUCTION
Peak bone mass, which is achieved soon after the end of sexual development, is the most important determinant of bone mass and osteoporosis later in life [1-3]. Children with disabilities such as cerebral palsy (CP) are particularly vulnerable to deficits in bone mass accretion due to decreased mobility and weight-bearing which reduces mechanical loading of the skeleton [4-9]. Children with CP are known to have low bone density and may have an increased propensity to fracture, especially at lower functional levels [4-6, 10, 11]. These children also have poor muscle strength and control which limits function and contributes to the lack of mechanical stimulation needed to build bone mass. The primary treatment for these children is physical therapy, which is time and labor intensive and may not be available as frequently as needed.
Whole body vibration has shown promise as an alternative method for stimulating both increases in bone mass and improvements in muscle performance [12-17]. Animal studies have demonstrated that low-magnitude, high-frequency vibration can increase bone mass and bone strength and prevent bone loss [18, 19]. Studies in humans have also shown a benefit to bone in post-menopausal women [12] and a benefit to both bone and muscle in young women, ages 15-20 years, with low bone density [16]. In children with disabilities, a small pilot study found that 6 months of low-magnitude, high-frequency (0.3g, 90 Hz) whole body vibration increased bone density and prevented bone loss in the proximal tibias of a heterogeneous group of participants [15].
The purpose of this study was to examine the effects of high frequency, low magnitude vibration on bone and muscle in pre-teen children with cerebral palsy. We were interested in this group because they are at the age when humans have the most potential to accumulate bone [20]. Our hypothesis was that this mechanical stimulus may be useful as a simple, non-invasive, non-pharmacological intervention for low bone mass and poor muscle function in these children.
METHODS
This was a prospective randomized study in which each subject participated in both a 6-month intervention and a 6-month control period. Participants were randomized in terms of the order of participation (i.e., intervention or control first) using computer-generated group assignments, balanced in sets of 8 and implemented using sealed envelopes. The observation periods were consecutive with no washout period because the outcome measures increase with growth regardless of intervention. Thus, outcomes must be measured in terms of the change in bone or muscle properties during each period. Greater increases during vibration indicate a positive effect of the intervention. The study was approved by the institutional review board (IRB) at our institution, and written informed assent and consent were obtained from the participants and their parents.
Participants
The participants were children with CP ages 6-12 years who were able to stand for 10 minutes with or without handheld support (such as a walker). Potential participants were excluded if they had high vertebral cancellous bone density (>295 mg/cm3, approximately 1 standard deviation above the average for typical development). Only 1 of the 37 children screened was excluded based on this criterion. These children were selected as the study population because, among those who are able to comply with the intervention protocol, they are among the most likely to show a response due to their young age and potential for bone mass accrual. Other exclusion criteria included surgery, casting, or botulinum-toxin injection in the last 12 months, metal rods or plates in the tibia or lumbar spine, scoliosis >20° or bowing of the tibia, concomitant medical conditions affecting bone or muscle, and use of corticosteroids or seizure medication. The participants were recruited by calling current and past patients from the orthopaedic clinics at a tertiary pediatric medical center.
The original sample size was determined by a power analysis based on a two-tailed two-group comparison assuming α = 0.05 and using pilot data for the change in vertebral bone density and muscle area measures after vibration in non-disabled children. This analysis indicated that 34 subjects would be needed (17 in each group) to detect a 10% difference with 90% power; two additional subjects were recruited to replace subjects who dropped out of the study while recruitment was still occurring.
Intervention
Participants were asked to stand on a vibrating platform (Juvent Medical Inc., Somerset, NJ) at home for 10 min/day for 6 months (vibration period) and without the vibrating platform for an additional 6 months (control period). The order of vibration or standing was randomized so that half the participants vibrated first (vibration first group) and the other half stood first (standing first group). The children were allowed to wear ankle-foot orthoses (AFOs) while standing if this made them feel more stable. The vibration platforms operated at 30 Hz with a peak acceleration of 0.3 g, which is barely perceptible. These parameters were selected to simulate the physiologic loading caused by muscle contractibility during postural control [21, 22]. The device had an internal computer that recorded the time and duration of usage. Participants were also asked to keep a daily log of their participation and were contacted regularly by phone to check on their compliance. Compliance was calculated as the number of days of standing or vibration divided by the total number of days between outcome assessments. The outcome assessments were performed at baseline, 6 months (after the first period of participation), and 12 months (after the second period of participation).
Dietary Intake Assessment
Nutritional status was assessed using written recall records of dietary intake [23]. Average daily calcium and vitamin D intake was calculated from these records using ESHA Food Processor (version 9.9, ESHA Research, Salem, OR).
CT Measurements of Bone and Muscle
All participants were assessed by CT using the same scanner (General Electric LightSpeed QX/i, Milwaukee, WI) and the same mineral reference phantom for simultaneous calibration (CT-T bone densitometry package; General Electric), and all studies were performed by the same technologist who was blinded to the participants’ group assignments. The time required to complete the CT scans in individual patients was approximately 10 minutes. In the axial skeleton, identification of the site to be scanned was performed with a lateral scout view. A single 10 mm slice was obtained at the midportion of the L3 vertebral body. Cancellous bone density (CBD, mg/cm3) and cross-sectional area (CSA, mm2) were determined at this level. The coefficients of variation (CV) for repeated CT measurements of vertebral density and CSA are 1-2% [24, 25].
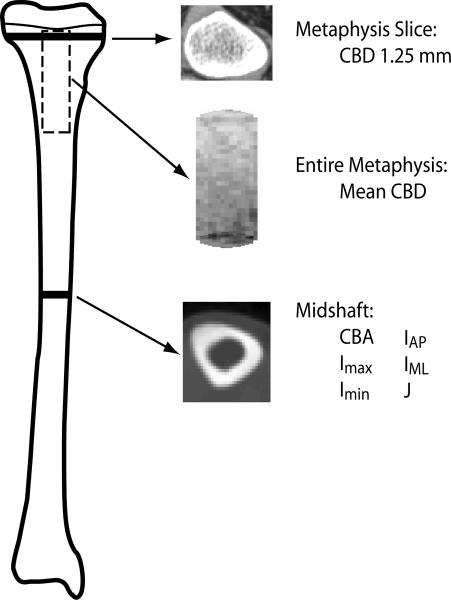
In the appendicular skeleton, location of the sites to be scanned was done by physical examination. Since it is sometimes difficult to align both limbs in the scanner simultaneously for children with CP, the right side was aligned for children with bilateral involvement, and the affected side was aligned for children with unilateral involvement. Contiguous 1.25 mm slices were obtained covering the proximal tibial metaphysis, and an additional 10 mm slice was obtained at the midshaft of the tibia [26]. The following measures were determined in the metaphysis: mean cancellous bone density (CBD, mg/cm3) and CBD in a single slice 1.25 mm below the growth plate [26] (Figure 1). These measures characterize trabecular bone across the entire metaphysis. The following parameters were measured at the midshaft of the tibia: cortical bone area (CBA, mm2), maximum and minimum principal moments of inertia (Imax and Imin, mm4), moments of inertia about anterior-posterior (AP) and medial-lateral (ML) axes (IAP and IML, mm4), and polar moment of inertia (J, mm4) (Figure 1). CBA is an indicator of the bone's compressive properties; Imax, Imin, IAP, and IML are indicators of its bending properties; J is an indicator of torsional properties. Calf muscle area (mm2) was also determined from the same CT cross-sectional images obtained at the midshaft of the tibia. The CVs for bone measurements in the appendicular skeleton are 0.3 to 2.8% [4, 27], and the CVs for muscle measures are 1 to 2% [28].
Figure 1.
Location of CT scans from the tibia.
Measurements from the aligned leg of each participant (affected leg for children with hemiplegia, right leg for other children) were used in the analysis. All image analysis was done using custom algorithms in Matlab version R2006b (Mathworks, Natick, MA) [29]. For the diaphysis, the geometric properties were calculated using contours from edge detection based on the density gradient between neighboring voxels [29]. For the metaphysis, a cylindrical core of cancellous bone was extracted along the entire length of the metaphysis, and densities were calculated as described previously [26].
Strength Measurements
Concentric and eccentric calf muscle strength was assessed using a Kin-Com 125AP dynamometer (Chattanooga Group, Hixson, TN). Calf strength was measured because previous studies have suggested that the vibration may improve muscle function and that muscle activity may be a mechanism for stimulating bone accretion [16, 21, 22]. With the participant lying supine on the dynamometer surface, the participant's foot was positioned on a footplate with the ankle joint axis aligned with the center of rotation of the dynamometer's lever arm. The ankle joint range of motion was established, and the joint was moved passively though its range of motion. The patient was then asked to actively assist the motion (push as hard as possible against the plate) as the ankle moved from dorsiflexion to plantarflexion to measure concentric strength. Similarly, the participant was asked to resist the motion (keep the plate from moving) as the dynamometer moved from plantarflexion to dorsiflexion to measure eccentric strength. Maximum active torque was calculated from the force and lever arm measurements after correction for the effects due to gravity and passive resistance. This method of measuring strength is the preferred method for participants who may not have the strength to move the dynamometer on their own [30].
Statistical Analysis
Linear regression was used to evaluate whether baseline vertebral bone density was lower than typical and to examine the influence of sex and GMFCS level on the baseline vertebral Z-scores. Pearson's correlation was used to investigate the relationship between compliance and outcomes. A significance level of α = 0.05 was used for all statistical tests.
Outcomes were examined using mixed model linear regression. The initial regression model included fixed terms for treatment (vibration or standing), period (first or second), and the interaction between treatment and period. The participant was included as a nested random effect. To account for growth of the participants during the course of the study, change in height was also included in the regression model. Finally, demographic, bone, muscle, and dietary measurements at the start of each period were examined for possible inclusion in the regression model. For models where convergence was not achieved, estimates were based on iterated expectation-maximization. Results were examined for all subjects combined and for GMFCS 1-2 and GMFCS 3-4 subgroups.
RESULTS
Thirty-six children enrolled in the study; 31 of these children completed the full year of participation and comprise the analysis cohort (Table 1). The 5 participants who dropped out did so because they moved out of town (3 participants), could no longer be contacted (1 participant), or decided they did not want to stand on the machine (1 participant). The distribution of functional levels according to the Gross Motor Function Classification System (GMFCS) was 10 participants in GMFCS level 1, 4 participants in level 2, 15 participants in level 3, and 2 participants in level 4. Seventeen of the participants walked with assistive devices, and 14 were independent ambulators.
Table 1.
Subject characteristics at start of study.
| Variable | Statistic | N = 31 |
|---|---|---|
| Age (yr) | Mean ± SD | 9.4 ± 1.4 |
| Sex | Male, N (%) | 18 (42%) |
| Female, N (%) | 13 (58%) | |
| GMFCS | 1 | 10 (32%) |
| 2 | 4 (13%) | |
| 3 | 15 (48%) | |
| 4 | 2 (6%) | |
| Involvement | Hemiplegia, N (%) | 4 (13%) |
| Diplegia, N (%) | 18 (58%) | |
| Triplegia, N (%) | 2 (6%) | |
| Quadriplegia, N (%) | 7 (23%) | |
| Height (cm) | Mean ± SD | 127 ± 10 |
| Weight (kg) | Mean ± SD | 29 ± 9 |
| BMI (kg/m2) | Mean ± SD | 18 ± 4 |
| Height (%) | Mean ± SD | 18 ± 22 |
| Weight (%) | Mean ± SD | 36 ± 33 |
| BMI (%) | Mean ± SD | 58 ± 32 |
% represents percentile for sex and age
At baseline, the participants had a mean ± standard deviation (SD) vertebral CBD of 135.8 ± 23.9 mg/cm3. This corresponded with a Z-score of -0.6 ± 0.7 (range: -1.7 to 0.9), where Z-score represents the number of SD an individual's CBD is above or below the mean of typical development. The Z-scores of the study participants were significantly lower than typical development (95% CI for difference from 0: -0.9 to -0.4; p < 0.001). Eleven of 31 (35%) participants had Z-scores between -1 and -2, and 14/31 (45%) had Z-scores between 0 and -1. The Z-scores did not differ significantly by sex (p = 0.98) or GMFCS level (p = 0.64) in this cohort.
Descriptive results for the bone and muscle measures are presented in Table 2A and Table 2B. There were no significant differences in baseline demographic or bone measures at the start of the vibration and standing periods (all p's > 0.40). There were also no significant differences in baseline muscle or strength measures (all p's > 0.46) or in calcium and vitamin D intake (both p's > 0.41). Therefore, baseline measures were not included in the model used to compare outcomes. In addition, the interaction term was not included in the final model because no significant interaction was observed (all p's > 0.15). The final model therefore included treatment (vibration or standing), period (first or second), change in height to account for growth during the study, and the participant random effect.
Table 2A.
Bone and muscle measures for Vibration First group. Values are mean (SD).
| Variable | N | Baseline | 6 months | 12 months |
|---|---|---|---|---|
| L3 vertebral body | ||||
| CBD (mg/cm3) | 16 | 133 (23) | 133 (22) | 132 (23) |
| CSA (mm2) | 16 | 6.8 (1.0) | 7.2 (1.0) | 7.7 (1.2) |
| | ||||
| Tibia metaphysis | ||||
| Mean CBD (mg/cm3) | 15 | 63 (24) | 61 (25) | 58 (26) |
| CBD 1.25mm (mg/cm3) | 15 | 103 (41) | 105 (44) | 101 (59) |
| | ||||
| Tibia midshaft | ||||
| CBA (mm2) | 16 | 139 (38) | 152 (40) | 159 (40) |
| Imax (mm4) | 16 | 4063 (2722) | 4715 (2969) | 5063 (2857) |
| Imin (mm4) | 16 | 2438 (1434) | 2792 (1552) | 2942 (1482) |
| IAP (mm4) | 16 | 2834 (1684) | 3258 (1591) | 3400 (1500) |
| IML (mm4) | 16 | 3668 (2476) | 4249 (3002) | 4606 (2901) |
| J (mm4) | 16 | 6502 (4137) | 7507 (4501) | 8005 (4314) |
| | ||||
| Calf muscle | ||||
| Muscle area (mm2) | 16 | 15.0 (6.3) | 15.2 (6.2) | 15.3 (5.5) |
| Concentric torque (N-m) | 15 | 840 (758) | 1441 (1240) | 1463 (1379) |
| Eccentric torque (N-m) | 15 | 1186 (804) | 1889 (1360) | 2007 (1406) |
Data are shown for subjects with complete data for each variable. One subject had missing tibia metaphysis data at 12 months due to motion artifact. One subject was unable to comply with muscle strength testing.
Table 2B.
Bone and muscle measures for Standing First group. Values are mean (SD).
| Variable | N | Baseline | 6 months | 12 months |
|---|---|---|---|---|
| L3 vertebral body | ||||
| CBD (mg/cm3) | 14 | 141 (24) | 142 (24) | 141 (25) |
| CSA (mm2) | 14 | 7.2 (1.1) | 7.5 (1.3) | 7.8 (1.3) |
| | ||||
| Tibia metaphysis | ||||
| Mean CBD (mg/cm3) | 14 | 64 (24) | 60 (22) | 53 (24) |
| CBD 1.25mm (mg/cm3) | 14 | 118 (41) | 111 (40) | 95 (49) |
| | ||||
| Tibia midshaft | ||||
| CBA (mm2) | 13 | 163 (39) | 170 (41) | 182 (44) |
| Imax (mm4) | 13 | 5489 (2931) | 5917 (3299) | 6957 (3755) |
| Imin (mm4) | 13 | 3342 (1546) | 3545 (1675) | 4089 (1996) |
| IAP (mm4) | 13 | 3993 (2137) | 4172 (2230) | 4687 (2254) |
| IML (mm4) | 13 | 4838 (2380) | 5289 (2793) | 6359 (3518) |
| J (mm4) | 13 | 8831 (4437) | 9462 (4931) | 11,045 (5707) |
| | ||||
| Calf muscle | ||||
| Muscle area (mm2) | 14 | 18.6 (8.4) | 17.7 (7.3) | 17.5 (5.1) |
| Concentric torque (N-m) | 15 | 1379 (1024) | 1750 (1181) | 1960 (1406) |
| Eccentric torque (N-m) | 15 | 2257 (1994) | 3009 (2312) | 3322 (2135) |
Data are shown for subjects with complete data for each variable. One subject had missing tibia midshaft data at baseline due to poor image quality. One subject did not have CT imaging at 12 months due to subject request.
The only significant difference in outcome measures between the vibration and standing periods was greater increases in the cortical bone properties during the vibration period (Table 3). The results were similar for absolute and percent change, except that IML showed a significant difference only for absolute change (p = 0.04). The results did not depend on the order of treatment (p > 0.43) (Table 4).
Table 3.
Percentage change in bone parameters during the vibration and control periods.
| Percent Change, mean (SD) | Vibration vs. Standing* | |||
|---|---|---|---|---|
| Variable | N | Vibration | Standing (Control) | |
| All Participants | ||||
| L3 vertebral body | ||||
| CBD (mg/cm3) | 30 | -0.2 (8.2) | 0.1 (8.8) | 0.71 |
| CSA (mm2) | 30 | 6.0 (4.6) | 5.2 (5.4) | 0.48 |
| Tibia metaphysis | ||||
| Mean CBD (mg/cm3) | 29 | -6.7 (22.8) | -4.3 (27.7) | 0.57 |
| CBD 1.25mm (mg/cm3) | 29 | -6.4 (30.7) | -4.1 (30.5) | 0.54 |
| Tibia midshaft | ||||
| CBA (mm2) | 29 | 8.5 (4.7) | 4.9 (6.1) | 0.02 |
| Imax (mm4) | 29 | 18.5 (14.8) | 9.6 (14.1) | 0.01 |
| Imin (mm4) | 29 | 15.9 (14.6) | 7.9 (15.1) | 0.03 |
| IAP (mm4) | 29 | 17.0 (16.1) | 6.9 (17.0) | 0.02 |
| IML (mm4) | 29 | 17.9 (18.3) | 11.4 (17.3) | 0.13 |
| J (mm4) | 29 | 17.4 (14.5) | 8.9 (14.3) | 0.02 |
| | ||||
| GMFCS 1 & 2 | ||||
| L3 vertebral body | ||||
| CBD (mg/cm3) | 13 | -2.6 (6.1) | -3.1 (5.2) | 0.88 |
| CSA (mm2) | 13 | 5.4 (3.8) | 6.8 (3.7) | 0.39 |
| Tibia metaphysis | ||||
| Mean CBD (mg/cm3) | 13 | -10.1 (20.1) | -1.7 (24.5) | 0.25 |
| CBD 1.25mm (mg/cm3) | 13 | -9.3 (16.3) | -6.3 (15.5) | 0.55 |
| Tibia midshaft | ||||
| CBA (mm2) | 12 | 7.2 (4.3) | 4.4 (4.1) | 0.03 |
| Imax (mm4) | 12 | 15.4 (12.5) | 8.9 (13.2) | 0.13 |
| Imin (mm4) | 12 | 12.9 (10.6) | 8.6 (10.8) | 0.21 |
| IAP (mm4) | 12 | 17.1 (15.1) | 5.8 (13.6) | 0.02 |
| IML (mm4) | 12 | 12.1 (17.4) | 12.6 (16.4) | 0.98 |
| J (mm4) | 12 | 14.4 (11.6) | 8.8 (12.2) | 0.15 |
| | ||||
| GMFCS 3 & 4 | ||||
| L3 vertebral body | ||||
| CBD (mg/cm3) | 17 | 1.6 (9.3) | 2.5 (10.2) | 0.73 |
| CSA (mm2) | 17 | 6.4 (5.2) | 3.9 (6.2) | 0.12 |
| Tibia metaphysis | ||||
| Mean CBD (mg/cm3) | 16 | -3.9 (25.1) | -6.5 (30.6) | 0.90 |
| CBD 1.25mm (mg/cm3) | 16 | -4.0 (39.2) | -2.3 (39.2) | 0.55 |
| Tibia midshaft | ||||
| CBA (mm2) | 17 | 9.4 (5.0) | 5.3 (7.2) | 0.07 |
| Imax (mm4) | 17 | 20.7 (16.3) | 10.1 (15.1) | 0.05 |
| Imin (mm4) | 17 | 18.0 (16.8) | 7.3 (17.9) | 0.08 |
| IAP (mm4) | 17 | 16.9 (17.3) | 7.6 (19.4) | 0.16 |
| IML (mm4) | 17 | 22.0 (18.4) | 10.5 (18.3) | 0.06 |
| J (mm4) | 17 | 19.6 (16.3) | 9.0 (16.0) | 0.06 |
p-value for treatment using model with period, change in height, and participant as a covariates.
Table 4.
Regression results for percent change in tibia cortical bone area.
| Variable | Coefficient | SE | P-value |
|---|---|---|---|
| Intervention | 3.3 | 1.4 | 0.02 |
| Period | -1.1 | 1.4 | 0.43 |
| Height change | 0.3 | 0.3 | 0.33 |
In the tibial diaphysis, cortical bone area and all moments of inertia increased during both periods (all p's ≤ 0.03). There were significantly larger changes during the vibration period for all diaphyseal measures except percentage change of IML (all p's ≤ 0.03) (Table 3). In the axial skeleton, the cross-sectional area of L3 increased during both periods (p < 0.001), but cancellous bone density did not change significantly during either period (p > 0.73). Similar results were obtained for both GMFCS subgroups, although p-values were slightly increased (Table 3).
Muscle area did not change significantly during either vibration or standing (p ≥ 0.10). Eccentric torque increased during both periods (p < 0.03), and concentric torque increased during vibration (p = 0.02), primarily due to increases in moment arm. This increase in torque was not observed in the GMFCS 3-4 subgroup (p > 0.26). There were no differences between vibration and standing for any of the muscle or strength variables (all p's > 0.10).
There was a wide range of compliance with an approximately even distribution from 24% to 99% compliance. There was no correlation between compliance and any of the outcome measures (all r's < 0.27; all p's > 0.15). This can be visualized for CBA in Figure 2. Similar results were obtained for the other outcome variables.
Figure 2.
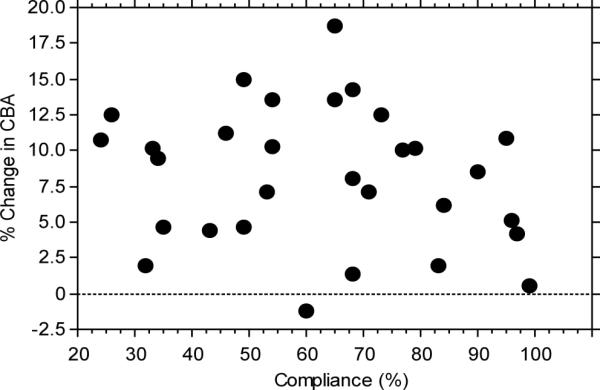
Percent change in tibia cortical bone area as a function of compliance.
DISCUSSION
In this study, we found that the primary benefit of the vibration intervention in children with CP was to cortical bone in the appendicular skeleton. At the midshaft of the tibia, more bone accretion occurred during the vibration intervention than during the control period. This led to increased cortical bone area and moments of inertia, which could potentially translate into decreased risk of long bone fractures for some children. These changes were not related to the time spent using the vibration device, and no effect was observed in the spine, tibial metaphysis, or calf muscles.
As in previous studies [12, 15, 16], we observed a wide range of compliance with the intervention from very little use of the vibration device to almost perfect compliance. However, we did not see an effect of compliance on the outcome measures. This is in contrast to previous studies which found greater benefits of the vibration in participants with higher compliance [12, 16]. Compliance is difficult to measure and verify. We attempted to obtain as accurate a measure of compliance as possible by using the vibration device to record usage time, but it is possible that the lack of compliance effect was due to inaccurate compliance values. It is also possible that it was due to the limited physical status of our cohort. In children with CP, the baseline loading on the skeleton is lower than in non-disabled populations, which may magnify the contribution of the mechanical stimulation provided by the intervention. If this is the case, then any exposure to the vibration may stimulate osteoblasts along the periosteal surface so that even a limited amount of vibration stimulation may be of benefit for children with physical disabilities such as CP. Alternatively, the increase in geometric properties of the tibia may be induced by a mechanism unrelated to the vibration intervention.
We did not observe a benefit of the intervention to cancellous bone in the proximal tibia and spine, in contrast to previous studies [12, 15, 16]. This may be due in part to differences in the patient populations. Our study examined 6 to 12 year old children with CP. The only other study involving participants with disabilities included children with muscular dystrophy in addition to CP [15]. Perhaps more importantly, the participants in previous studies were either already skeletally mature [12, 16] or very heterogeneous in age (range 4-19 yr, with 5/10 participants postpubertal in control group) [15]. Age greatly affects the rate of bone accretion or loss. In the previous studies, benefits to cancellous bone were primarily small gains compared with losses in the control group. In contrast, in our age group (6-12 yr), cancellous bone density is expected to be stable. We have previously shown that cancellous density does not begin to increase until approximately 12 years of age [31]. It is therefore not surprising that cancellous density did not substantially increase or decrease in our study.
A limitation of the current study is that it involved only relatively functional children with CP. Because the configuration of the vibration device required the participants to be able to stand on the platform, our study sample excluded non-ambulatory children and included only two children in GMFCS level 4. More involved children have the greatest risk of fractures and might be expected to benefit most from the intervention. Recent research suggests that the key mechanical stimulus may be the acceleration induced by the vibration, rather than longitudinal strains [32], making possible adaptation of the intervention for patients who cannot stand. In fact, specific customizations have been created to allow individual patients to be supported in standers or to receive the intervention in a sitting position. The effectiveness of vibration in these configurations would need to be tested in a separate study. Since bone deficits are greater in less functional children with CP [6], the intervention could have larger effects in a broader CP population that includes non-ambulatory children. The results of the current study should be considered preliminary until larger and more inclusive studies can be conducted.
The positive effects of the intervention are presumably associated with increased mechanical stimulation of the bone. However, this increased stimulation does not appear to result from gross changes in muscle size or strength. Rather, it may be due to changes in neuromuscular control [14] and/or direct transmission of the vibration stimulus to the bone [33]. Additional research is needed to further elucidate these mechanisms.
In summary, we found that 6 months of high frequency, low magnitude vibration increased cortical bone area and moments of inertia in the tibial diaphysis of pre-teen children with cerebral palsy. This benefit was not dependent on the number of days per week that the vibration device was used and did not result from increases in muscle mass or strength. No effect was seen on cancellous bone in this study. More research is needed to confirm these findings, to establish their clinical significance, to elucidate the mechanisms of the intervention, and to determine the most effective age and duration for the treatment.
ACKNOWLEDGEMENTS
Support for this research was provided by grant number 5 R21 AR051564 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) at the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hansen MA, Overgaard K, Riis BJ, et al. Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. Bmj. 1991;303:961–4. doi: 10.1136/bmj.303.6808.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui SL, Slemenda CW, Johnston CC., Jr. Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988;81:1804–9. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilsanz V, Gibbens DT, Carlson M, et al. Peak trabecular vertebral density: a comparison of adolescent and adult females. Calcif Tissue Int. 1988;43:260–2. doi: 10.1007/BF02555144. [DOI] [PubMed] [Google Scholar]
- 4.Binkley T, Johnson J, Vogel L, et al. Bone measurements by peripheral quantitative computed tomography (pQCT) in children with cerebral palsy. J Pediatr. 2005;147:791–6. doi: 10.1016/j.jpeds.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Tasdemir HA, Buyukavci M, Akcay F, et al. Bone mineral density in children with cerebral palsy. Pediatr Int. 2001;43:157–60. doi: 10.1046/j.1442-200x.2001.01352.x. [DOI] [PubMed] [Google Scholar]
- 6.Henderson RC, Lark RK, Gurka MJ, et al. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics. 2002;110:e5. doi: 10.1542/peds.110.1.e5. [DOI] [PubMed] [Google Scholar]
- 7.Henderson RC, Lin PP, Greene WB. Bone-mineral density in children and adolescents who have spastic cerebral palsy. J Bone Joint Surg Am. 1995;77:1671–81. doi: 10.2106/00004623-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Shaw NJ, White CP, Fraser WD, et al. Osteopenia in cerebral palsy. Arch Dis Child. 1994;71:235–8. doi: 10.1136/adc.71.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houlihan CM, Stevenson RD. Bone density in cerebral palsy. Phys Med Rehabil Clin N Am. 2009;20:493–508. doi: 10.1016/j.pmr.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevenson RD, Conaway M, Barrington JW, et al. Fracture rate in children with cerebral palsy. Pediatr Rehabil. 2006;9:396–403. doi: 10.1080/13638490600668061. [DOI] [PubMed] [Google Scholar]
- 11.Henderson RC. Bone density and other possible predictors of fracture risk in children and adolescents with spastic quadriplegia. Dev Med Child Neurol. 1997;39:224–7. doi: 10.1111/j.1469-8749.1997.tb07415.x. [DOI] [PubMed] [Google Scholar]
- 12.Rubin C, Recker R, Cullen D, et al. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343–51. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 13.Rubin C, Turner AS, Bain S, et al. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412:603–4. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 14.Torvinen S, Kannus P, Sievanen H, et al. Effect of 8-month vertical whole body vibration on bone, muscle performance, and body balance: a randomized controlled study. J Bone Miner Res. 2003;18:876–84. doi: 10.1359/jbmr.2003.18.5.876. [DOI] [PubMed] [Google Scholar]
- 15.Ward K, Alsop C, Caulton J, et al. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–9. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 16.Gilsanz V, Wren TA, Sanchez M, et al. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464–74. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 17.Bosco C, Colli R, Introini E, et al. Adaptive responses of human skeletal muscle to vibration exposure. Clin Physiol. 1999;19:183–7. doi: 10.1046/j.1365-2281.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 18.Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. Faseb J. 2001;15:2225–9. doi: 10.1096/fj.01-0166com. [DOI] [PubMed] [Google Scholar]
- 19.Rubin CT, Sommerfeldt DW, Judex S, et al. Inhibition of osteopenia by low magnitude, high-frequency mechanical stimuli. Drug Discov Today. 2001;6:848–858. doi: 10.1016/s1359-6446(01)01872-4. [DOI] [PubMed] [Google Scholar]
- 20.Gilsanz V, Gibbens DT, Roe TF, et al. Vertebral bone density in children: effect of puberty. Radiology. 1988;166:847–50. doi: 10.1148/radiology.166.3.3340782. [DOI] [PubMed] [Google Scholar]
- 21.Fritton SP, McLeod KJ, Rubin CT. Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J Biomech. 2000;33:317–25. doi: 10.1016/s0021-9290(99)00210-9. [DOI] [PubMed] [Google Scholar]
- 22.Huang RP, Rubin CT, McLeod KJ. Changes in postural muscle dynamics as a function of age. J Gerontol A Biol Sci Med Sci. 1999;54:B352–7. doi: 10.1093/gerona/54.8.b352. [DOI] [PubMed] [Google Scholar]
- 23.Mora S, Gilsanz V. Establishment of peak bone mass. Endocrinol Metab Clin North Am. 2003;32:39–63. doi: 10.1016/s0889-8529(02)00058-0. [DOI] [PubMed] [Google Scholar]
- 24.Gilsanz V. Quantitative computed tomography. In: Siegel M, editor. Pediatric Body CT. Churchill Livingstone; New York: 1988. pp. 349–69. [Google Scholar]
- 25.Mora S, Goodman WG, Loro ML, et al. Age-related changes in cortical and cancellous vertebral bone density in girls: assessment with quantitative CT. Am J Roentgenol. 1994;162:405–9. doi: 10.2214/ajr.162.2.8310936. [DOI] [PubMed] [Google Scholar]
- 26.Lee DC, Gilsanz V, Wren TA. Limitations of Peripheral Quantitative Computed Tomography Metaphyseal Bone Density Measurements. J Clin Endocrinol Metab. 2007;92:4248–4253. doi: 10.1210/jc.2007-0126. [DOI] [PubMed] [Google Scholar]
- 27.Hangartner TN, Gilsanz V. Evaluation of cortical bone by computed tomography. J Bone Miner Res. 1996;11:1518–25. doi: 10.1002/jbmr.5650111019. [DOI] [PubMed] [Google Scholar]
- 28.Arfai K, Pitukcheewanont PD, Goran MI, et al. Bone, muscle, and fat: sex-related differences in prepubertal children. Radiology. 2002;224:338–44. doi: 10.1148/radiol.2242011369. [DOI] [PubMed] [Google Scholar]
- 29.Lee DC. Biomedical Engineering. University of Southern California; Los Angeles, CA: 2009. Optimizations in the Assessment of Pediatric Bone. [Google Scholar]
- 30.Engsberg JR, Ross SA, Olree KS, et al. Ankle spasticity and strength in children with spastic diplegic cerebral palsy. Dev Med Child Neurol. 2000;42:42–7. doi: 10.1017/s0012162200000086. [DOI] [PubMed] [Google Scholar]
- 31.Gilsanz V, Nelson DA. Childhood and adolescence. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. American Society for Bone and Mineral Research; Washington, D.C.: 2003. pp. 71–79. [Google Scholar]
- 32.Judex S, Lei X, Han D, et al. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40:1333–9. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Rubin C, Pope M, Fritton JC, et al. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine. 2003;28:2621–7. doi: 10.1097/01.BRS.0000102682.61791.C9. [DOI] [PubMed] [Google Scholar]