Summary
Several of the nucleos(t)ide analogues used to treat HIV also inhibit hepatitis B virus (HBV) replication, with lamivudine being the oldest of this group. Thus, prior to licensing of tenofovir, many HIV-HBV co-infected subjects received lamivudine as the only active drug against HBV as part of the anti-HIV regimen setting the stage for emergence of drug-resistant HBV. In co-infected persons, lamivudine-resistant HBV develops more rapidly than in HBV moninfected persons, but it is not known if this is true for the newer agents. Due to overlapping reading frames of the HBV Polymerase and Surface antigens, drug-resistant changes in HBV Pol can lead to mutations in the envelope. This review will discuss studies of drug-resistant HBV in HIV-infected persons including drug-resistant mutations that have been identified and clinical sequelae of these mutations.
Introduction
For the estimated 4 million HIV-infected individuals who are co-infected with hepatitis B virus (HBV), drug-resistant HBV is a common problem because several of the nucleos(t)ide analogues used to treat HIV are also active against HBV. This dual activity of drugs is especially problematic when the HBV status is not known prior to administering HIV therapy since anti-HBV drugs with a low genetic barrier to resistance, such as lamivudine, may be given as part of the anti-HIV regimen. Once drug-resistant HBV emerges, it can be problematic to treat because only a few classes of anti-HBV drugs are available and cross-resistance between the drugs and even between classes occurs.
In HIV-infected individuals, the treatment of HBV is important because HIV increases the risk for liver-related mortality(1). Furthermore, liver disease is a leading cause of mortality in HIV-infected patients on highly active antiretroviral therapy (HAART). In the D:A:D study, which followed 23,441 subjects with HIV who received HAART for a median of 3.5 years, there were 1246 (5.3%) deaths (2). The second leading cause of death was liver disease, which accounted for 14.5% of all deaths. About 25% of the liver deaths could be attributed to an HBV infection. For this reason, in HIV-infected individuals hepatitis B should be treated and consideration should be given to minimizing the risk of drug-resistant HBV. In this article, we will review what is known about development of drug-resistant HBV in HIV-infected subjects on HAART and discuss the clinical implications.
Virology
Although HBV is a DNA virus, it has an RNA intermediate and an error-prone reverse transcriptase; thus, substitutions are made at each base daily allowing for development of drug-resistant variants (see article by Locarnini in this issue). These variants emerge at different rates due to varying potencies and genetic barriers of resistance of the drugs as well as the ability of the drug-resistant virus to replicate compared to wild type (replication capacity). In HIV co-infected individuals, drug-resistant variants have not been extensively studied in controlled trials; thus, our knowledge is from a few cohort studies and individual case reports.
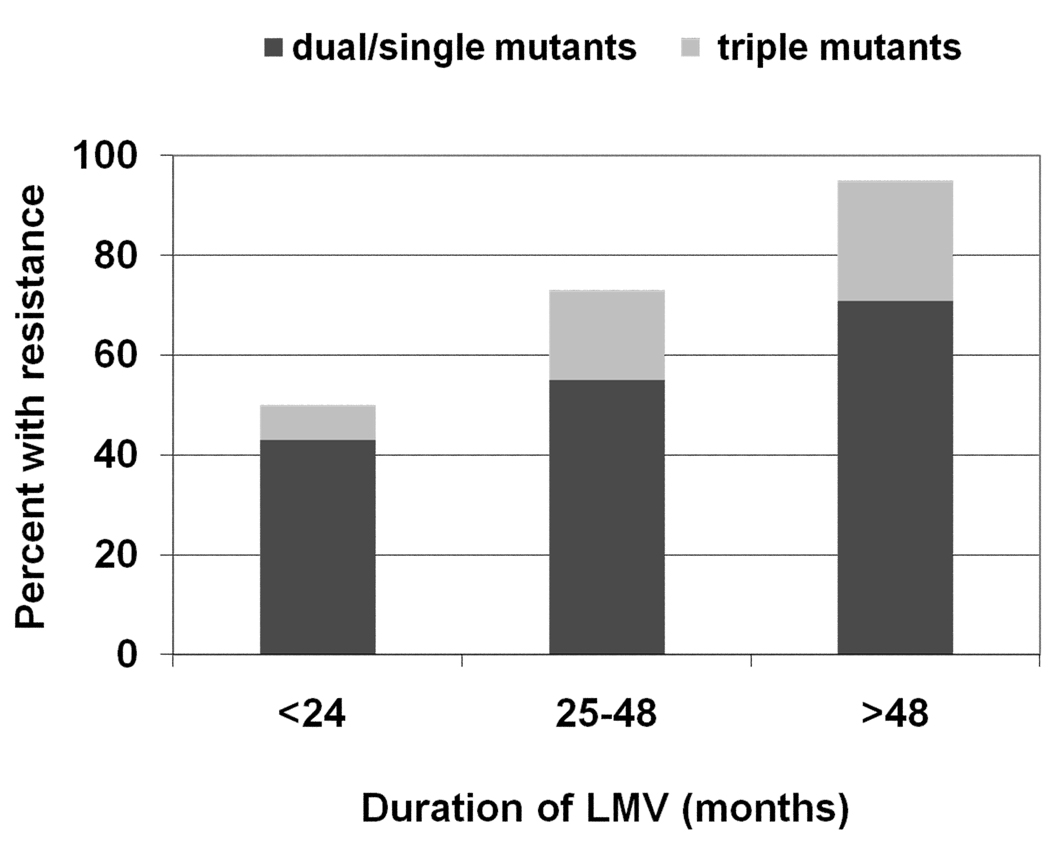
Lamivudine has been used most extensively since it was one of the first drugs available to treat HIV infection. Individuals who received lamivudine prior to the approval of tenofovir disoproxil fumarate (TDF) in 2001 are at greatest risk for having lamivudine-resistant HBV since lamivudine was the only drug active against HBV in HIV regimens. Benhamou et al demonstrated that in an HIV-HBV co-infected cohort, 53% had lamivudine-resistant HBV after two years of lamivudine monotherapy (3), which is higher than the 38% in HBV monoinfected subjects (4). He also estimated that after 4 years of therapy 90% would have lamivudine-resistant HBV, which is higher than 67% in HBV monoinfection. Matthews et al confirmed this rapid development of lamivudine-resistant HBV in the setting of HIV co-infection (5). They studied 53 HIV-HBV co-infected individuals on lamivudine monotherapy for > 6 months and found the prevalence of lamivudine-resistant HBV to be 50% in those with <24 months of lamivudine and 94% in those with > 48 months of lamivudine monotherapy (Figure 1). This study also demonstrated that at each time point the majority had a single (rtM204V/I) or double mutant (rtM204V+ rtL180M) and the minority had the triple mutant (rtV173L+rt L180M+rtM204V). However, the overall prevalence of the triple mutant was 17%, which is about double the prevalence in HBV monoinfection (6). Due to the overlapping reading frames of the HBV Pol and the HBV envelope genes, the triple mutant leads to the surface mutations sE164D/sI195M. In vitro, these surface mutations behave as a vaccine escape mutant, so there is concern that this mutant virus could be transmitted to individuals who have been vaccinated against HBV(7). To date, this has not been documented in humans. However, in chimpanzees vaccinated against HBV and then inoculated with the triple mutant, Kamili et al found that these chimpanzees developed antibodies to the hepatitis B core antigen and T-cell responses against the HBV Pol suggesting that there may have been some replication of the mutant virus (8). However, HBV DNA was not detectable in either chimp.
Figure 1. Frequency of lamivudine-resistant HBV in 53 HIV-HBV co-infected subjects with HBV viremia.
The frequency of both the single/dual (rtM204V/I ± rtL180M) and triple mutants (rtL173T+ rtL180M+ rtM204V/I) increase with duration of lamivudine therapy.
Once drug-resistant viruses have developed on lamivudine, continued lamivudine therapy can lead to accumulation of additional mutations, which could compromise other future therapies. In the study by Matthews et al discussed above, the authors found that 11% had other potentially significant HBV Pol mutations including mutations at entecavir resistance sites. One individual had the rtQ215S, which was associated with lamivudine and adefovir resistance in one study (9). However, a recent in vitro study demonstrated that HBV with this variant was still susceptible to lamivudine and adefovir, but differences between the mutant and wild type constructs in the EC50 could not be excluded (10).
The next most commonly used anti-HBV drug in HIV co-infected patients is TDF given its potent activity against HIV. However, since TDF has a high genetic barrier to resistance for HBV and few studies have examined the development of resistance in HIV co-infection, little is known about the patterns and frequencies of TDF-resistant HBV. The only study to find any potential TDF-resistant mutations is one of 43 HIV-HBV co-infected patients on TDF and ongoing lamivudine who had detectable viremia after a mean of 11.2 months on TDF (11). About half the subjects had wild type virus and the other half had mutations that conferred lamivudine resistance. In addition, a novel mutation, rtA194T, was found in two subjects. In one patient, its emergence coincided with an ALT flare and a 1.5 log copy/ml increase in HBV DNA after 62 weeks of TDF. In the other patient, there were no clinical symptoms and no rise in HBV DNA, which does not meet the current definition of resistance as a 1 log increase in HBV DNA. There are conflicting in vitro data regarding whether this rtA194T mutant is resistant to TDF. In a recent study, Olyaee et al made replication-competent constructs with the rtA194T and found that it impaired replication and led to a 5–6 fold decreased susceptibility to TDF (12). Interestingly, when the basal core promoter mutants A1762T and G1764A or the precore mutants G1896A or C1858T were included in the constructs, the replication capacity of the mutant increased.
There is a case report of an interesting envelope mutant that developed on TDF (13). The report was of a HIV-HBV co-infected individual on HIV therapy with TDF and lamivudine who at 29 weeks of therapy was found to have an rtV191I without clinical symptoms or a rise in HBV DNA. He was however, HBsAg negative since the mutation leads to a truncation of the HBsAg deleting the last 44 amino acids. The authors found that this mutation leads to decreased lamivudine susceptibility in vitro but not to decreased TDF susceptibility.
Overall, there have been six studies published in HIV-HBV co-infection examining TDF resistance (Table 1). The duration of follow-up in these studies is short, so TDF resistance is unlikely with the longest one being 104 weeks. The only study to demonstrate any mutations is the one discussed above with the rtA194T.
Table 1.
Published studies of TDF-resistant HBV in HIV co-infected subjects.
| Number of subjects |
Duration of TDF (wks) |
Number (%) with detectable HBV DNA |
Number with TDF mutations |
Reference |
|---|---|---|---|---|
| 31 | 48 | 4 (12.9) | 0 | (26) |
| 43 | 45 | 43(100)* | 2 | (11) |
| 65 | 52 | 2(3.1) | 0 | (27) |
| 30 | 52 | 11(36.7) | 0 | (28) |
| 28 | 104 | 4(14.3) | 0 | (29) |
subjects selected for study based on having a detectable HBV DNA
Adefovir has not been extensively used to treat HBV in HIV-infected individuals since the related drug, TDF, is active against both viruses. One cohort study of 35 HIV-HBV co-infected individuals with adefovir added to lamivudine-based HAART did not demonstrate any resistance mutations after 144 weeks of adefovir (14). There is one case report of the rtA181T emerging after 30 months of adefovir(15). This mutant is interesting because it leads to a stop codon in the overlapping envelope gene, which results in defective secretion of viral particles (16). In vitro, co-expression of this mutant with wild type demonstrates that it has a dominant negative effect on virion secretion.
Entecavir was recently recognized to have anti-HIV activity that can result in emergence of drug-resistant HIV (17;18); thus, its use in HIV-HBV co-infection is restricted to individuals who are on a suppressive HIV regimen. One study enrolled 68 HIV-HBV co-infected subjects with HIV RNA <400 cp/ml who were on lamivudine-based HAART for at least 24 weeks with a detectable HBV DNA; thus had lamivudine-resistant HBV (19). Subjects were randomized into a double blind study of 1.0 mg of entecavir versus placebo with ongoing lamivudine for 24 weeks. After 24 weeks, open label entecavir was given. Not surprisingly, entecavir led to a significant decline in HBV DNA; however, the response was less than has been seen in HBV-infected subjects without HIV co-infection. At week 48, only 8% of the group who received 48 weeks of entecavir had HBV DNA <30 cp/ml and none in the placebo group. This 8% is lower than what has been reported in HBV monoinfection, which is about 33% (20); thus, there are a large number of individuals at increased risk for developing entecavir resistance mutations. Of those who received 48 weeks of entecavir, 43 of 47 subjects were sequenced and 5% had entecavir-resistance substitutions. This is similar to what is reported in HBV monoinfection, but further follow-up is needed to see if resistance develops more rapidly as has been seen with lamivudine.
One issue that is still not clear is whether drug-resistant mutants are present prior to therapy. In one study of 20 HIV-HBV co-infected subjects in South Africa, 10 (50%) had the rtM204I prior to therapy (21). In the 15 HBV monoinfected subjects, three (20%) had lamivudine-resistant HBV. Since this was a small study, additional studies are needed to determine if the prevalence of lamivudine-resistant HBV in therapy naïve subjects is truly this high.
Clinical sequelae of drug-resistant HBV
In order to slow liver disease progression from HBV infection, effective anti-HBV therapy is needed. However, one complication of drug-resistant HBV is the limitation of treatment options because the structural similarity amongst several of the anti-HBV drugs results in overlapping resistance patterns. Thus, if drug-resistant mutations emerge on one drug, other drugs will have decreased or no susceptibility. For example, the rtM204V/I mutant either by itself or with the rtL180M or rtL173T often develops on lamivudine monotherapy, which leads to resistance to telbivudine and emtricitabine as well as reducing susceptibility to entecavir about 30-fold. Likewise, the mutations that lead to entecavir resistance also lead to resistance to lamivudine, emtricitabine, and telbivudine.
Similarly, the adefovir resistance mutation rtN236T is still susceptible to TDF, but clinically these mutant viruses do not respond to TDF as well. In a multicenter study of HBV monoinfected patients, von Bommel et al retrospectively studied 127 patients on TDF monotherapy for a median of 20 months (6–54) of whom 19% had adefovir-resistant virus (22). In a Kaplan-Meier analysis, the probability of reaching HBV DNA <400 copies/ml was significantly lower in those with adefovir-resistant virus. Thus, adefovir-resistant HBV is clinically more difficult to treat with TDF than HBV without adefovir mutations.
Drug-resistant HBV could result in more liver disease progression since the drug-resistant virus is difficult to treat. In HIV-HBV co-infection there are no long-term studies that directly address this issue. One study from the Multicenter AIDS Cohort Study (MACS) sheds some light on this. Hoffmann et al compared 350 MACS participants without markers for a HBV infection to 45 with chronic hepatitis B who initiated HAART (23). When looking at the outcome of non-AIDS deaths, those with HBV had a rate of 22/1000 person-years (PYs) compared to 2.4/1000 PYs in those without HBV markers. The liver-related mortality rate in those with HBV infection was 17/1000 PYs, which was similar to the liver-mortality rate in the pre-HAART era of 14/1000 PYs (1). In the four men who died of liver disease, HBV DNA was tested on three of them and two were detectable with lamivudine-resistant virus. Further studies are needed to determine if those with lamivudine-resistant virus have similar liver mortality rates to those receiving HIV therapy that includes more potent anti-HBV agents such as TDF.
Although development of mutant virus usually occurs in the context of ALT flares, in HIV-coinfected individuals flares may not occur. Bhattacharya et al looked at the ALT values and the presence of drug-resistant virus in 45 different samples from 11 patients (24). In those samples with only wild type virus the median ALT was 43 IU/ml whereas in those with drug-resistant variants the median ALT was 44 IU/ml. This lack of flare may be due to a compromised immune system. This study emphasizes the need to monitor with both HBV DNA and ALT for the emergence of drug-resistant virus in HIV-infected patients.
The other potential concern in HIV co-infected subjects is that immune reconstitution syndrome could occur with the emergence of a drug-resistant variant, potentially resulting in fulminant hepatic disease. Gouskos et al reported a case of severe and prolonged HBV-specific CD8 T-cell response associated with emergence of a lamviudine-resistant HBV that resulted in a severe flare of liver disease with ALT >1000 IU/L (25).
Conclusions
HBV that is resistant to the L-nucleosides is common in HIV-infected individuals due to the more rapid emergence of drug-resistant virus and due to the long-standing use of lamivudine monotherapy in the early years of effective antiretroviral therapy. If lamivudine-resistant HBV is present, continued therapy with lamivudine leads to emergence of additional mutations which can compromise use of other anti-HBV agents. Whether drug-resistance occurs more rapidly in HIV infection to some of the newer agents is not known. Due to the overlapping polymerase and envelope reading frames, drug-resistant variants can lead to surface antigen negative mutants. Clinical monitoring of ALT and HBV DNA is necessary to detect emergence of drug-resistant variants. Immune reconstitution syndrome to drug-resistant variants may also occur.
Acknowledgements
This work was supported by NIH grants AI060449 and AI071820. The contents of this manuscript were presented at the Drug-Resistant and Vaccine-Escape Hepatitis B Virus Mutants meeting on June 4–5, 2009 at CDC, Atlanta, Georgia.
Footnotes
Disclosure statement
CLT has no conflicts of interest.
Reference List
- 1.Thio CL, Seaberg EC, Skolasky RL, Phair J, Visscher B, Munoz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter AIDS Cohort Study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 2.Weber R, Sabin CA, Friis-Moller N, Reiss P, El Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166(15):1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 3.Benhamou Y, Bochet M, Thibault V, Di M V, Caumes E, Bricaire F, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology. 1999;30(5):1302–1306. doi: 10.1002/hep.510300525. [DOI] [PubMed] [Google Scholar]
- 4.Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125(6):1714–1722. doi: 10.1053/j.gastro.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Matthews GV, Bartholomeusz A, Locarnini S, Ayres A, Sasaduesz J, Seaberg E, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS. 2006;20(6):863–870. doi: 10.1097/01.aids.0000218550.85081.59. [DOI] [PubMed] [Google Scholar]
- 6.Delaney WE, Yang H, Westland CE, Das K, Arnold E, Gibbs CS, et al. The hepatitis B virus polymerase mutation rtV173L is selected during lamivudine therapy and enhances viral replication in vitro. J Virol. 2003;77(21):11833–11841. doi: 10.1128/JVI.77.21.11833-11841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torresi J, Earnest-Silveira L, Deliyannis G, Edgtton K, Zhuang H, Locarnini SA, et al. Reduced antigenicity of the hepatitis B virus HBsAg protein arising as a consequence of sequence changes in the overlapping polymerase gene that are selected by lamivudine therapy. Virology. 2002;293(2):305–313. doi: 10.1006/viro.2001.1246. [DOI] [PubMed] [Google Scholar]
- 8.Kamili S, Sozzi V, Thompson G, Campbell K, Walker CM, Locarnini S, et al. Efficacy of hepatitis B vaccine against antiviral drug-resistant hepatitis B virus mutants in the chimpanzee model. Hepatology. 2009;49(5):1483–1491. doi: 10.1002/hep.22796. [DOI] [PubMed] [Google Scholar]
- 9.Bartholomeusz A, Locarnini S, Ayres A, Thompson G, Angus P, Sievert W, et al. Molecular modelling of hepatitis B polymerase and adefovir resistance identifies three clusters of mutations. Hepatology. 2004;40 4, Suppl 1:246A. [Google Scholar]
- 10.Amini-Bavil-Olyaee S, Herbers U, Mohebbi SR, Sabahi F, Zali MR, Luedde T, et al. Prevalence, viral replication efficiency and antiviral drug susceptibility of rtQ215 polymerase mutations within the hepatitis B virus genome. J Hepatol. 2009 doi: 10.1016/j.jhep.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Sheldon J, Camino N, Rodes B, Bartholomeusz A, Kuiper M, Tacke F, et al. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antiviral Therapy. 2005;10(6):727–734. [PubMed] [Google Scholar]
- 12.Amini-Bavil-Olyaee S, Herbers U, Sheldon J, Luedde T, Trautwein C, Tacke F. The rtA194T polymerase mutation impacts viral replication and susceptibility to tenofovir in hepatitis B e antigen-positive and hepatitis B e antigen-negative hepatitis B virus strains. Hepatology. 2009;49(4):1158–1165. doi: 10.1002/hep.22790. [DOI] [PubMed] [Google Scholar]
- 13.Amini-Bavil-Olyaee S, Sheldon J, Lutz T, Trautwein C, Tacke F. Molecular analysis of an HBsAg-negative hepatitis B virus mutant selected in a tenofovir-treated HIV-hepatitis B virus co-infected patient. AIDS. 2009;23(2):268–272. doi: 10.1097/QAD.0b013e3283224316. [DOI] [PubMed] [Google Scholar]
- 14.Benhamou Y, Thibault V, Vig P, Calvez V, Marcelin AG, Fievet MH, et al. Safety and efficacy of adefovir dipivoxil in patients infectedwith lamivudine-resistant hepatitis B and HIV-1. J Hepatol. 2006;44(1):62–67. doi: 10.1016/j.jhep.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Lacombe K, Ollivet A, Gozlan J, Durantel S, Tran N, Girard PM, et al. A novel hepatitis B virus mutation with resistance to adefovir but not to tenofovir in an HIV-hepatitis B virus-co-infected patient. AIDS. 2006;20(17):2229–2231. doi: 10.1097/01.aids.0000252061.35422.84. [DOI] [PubMed] [Google Scholar]
- 16.Warner N, Locarnini S. The antiviral drug selected hepatitis B virus rtA181T/sW172* mutant has a dominant negative secretion defect and alters the typical profile of viral rebound. Hepatology. 2008;48(1):88–98. doi: 10.1002/hep.22295. [DOI] [PubMed] [Google Scholar]
- 17.McMahon MA, Jilek BL, Brennan TP, Shen L, Zhou Y, Wind-Rotolo M, et al. The HBV drug entecavir - effects on HIV-1 replication and resistance. N Engl J Med. 2007;356(25):2614–2621. doi: 10.1056/NEJMoa067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasadeusz J, Audsley JA, Mijch A, Baden R, Caro J, Hunter H, et al. The anti-HIV activity of entecavir: a multicenter evaluation of lamivudine-experienced and lamivudine-naive patients. AIDS. 2008;22(8):947–955. doi: 10.1097/QAD.0b013e3282ffde91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pessoa MG, Gazzard B, Huang AK, Brandao-Mello CE, Cassetti I, Mendes-Correa MC, et al. Efficacy and safety of entecavir for chronic HBV in HIV/HBV coinfected patients receiving lamivudine as part of antiretroviral therapy. AIDS. 2008;22(14):1779–1787. doi: 10.1097/QAD.0b013e32830b3ab5. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki F, Toyoda J, Katano Y, Sata M, Moriyama M, Imazeki F, et al. Efficacy and safety of entecavir in lamivudine-refractory patients with chronic hepatitis B: randomized controlled trial in Japanese patients. J Gastroenterol Hepatol. 2008;23(9):1320–1326. doi: 10.1111/j.1440-1746.2008.05455.x. [DOI] [PubMed] [Google Scholar]
- 21.Selabe SG, Lukhwareni A, Song E, Leeuw YG, Burnett RJ, Mphahlele MJ. Mutations associated with lamivudine-resistance in therapy-naive hepatitis B virus (HBV) infected patients with and without HIV co-infection: implications for antiretroviral therapy in HBV and HIV co-infected South African patients. J Med Virol. 2007;79(11):1650–1654. doi: 10.1002/jmv.20974. [DOI] [PubMed] [Google Scholar]
- 22.VanBommel F, deMan RA, Stein K, Huppe D, Petersen J, Buggisch P, et al. A multicenter analysis of antiviral response after one year of tenofovir monotherapy in HBV-monoinfected patients with prior nucleos(t)ide analogue experience. J Hepatol. 2008;48 Suppl 2:S32. [Google Scholar]
- 23.Hoffmann CJ, Seaberg EC, Young S, Witt MD, D'Acunto K, Phair J, et al. Hepatitis B and long-term HIV outcomes in co-infected HAART recipients. AIDS. 2009 doi: 10.1097/QAD.0b013e32832e463a. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhattacharya D, Katzenstein D, Wong A, Israelski D, Imperial JC, Keeffe EB, et al. Alanine aminotransferase levels are not significantly elevated in patients with HIV/HBV co-infection and lamivudine resistance. Int J STD AIDS. 2008;19(11):780–781. doi: 10.1258/ijsa.2008.008020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gouskos T, Wightman F, Chang J, Earnest-Silveira L, Sasadeusz J, Lewin SR, et al. Severe hepatitis and prolonged hepatitis B virus-specific CD8 T-cell response after selection of hepatitis B virus YMDD variant in an HIV/hepatitis B virus-co-infected patient. AIDS. 2004;18(12):1734–1737. doi: 10.1097/01.aids.0000131384.53350.59. [DOI] [PubMed] [Google Scholar]
- 26.Stephan C, Berger A, Carlebach A, Lutz T, Bickel M, Klauke S, et al. Impact of tenofovir-containing antiretroviral therapy on chronic hepatitis B in a cohort co-infected with human immunodeficiency virus. J Antimicrob Chemother. 2005;56(6):1087–1093. doi: 10.1093/jac/dki396. [DOI] [PubMed] [Google Scholar]
- 27.Benhamou Y, Fleury H, Trimoulet P, Pellegrin I, Urbinelli R, Katlama C, et al. Anti-hepatitis B virus efficacy of tenofovir disoproxil fumarate in HIV-infected patients. Hepatology. 2006;43(3):548–555. doi: 10.1002/hep.21055. [DOI] [PubMed] [Google Scholar]
- 28.Jain MK, Comanor L, White C, Kipnis P, Elkin C, Leung K, et al. Treatment of hepatitis B with lamivudine and tenofovir in HIV/HBV-coinfected patients: factors associated with response. J Viral Hepat. 2007;14(3):176–182. doi: 10.1111/j.1365-2893.2006.00797.x. [DOI] [PubMed] [Google Scholar]
- 29.Audsley J, Arrifin N, Yuen LK, Ayres A, Crowe SM, Bartholomeusz A, et al. Prolonged use of tenofovir in HIV/hepatitis B virus (HBV)-coinfected individuals does not lead to HBV polymerase mutations and is associated with persistence of lamivudine HBV polymerase mutations. HIV Med. 2009;10(4):229–235. doi: 10.1111/j.1468-1293.2008.00675.x. [DOI] [PubMed] [Google Scholar]