Abstract
Hypothesis
Due to auditory plasticity there can be experience-dependent acquisition and refinement of spatial hearing skills.
Background
A growing number of children who are deaf are receiving bilateral cochlear implants (BICIs), in an attempt to provide them with acoustic cues known to be important for spatial hearing. A feasible and reliable task for children is the right-left discrimination task, which enables measurement of the smallest angle from midline that can be reliably discriminated (minimum audible angle, MAA).
Methods
Ten children (5 to 10 yrs of age) were followed longitudinally during their transition from one to two cochlear implants (CIs), with testing prior to bilateral activation, as well as 3- and 12-months post-bilateral activation. Testing at 3- and 12-months post-bilateral was conducted under bilateral and first-CI listening modes. During testing, stimuli were presented from an array of loudspeakers. On each trial the child reported whether the sound was to the right or left, with feedback. Percent correct was measured in blocks of trials for numerous angle values.
Results
At baseline, some children were unable to perform the right-vs-left task, but group mean MAA was 44.8°. MAA in the bilateral listening mode improved to 20.4° at 3-months and 16.8° at 12-months post-bilateral activation. No improvement was seen in the unilateral listening mode. Bilateral performance was better than unilateral.
Conclusion
Spatial hearing skills in sequentially implanted children develop in an experience-dependent manner, perhaps due to the ability of the auditory system to utilize newly acquired electrical stimulation presented to the two ears.
INTRODUCTION
A growing number of children who are deaf are receiving bilateral cochlear implants (BICIs), in an attempt to provide them with acoustic cues that are known to be important for spatial hearing. The benefits of BICI in adults are well documented. When both cochlear implants (CIs) are activated compared with when a single CI is used there appear to be significant improvements in the ability of adult patients to understand speech in noise (e.g., van Hoesel, 2004; Schleich et al., 2004; Litovsky et al., 2006a), and to localize sounds (e.g., Nopp et al., 2004; Litovsky et al., 2009). The ability to identify source locations is the topic of interest in this study, with a particular focus on the minimum audible angle (MAA), i.e., a left vs. right discrimination task that aims to measure the smallest angle from midline that can be reliably discriminated (Mills, 1958; Litovsky and Macmillan, 1994; Litovsky, 1997). We have previously reported that MAA thresholds in children with BICIs are significantly smaller when bilateral devices are used than with the CI in the first-implanted ear (Litovsky et al., 2006b). In a few of these children we also documented the finding that MAA thresholds improved over time. While bilateral implantation has become more common in children in recent years, many clinical questions arise, such as whether simultaneous or sequential implantation might offer similar outcomes. Children who are implanted sequentially experience several changes in auditory input during childhood, as they typically transition from being bilaterally deaf to being unilaterally implanted, and then bilaterally implanted. In addition, some children are fitted with a hearing aid in the non-implanted ear, thus they transition from having bimodal (acoustic+electric) hearing to having bilateral electric hearing. Other children transition from being unilaterally implanted with no input in the contralateral ear to having bilateral electric hearing. This study was concerned with the emergence of spatial hearing abilities in a group of children who experienced these transitions. The study was prospectively designed to capture MAA thresholds while the children were unilateral CI users, and at two subsequent intervals following activation of the second CI: 3-months and 12-months. We tested the hypothesis that, due to auditory plasticity, i.e., the ability of the auditory system to integrate novel stimulation from the two ears, there can be experience-dependent acquisition and refinement of spatial hearing skills. Change in performance over time was tracked for both unilateral and bilateral listening modes. Because the same group of children were tested prior to, and following activation of the second CI, they served as their own unilateral control.
MATERIALS AND METHODS
Subjects
Ten children (5 to 10 yrs of age) were followed longitudinally during their transition from one to two CIs. Upon enrollment in the study (i.e., baseline visit), each of the children had at least one year of experience with a single CI, used oral communication, and was mainstreamed in his/her school setting. Six of the children wore a hearing aid in the ear contralateral to her/his CI during waking hours (CI-HA). The other four children received no consistent stimulation to the contralateral ear (UniCI). At the baseline visit, testing was completed in either CI-HA or UniCI listening mode, depending on the child. Following activation of the second CI the children returned for further participation, after having had 3- and then 12-months of listening experience with bilateral CIs. Due to logistical issues, one subject, CIAY, participated at 3 and 9- month intervals. Demographic information is presented in Table 1.
Table 1.
| Subject | Gender | Etiology | Age at 1st CI Activation Yr;Mo |
Age at 2nd CI Activation Yr;Mo |
Time between 1st & 2nd CI Yr;Mo |
Contralateral HA at Baseline |
1st CI | 2nd CI | |
|---|---|---|---|---|---|---|---|---|---|
|
CI-HA at Baseline |
CIAP | F | Progressive, cause unknown |
3;6 | 5;2 | 1;8 | Oticon DigiFocusSP; Left ear |
Nucleus 24C Advance; Right ear |
Nucleus 24C Advance; Left ear |
| CIAQ | M | Connexin-26 | 3;1 | 8;1 | 5;0 | Widex Senso P38; Left ear |
Nucleus 24C; Right ear |
Nucleus 24C Advance; Left ear |
|
| CIBA | M | Connexin-26 | 3;7 | 10;2 | 6;7 | Phonak Supero 412; Right ear |
Nucleus 24; Left ear |
Nucleus Freedom; Right ear |
|
| CIBH | M | Mondini Malformation |
2;5 | 7;0 | 4;7 | Phonak P4AZ; Right ear |
Med-El Combi40+; Left ear |
Med-El Pulsar; Right ear |
|
| CIBK | M | Connexin-26 | 2;1 | 7;1 | 5;0 | Oticon DigiFocus II; Left ear |
Nucleus 24C; Right ear |
Nucleus Freedom; Left ear |
|
| CIBM | M | Progressive, viral cause suspected |
3;9 | 8;0 | 4;1 | Sonic Innovations Digital BTE; Right ear |
Nucleus 24; Left ear |
Nucleus Freedom; Right ear |
|
|
UniCI at Baseline |
CIAW | M | Prenatal CMV exposure |
1;2 | 5;5 | 4;3 | NA | Nucleus 24C; Right ear |
Nucleus Freedom; Left ear |
| CIAY | M | Progressive, bilateral ear infection |
5;2 | 5;11 | 0;9 | NA | Nucleus 24C Advance; Right ear |
Nucleus 24C Advance; Left ear |
|
| CIBG | M | Unknown | 1;2 | 5;5 | 3;3 | NA | Nucleus 24; Right ear |
Nucleus Freedom; Left ear |
|
| CIBJ | F | Progressive, cause unknown |
3;9 | 8;0 | 4;1 | NA | Advanced Bionics CII/HiFocus; Left ear |
Advanced Bionics HiRes/90K; Right ear |
|
Implant Devices
The children were recruited from a wide array of geographic locations and CI centers across the United States. The device manufacturer was not a controlled variable in this study. Device types for each child are listed in Table 1. Due to the sequential nature of the surgeries and device manufacturer upgrades, seven of the ten children received a newer internal device model (electrode array and receiver) for their second CI as compared with their first CI. Device programming was completed by each child’s audiologist during regularly scheduled appointments. In most cases, device programming was done independently for each ear, as was customary in those clinics. The program most often used in daily listening situations, based on parent report and audiologist recommendation, was the one chosen for all aspects of research participation. On the first day of study participation, volume control and/or sensitivity were adjusted in order to equalize (as much as possible) the loudness perception produced by the two implants. The loudness balancing procedure used subjective report from the participant.
Testing Environment
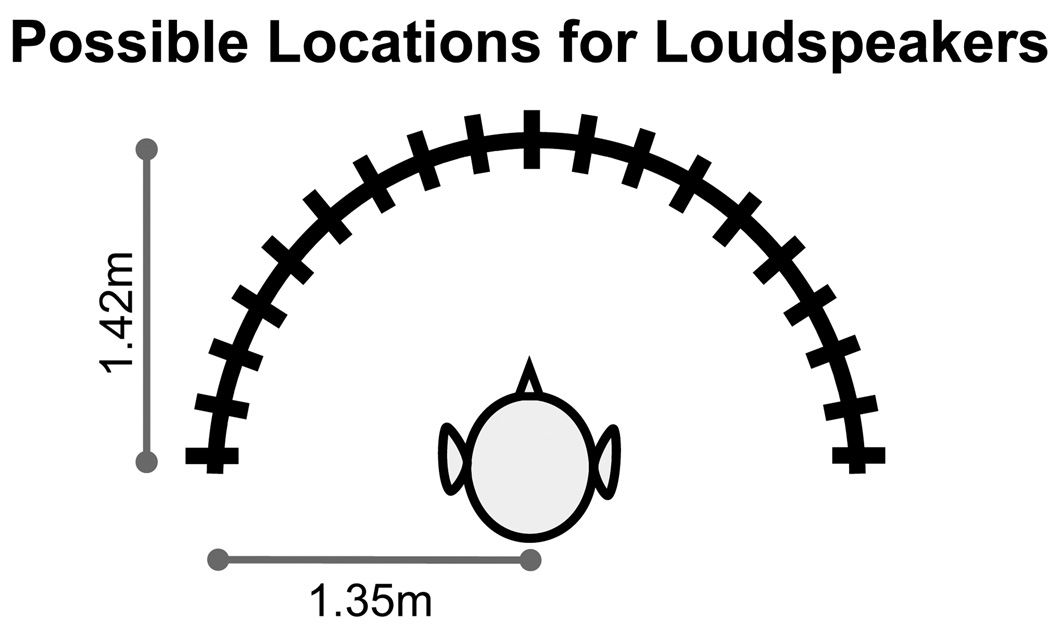
Testing was conducted inside a standard IAC double-walled sound booth with inner dimensions of 2.8m × 3.25m. During testing, the child was seated at a small foam-covered table, facing a semicircular arc. Frequency-matched loudspeakers [Cambridge Soundworks Center Surround IV] were mounted at ear level and were positioned along the arc at 10° intervals ranging from −90° to +90°. The child’s head was always 1.42m from the 0° loudspeaker (See Figure 1). The children were instructed to face and look at the computer monitor positioned underneath the center loudspeaker and to refrain from moving their heads during stimulus presentation.
Figure 1.
Testing apparatus for MAA included loudspeakers placed at 10° increments from −90° to +90°. The child’s head was always 1.42m from the 0° loudspeaker and 1.35m from the loudspeakers at −90° and +90°.
The stimulus used was the spondee “baseball” recorded using a male voice at a sampling rate of 44,000 Hz and digitized as a .wav file. Stimuli were amplified and sent to the loudspeakers via Tucker Davis Technologies (TDT) System III hardware with a PC host. Stimulus levels were set to an average level of 60 dB SPL, with random roving between 56 and 64 dB SPL (i.e., ±4 dB), in order to minimize monaural level cues. The reader is referred to Litovsky et al. (2006b) for a discussion of the use of this spondaic stimulus for this task. Testing was conducted through a computer “listening game” platform whereby the child was presented with response options via a computer monitor and mouse. The computer monitor was positioned underneath the front loudspeaker at 0° to avoid interference with the stimuli as they arrived at the ears; the mouse was placed on the small table in front of the child. A trained tester was present in the sound booth at all times. During testing, feedback was provided through flashing icons presented by the computer. In addition, reinforcement was provided on a trial-by-trial basis using computerized puzzle pictures revealed one piece at a time. Stickers and prizes were awarded between test measures as further reinforcement.
Psychophysical Measurement
Testing was conducted using a 2-AFC procedure. On each trial the stimulus was presented from a loudspeaker to the child’s left or right. The child indicated a response either using the computer mouse or by pointing to the right/left hemifield. After each response, feedback was provided whereby the correct hemifield was revealed to the child via a blinking response icon on the computer monitor. Testing was conducted in blocks of 20 trials, during which a pair of loudspeakers on the arc that were equidistant from the center were selected and labeled with icons matching the computer response icons. The angular separation of the loudspeakers from the center was varied between trial blocks using a modified adaptive rule based on the child’s performance (see Litovsky et al., 2006b): Testing was initiated with angles ranging from 40–90° from center, with exact angle guided by pilot testing or prior data obtained with the child. Following blocks in which the child obtained ≥75% correct, angle size was decreased, otherwise the angle size was increased. Decreases in angle size were initially in steps of 20°, with smaller increments (smallest being 2.5°) used on successive blocks of trials. Testing was terminated when testing with a successive pair of angular separations yielded performance of ≥75% correct and <70% correct. Psychometric functions were extracted from the data, and Minimum Audible Angle (MAA) threshold was estimated by finding the smallest angular separation on the psychometric function where performance yielded 72.4% correct (i.e., two standard deviations above chance performance).
At the baseline visit, participants completed MAA testing in either the CI-HA or UniCI listening mode. In subsequent visits, participants completed MAA testing while in BICI and UniCI (with only the first CI turned on) listening modes. During the baseline visit and the 3-month bilateral visit, children underwent training on the task using a fixed stimulus presentation level of 60 dB SPL. If they were able to perform the task at large angle separations with the criterion of ≥75%, they were subsequently tested with overall sound level varying randomly from 56 to 64 dB SPL in order to minimize monaural level cues. Three children (CIAP, CIAW, CIBG) experienced difficulty with the task and were therefore not tested on additional conditions in which the level was roved during either the baseline or 3-month visit.
RESULTS
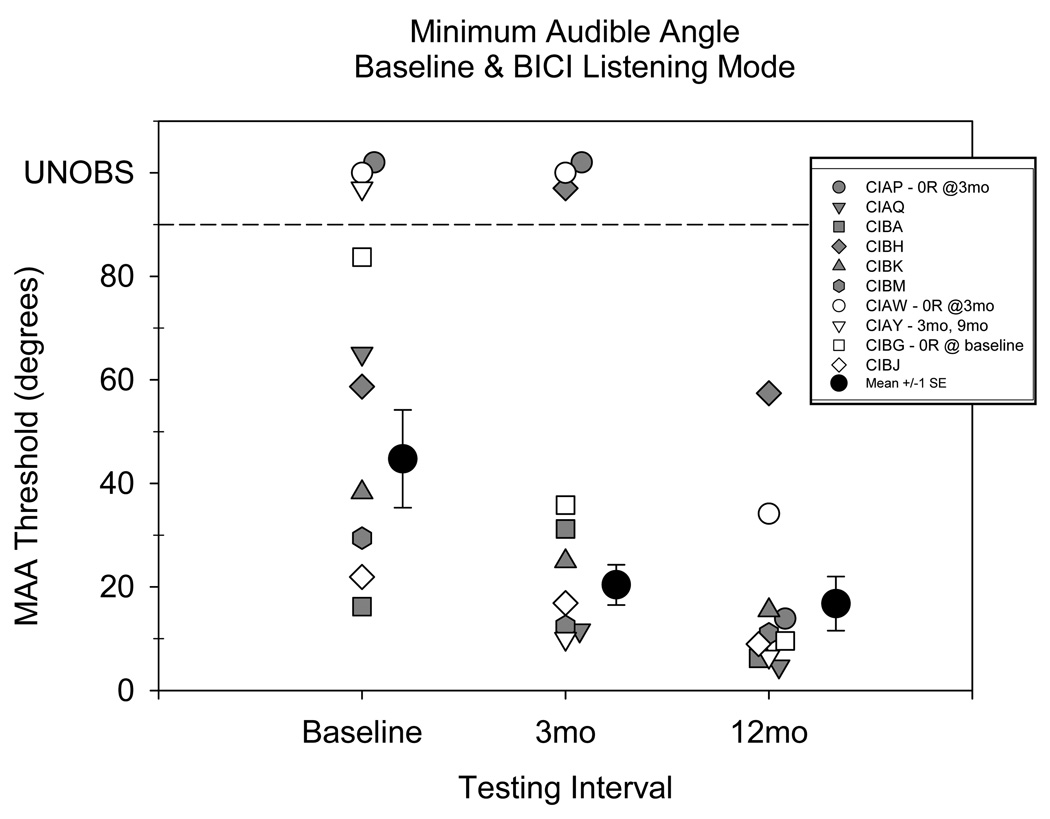
Figure 2 shows individual MAA thresholds for the three testing intervals (baseline, 3- and 12-months), in the BICI listening mode. During the baseline testing interval some children were able to identify the correct hemifield with greater than 75% accuracy with no training. Other children had greater difficulty with the task and underwent several practice blocks at wide angular displacements (>60°). Following practice blocks, 7 children were able to perform the task with >75% accuracy on wide angle separation blocks, while 3 children were unable to perform the task, even at the widest separation of ±90° (CIAP, CIAW, CIBG). MAA threshold for these participants at the baseline interval, therefore, was considered unobserved data in the statistical analysis. In Figure 2, unobserved data points are denoted by the y-axis label UNOBS. The number of children with observed data was 7 at baseline, 7 at 3-months, and 10 at 12-months.
Figure 2.
Individual and group mean MAA are shown for baseline (CI-HA or UniCI) and for the BICI listening mode at 3- and 12-month test intervals. Filled symbols indicate participants who were tested in CI-HA listening mode at baseline; unfilled symbols indicate participants tested in UniCI listening mode at baseline. Means for each interval were computed using only data from participants whose results were measureable at that interval. Number of unobserved datapoints at each interval were: baseline = 3, 3-mo = 3, 12-mo = 0.
As is shown in Figure 2, baseline thresholds are highly variable amongst the 10 subjects, ranging from 16.2° to 83.7° for the 7 children who were able to complete the task. Variability decreased at the 3- and 12-month test intervals, as is evident from the error bars and spread in the data. By the 12-month interval, all participants were able to perform the task at one or more angles at a level of performance >75% correct. In addition, at 12-months, all but 2 children achieved MAA thresholds of 15° or smaller. Group means, (± 1 SE) plotted beside the individual data, were calculated at each testing interval based only on data from participants who were able to complete the task (no unobserved data). Average MAA thresholds decreased steadily during the intervals tested, from 44.8° at baseline to 20.4° at 3-months, and finally to 16.8° at 12-months.
It is interesting to note that the rate and pattern of improvement on the task varied by individual. As was mentioned above, at baseline, performance ranged from 16.2° to Unobserved for the 10 participants, and was not predicted by whether the participants entered the study with CI-HA or UniCI listening experience. At the 3-month visit, while most of the participants showed improvements in MAA Threshold, 3 of the 6 children who entered with CI-HA listening experience (CIAP, CIBH, CIBA) showed no change or a decrement in performance. By the 12-month visit, the decrement measured early on appears to have resolved. Furthermore, all but one participant (CIBH) showed gains in performance compared with baseline. It is possible that these children experienced a period of adjustment to bilateral electrical stimulation following bimodal (acoustic + electrical) stimulation that results in performance decrements during the initial months following activation of the 2nd CI.
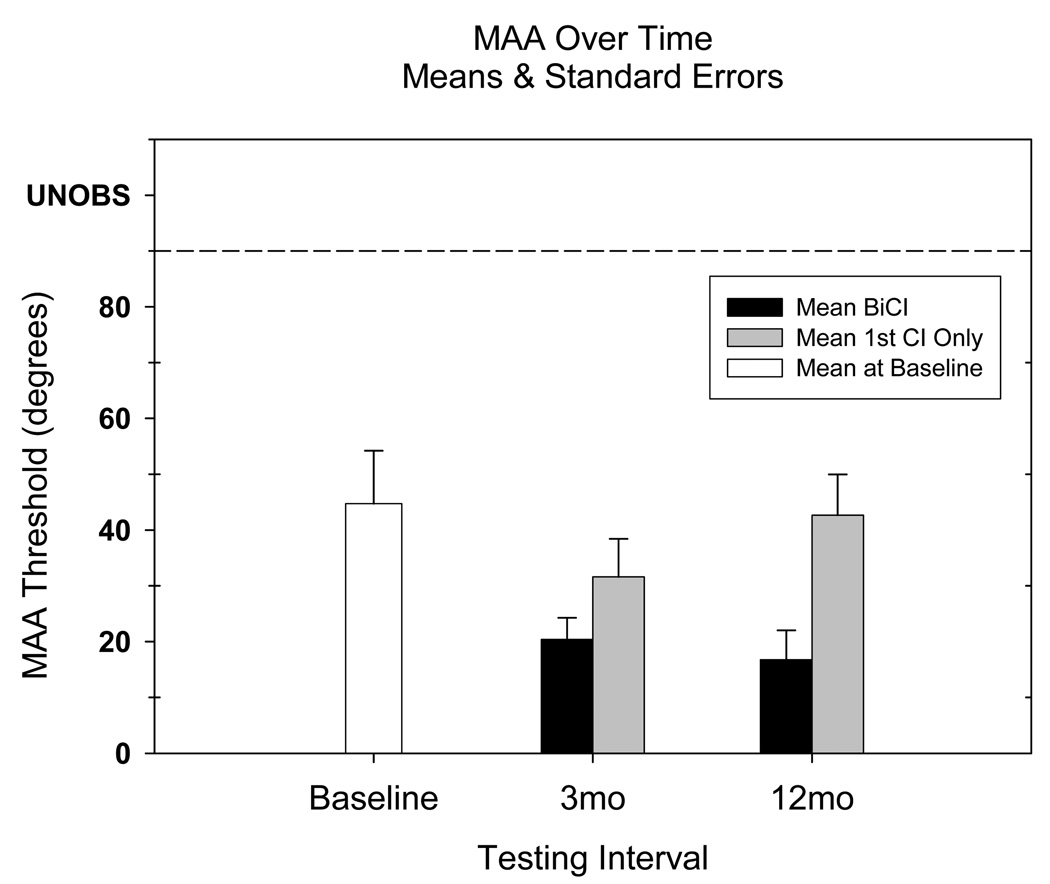
In Figure 3, group averages from Figure 2 (BICI listening mode) are re-plotted for comparison with data from the same children collected in the UniCI listening mode, at the 3- and 12-month intervals, along with the baseline data. Group averages for each listening mode at each test interval were computed using only data from participants whose results were measurable at that interval. For UniCI mode the number of data points were 4 at 3-months and 9 at 12-months. Compared with the average MAA at baseline of 44.8°, average UniCI MAA thresholds remained high following activation of the second CI: 31.6° at 3-months and 42.7° at 12-months.
Figure 3.
Mean MAA for all participants at each of the three testing intervals. At baseline, 6 children were tested with CI-HA and 4 children were tested with UniCI only. At the 3- and 12-mo intervals, all children were tested both with BICI and UniCI (1st CI only). Group means were computed using only data from participants whose results were measureable at that interval. Number of unobserved datapoints at each interval were: baseline=3, 3-mo=3(BICI) 6(UniCI), 12-mo=0(BICI) 1(UniCI).
Due to the unobserved data points contained in the dataset, standard statistical methods could not be employed. In order to evaluate the differences in gains between the BICI and UniCI modes from baseline to 12-months, a random coefficient model was fit to the data from 10 subjects with respect to a baseline condition (common to both CI-HA and UniCI modes), and both UniCI and BICI modes at 3-months and 12-months. The angular outcome measure was log-transformed to achieve approximate normality and homogeneity of variance across listening modes. The random coefficient model used in the current analysis characterized individual differences in reference to three parameters-an intercept (representing expected score at baseline), a linear slope related to the UniCI mode, and a linear slope related to the BICI mode. The two linear slopes represent expected gains (per month) with respect to log-angular measure under the UniCI and BICI modes, respectively. We tested the hypothesis that the mean difference in slopes between the BICI and UniCI modes would be negative, implying greater gains in the BICI mode. We compared two nested models: (1) a baseline model in which the difference in the mean slopes across BICI and UniCI modes is allowed to be nonzero, and (2) a comparison model in which the difference in the mean slopes is constrained to be 0. In both models, the residual variances associated with each of the five repeated measures (baseline, UniCI at 3-months, UniCI at 12-months, BICI at 3-months, BICI at 12-months) are constrained to be equal.
Both the baseline and comparison models were fit using Mplus, version 4 (Muthen and Muthen, 1998−2007), and compared using a chi-square difference test. Data points that were unobserved due to the subject not being able to complete the task were treated as censored from above. Mplus uses a weighted least squares missing value (WLSMV) estimator for both models.
The model fit observed for the baseline model was quite good (Chi-square=1.854, df=4, p=.763; CFI=1.00; TLI=1.01; RMSEA=0.000; WRMR=.414). The results of the chi-square difference test comparing the baseline and comparison models was significant (Chi-square=12.323, df=1, p<.001), implying a significant mean difference in the slopes between the UniCI and BICI modes. From the baseline model, the mean slope for the UniCI mode (−.031, se=.020, t=−1.525, ns) is significantly above that for the BICI mode (−.138, se=.028, t=−4.989, p<.01) suggesting significantly greater improvement for the BICI mode. Moreover, the pattern of statistical significance for the individual parameter estimates implies no detectable gains under the UniCI mode, but detectable gains under the BICI mode.
To further investigate the presence of differences in the UniCI and BICI modes at both 3-months and 12-months, a random coefficient model was specified in which all measures were associated with a common slope, but the intercepts associated with the BICI mode measures at 3-months and at 12-months were allowed to be nonzero. In this way, we can test whether the mean scores observed at these time points for the BICI mode differ from those expected under the UniCI mode. The intercept for the BICI mode was estimated at −.499 (se=.562, t=−0.877, ns) at 3 months, and at −1.270 (se=.349, t=−3.645, p<.01) at 12 months. Thus, significantly better scores were only detected at the 12-month time point under the BICI mode, suggesting that following 1 year of bilateral experience, spatial hearing improves significantly in the BICI mode.
DISCUSSION
The purpose of this study was to evaluate the emergence of spatial hearing skills in sequentially implanted children, and to test the hypothesis that due to auditory plasticity there can be experience-dependent acquisition and refinement of spatial hearing skills. This work was motivated by the fact that, clinically, the number of children who are receiving BICIs is growing, yet little is known about the extent to which sequential vs. simultaneous implantation and activation of the devices will impact outcomes. By testing children who are 5–10 years of age we were able to focus on the issue of early binaural deprivation and to look at its impact on spatial hearing.
Results showed that children performed better when listening with both CIs than unilaterally. In addition, at baseline, i.e., with the use of a single CI and either a HA in the other ear or no stimulation in the other ear, performance was generally poorest. Group mean MAAs improved somewhat by 3-months, but statistically significant improvement in the BICI condition was noted at 12-months post-bilateral activation. These results suggest that nearly all children tested here, who received the second CI between the ages of 5–10 years, were able to perform better on a measure of sound localization acuity after one year of listening with both of their devices. Some of the children experienced progressive hearing loss, thus they may have had exposure to binaural acoustic hearing prior to becoming deaf. However, other children with Connexin-26 had profound hearing loss from birth and had experienced up to 10 years of auditory deprivation in the second-implanted ear. Subjects CIBA, CIAQ and CIBK had bilateral auditory deprivation until ages 3;7, 3;1 and 2;1, respectively. They then remained unilaterally implanted until ages 10;2, 8;1 and 7;1, respectively. Nonetheless, their MAA thresholds at the 12 month interval were 6.2°, 4.7° and 15.5°, all at or below the average MAA for the 12-month interval. This finding supports the notion that the auditory system of children who are born deaf and do not receive bilateral hearing for a number of years is highly capable of processing spatial cues relevant for sound location discrimination. Evidence for auditory plasticity exists in other species (Kacelnik et al., 2006; Keuroghlian and Knudsen, 2007; King et al., 2007) and lends support to the notion that bilateral implantation may have a protracted window of opportunity for emergence of spatial hearing benefits. What remains unclear is whether these children would perform just as well as their peers with earlier onset of bilateral activation on other important measures, such as sound localization in more challenging tasks, speech-in-noise, language and speech acquisition, as well as non-auditory abilities. It has been shown, for example, that the age at which the second CI is activated can have significant effects on of speech perception in quiet and in noise. Peters et al. (2007) demonstrated that children who receive a second CI by age 3–5 are more likely to have speech scores in the second-implanted ear that catch up to speech scores in the first-implanted ear than children whose second CI is activated at ages 5–8, and more so compared with activation at age 8–13 years.
A further observation regarding the baseline condition applies to a unilateral child without a HA who was most likely able to use subtle monaural level cues (CIBJ) to perform the task, and several children with a HA in the non-implanted ear, who were likely able to extract binaural cues from bimodal stimulation (CIBA, CIBK and CIBM). While they all improved from baseline to 12-months, the amount of improvement that could be measured for these “better initial performers” was limited by a floor effect with the MAA task. A more rigorous measure of spatial hearing, such as sound localization in a multi-loudspeaker listening situation, would be required in order to determine the extent to which this cohort of children may have also benefited from bilateral implantation.
It is further important to note that the improvement on the MAA task documented here was seen in the bilateral listening mode; however, there was no improvement when testing was done in the unilateral listening mode. One might argue that testing these children with a single CI once they have been bilaterally implanted disadvantages the unilateral listening mode since they are no longer accustomed to using a single CI in their daily lives. Nonetheless, given that they had numerous years of use with that single CI prior to being bilaterally activated, the unilateral testing mode provides an opportunity for examining overall change in performance.
One child (CIBH) did not show improvement from baseline to 12-months. As can be seen from the participant characteristics in Table I, the factors that contributed to lack of improvement in this child’s performance are not easily identifiable. This child had a similar age at first implantation and similar amount of delay between activation of the two CIs to other participants who did show improvement. Thus, it may be the case that with additional bilateral experience this child would have caught up with the other participants and shown improvement on the MAA task, as has previously been shown (Litovsky et al., 2006b). Alternatively, auditory deprivation in the second-implanted ear may have resulted in loss of ability to effectively utilize information in that ear, or to combine input in this ear with that of the first-implanted ear. Future work with a larger population of children who meet these recruitment criteria may be necessary for understanding the factors that contribute to this finding.
In conclusion, spatial hearing skills as measured with a right-left discrimination task emerge in sequentially implanted children in an experience-dependent manner, perhaps due to the ability of the auditory system to utilize newly acquired electrical stimulation presented to the two ears.
Acknowledgments
Sources of support that require acknowledgement
Disclosure of funding received for this work from NIH, Wellcome Trust, Howard Hughes Medical Institute, and others
The procedures followed in this work were in accordance with the ethical standards of the responsible committee on human experimentation (Human Subjects IRB, Univ. of Wisconsin-Madison) and with the Helsinki Declaration (JAMA 2000;284:3043–3049).
Funding: This work was funded by the NIH-NIDCD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shelly M. Godar, Waisman Center, University of Wisconsin – Madison, USA.
Ruth Y. Litovsky, Waisman Center, University of Wisconsin – Madison, USA
REFERENCES
- 1.van Hoesel RJ. Exploring the benefits of bilateral cochlear implants. Audiol Neurootol. 2004;9:234–246. doi: 10.1159/000078393. [DOI] [PubMed] [Google Scholar]
- 2.Schleich P, Nopp P, D’Haese P. Head shadow, squelch, and summation effects in bilateral users of the MED-EL COMBI 40/40 cochlear implant. Ear Hear. 2004;25:197–204. doi: 10.1097/01.aud.0000130792.43315.97. [DOI] [PubMed] [Google Scholar]
- 3.Litovsky RY, Parkinson A, Arcaroli J, et al. Clinical study of simultaneous bilateral cochlear implantation in adults: A multicenter study. Ear Hear. 2006a;27(6):714–731. doi: 10.1097/01.aud.0000246816.50820.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nopp P, Schleich P, D'Haese P. Sound localization in bilateral users of MED-EL COMBI 40/40+ cochlear implants. Ear Hear. 2004;25:205–214. doi: 10.1097/01.aud.0000130793.20444.50. [DOI] [PubMed] [Google Scholar]
- 5.Litovsky RY, Parkinson A, Arcaroli J. Spatial hearing and speech intelligibility in bilateral cochlear implant users. Ear Hear. 2009 doi: 10.1097/AUD.0b013e3181a165be. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills AW. On the minimum audible angle. J Acoust Soc Am. 1958;30:237–246. [Google Scholar]
- 7.Litovsky R, Macmillan N. Minimum auditory angle for clicks with simulated echoes: Effects of azimuth and standard. J Acoust Soc Am. 1994;96(2):752–758. doi: 10.1121/1.411390. [DOI] [PubMed] [Google Scholar]
- 8.Litovsky R. Developmental changes in the precedence effect: Estimates of minimal audible angle. J Acoust Soc Am. 1997;102:1739–1745. doi: 10.1121/1.420106. [DOI] [PubMed] [Google Scholar]
- 9.Litovsky RY, Johnstone PM, Godar S, et al. Bilateral cochlear implants in children: localization acuity measured with minimum audible angle. Ear Hear. 2006b;27(1):43–59. doi: 10.1097/01.aud.0000194515.28023.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muthen LK, Muthen BO. Mplus User’s Guide. Fifth Edition. Los Angeles: Muthen & Muthen; 1998–2007. [Google Scholar]
- 11.Kacelnik O, Nodal FR, Parsons CH, et al. Training-induced plasticity of auditory localization in adult mammals. PLoS Biol. 2006;4(4):e71. doi: 10.1371/journal.pbio.0040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keuroghlian AS, Knudsen EI. Adaptive auditory plasticity in developing and adult animals. Prog Neurobiol. 2007;82(3):109–121. doi: 10.1016/j.pneurobio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 13.King AG, Bajo VM, Bizley JK, et al. Physiological and behavioral studies of spatial coding in the auditory cortex. Hear Res. 2007;229(1–2):106–115. doi: 10.1016/j.heares.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters R, Litovsky RY, Parkinson A, et al. Importance of age and post-implantation experience on performance in children with sequential bilateral cochlear implants. Oto. Neurotol. 2007;28(5):649–657. doi: 10.1097/01.mao.0000281807.89938.60. [DOI] [PubMed] [Google Scholar]