Abstract
Antidromically propagated action potentials can be recorded in the proximal end of the severed medial articular nerve (MAN) on mechanical stimulation of an inflamed knee in rats and are referred to as dorsal root reflex (DRR) activity. The absence of DRR activity in normal rats suggests that the activity could be the result of hyperexcitability of spinal neurons induced by inflammation. In this study, the role of spinal type 1 metabotropic glutamate (mGlu1) receptors in the generation of DRR activity in the MAN during acute knee inflammation was investigated. Four hours after an injection of a mixture of kaolin and carrageenan (k/c) into a knee joint, DRR activity could be evoked in the ipsilateral MAN by mechanical stimulation of the inflamed limb. Spinal application of a selective mGlu1 receptor antagonist, [RS]-1-Aminoindan-1,5-dicarboxylic acid/UPF 523 (AIDA), or a potent, but less specific mGlu1 receptor antagonist, LY393053, both depressed the DRR activity significantly. AIDA and LY39053 had no effect on recordings in the MAN from noninflamed control animals. However, spinal administration of AIDA did suppress DRR activity generated by infusion of 4-aminopyridine (4-AP), a K+ channel blocker, into the dorsal horn of noninflamed animals. These observations suggest that mGlu1 receptors support the generation of DRR activity in the MAN following sensitization of spinal cord neurons.
Keywords: Metabotropic glutamate receptor, dorsal root reflex, 4-AP, arthritis, pain
Experimental arthritis induced by injection of a mixture of kaolin and carrageenan (k/c) into the knee joint results in localized knee joint swelling, limping, and guarding of the affected limb. Increased responsiveness to thermal stimuli applied to the hindpaw (secondary hyperalgesia) begins after 3 hours and reaches a maximum by 4 hours. These symptoms are maintained for 24 hours and subside by 48 to 72 hours.1 Temporal and correlative relationships have been established between the increasing concentrations of glutamate and some peptides in the spinal dorsal horn and the development of spontaneous persistent nociception and knee joint inflammation,1,2,3,4 including the development of primary and secondary hyperalgesia and allodynia. The increasing glutamate in the dorsal horn undoubtedly contributes to the sensitization of dorsal horn neurons. Nociceptive spinal neurons show an enhanced responsiveness to low-threshold mechanical stimulation of the knee joint,5,6,7 increased neuronal background activity, and enlarged receptive fields. 8,9
Further, plastic changes in the dorsal horn have been shown to generate the dorsal root reflex (DRR) originating at the primary afferent endings in the dorsal horn and propagating back out to the periphery through the afferent nerves.10 The DRR has been proposed to be the result of primary afferent depolarization (PAD) caused by the activation of γ-aminobutyric acid (GABAA) receptors on the primary afferent terminals.11 Recordings of DRR activity in the medial articular nerve (MAN) after induction of inflammation of the knee joint can be eliminated by administration of non-N-methyl-D-aspartate (NMDA) and GABAA receptor antagonists into the spinal cord dorsal horn. Unilateral dorsal rhizotomy, but not sympathectomy, results in a significant reduction of DRR activity 6,12 All the evidence indicates that the DRR is the result of central sensitization in the dorsal horn involving ionotropic glutamate (iGlu) and GABAA receptor-mediated events.
It has been shown also that the long-term release of glutamate and secondary hyperalgesia can be blocked by non-NMDA and NMDA receptor antagonists,3 revealing the involvement of iGlu receptors in central sensitization. The involvement of the other class of glutamate receptors, the G protein-coupled metabotropic glutamate receptors (mGlu), in central sensitization after inflammation also has been suggested. 13,14,15,16,17
Eight mGlu receptors have been cloned and can be divided into 3 groups based on their pharmacological profile, signal transduction pathways, and amino acid homology. The group I receptors comprising mGlu1 and mGlu5 are coupled to phosphoinositide hydrolysis/intracellular calcium mobilization. The group III (mGlu2 and mGlu3) and group III (mGlu4,6,7,8) receptors are negatively linked to adenyl cyclase; thus, they reduce cyclic adenosine monophosphate (cAMP) formation.18 Many of the mGlu receptors have been localized in the spinal cord.19–25 The mGlu receptors, particularly the Group I receptors, have been implicated in nociceptive responses.15–17,26,27 Agonists selective for group I mGlu receptors depolarize spinal neurons28,29 and potentiate responses to iGlu agonists in dorsal horn and ventral horn neurons.30–34 Agonists selective for group II or group III mGlu receptors depress synaptic transmission in the spinal cord with a dominant presynaptic action.35–37 The physiological role of these receptors is probably to depress glutamate release during high-frequency stimulation.38
The aim of this study was to record DRR activity in the MAN as a functional index to investigate the role of mGlu1 receptors in supporting dorsal horn sensitization after knee joint inflammation. To study the mGlu receptors in the spinal cord, a microdialysis delivery system was used to infuse mGlu antagonists directly into the dorsal horn. However, the microdialysis delivery system requires the use of water-soluble mGlu antagonists to cross the dialysis fiber to achieve an effective concentration in the surrounding tissue. After in vitro assessments, the selective mGlu1 receptor antagonist [RS]-1-Aminoindan-1,5-dicarboxylic acid/UPF 523 (AIDA)39 and the less selective mGlu1 receptor antagonist LY393053 ([+]-2-amino-2-93-cis and trans-car-boxycyclobutyl-3-[9-thioxanthyl]propionic acid), were chosen for these experiments. LY393053 is a novel, potent mGlu receptor antagonist with highest potency on the group I mGlu receptors, imGlu1 and mGlu5 with an IC50 of about Iμmol/L.29
Although it also has some antagonistic potency on mGlu2 and mGlu8 receptors, it is the most potent and water-soluble group I mGlu receptor antagonist available so far.
We also have examined the generation of spontaneous DRR activity after the infusion of 4-aminopyridine (4-AP) into the spinal cord dorsal horn of the noninflamed rats. The 4-AP is a K+ channel blocker and has been used extensively in experiments to induce a hyperexcitable state in spinal cord neurons.40,41 The role of mGlu1 receptors in 4-AP-induced increases of spontaneous DRR activity was investigated, and the rationale behind the use of 4-AP-induced DRR as an experimental model of central sensitization is discussed.
Materials and Methods
The experiments were approved by the University Animal Care and Use Committee and were consistent with the ethical guidelines of the National Institutes of Health and of the International Association for the Study of Pain.
Animal Knee Joint Inflammation
Experiments were carried out on 28 male Sprague-Dawley rats, weighing between 400 and 420 g. Acute knee joint inflammation was induced by an injection of a mixture of 3% kaolin and 3% carrageenan (k/c) (0.1 mL in saline) into 1 knee joint of each rat, while the animal was anesthetized with a short acting anesthetic, Brevital (60 mg/kg, intraperitoneal [i.p.]). In this acute arthritis model, localized inflammation of the knee joint reached its maximum by 4 hours and remained at this level for 24 hours.42
Electrophysiological Recording
The animals were anesthetized with urethane (1.3 g/kg) for electrophysiological recording, 3 hours after injection of k/c. In each rat, the jugular vein was cannulated, and a tracheotomy was performed. The rat was paralyzed and anesthetized with a continuous intravenous infusion of a mixture of pancuronium bromide (Astra Pharmaceuticals, Wayne, PA; 4.5 mg/kg/h) and urethane (Sigma-Aldrich, St. Louis, MO; 75 mg/kg/h), and artificially ventilated. End-tidal CO2 was monitored and maintained at 3.5% to 4.5%. The depth of anesthesia was judged by monitoring the heart rate and was kept stable during the whole experiment. Core body temperature was maintained at 37.5°C with a homeothermic blanket control unit linked to a rectal probe. The skin on the medial side of the right thigh was incised rostrally from the inguinal fossa to a point just below the medial condyle of the tibia. The MAN was dissected free and cut distally, the skin flap was retracted to form a pool filled with warmed mineral oil to prevent desiccation of the nerve. The whole MAN was placed across a bipolar silver electrode. The evoked antidromic compound action potentials in an arthritic rat were recorded (bin width, 1 second). In a noninflamed control group of rats with microdialysis fiber inserted into the spinal cord for 24 hours, the monopolar silver electrode was used to record spontaneous background activity from the proximal end of the whole MAN (bin width, 1 second). All action potentials were amplified with an AC amplifier (World Precision Instruments, Sarasota, FL), displayed on an oscilloscope and digitized using a data-logging interface system (CED micro 1401, Cambridge Electronic Design Limited, Cambridge, United Kingdom). The data was then captured and stored in a Pentium (Dell) computer for later analysis. Spike rate histograms and interspike interval histograms used in this study were plotted using the Spike2 program (Cambridge Electronic Design Limited).
Administration of Drugs
Delivery of chemicals directly into the dorsal horn was achieved using a microdialysis fiber (HOSPAL, Meyzieu, France) (200 μm outer diameter, 45,000 MW cut-off, Hospal AN69) coated with epoxy glue except for a 2 mm permeable length. The permeable area of the fiber was inserted into the spinal dorsal horn laminae III or IV of L4-L6 segments. This allowed a reasonable spread of the agents throughout these spinal segments without damage of nociceptive afferents. After fiber insertion, artificial cerebrospinal fluid (aCSF) was infused for 1 hour, and then the animals were allowed to recover overnight. The detailed insertion of the microdialysis fiber was described in a previous article.42 During experiments, aCSF was infused through the fiber at a rate of 5 μL/min. The composition of the aCSF was (in mmol/L): NaCl, 125: CaCl 2, 1.3: KCl, 2.6: MgCl2, 0.9: NaHCO3, 21: Na2HPO4, 1.3: and glucose, 3.5 (pH 7.4).
Both of the 2 mGlu1 receptor antagonists, AIDA (Tocris) and LY393053 (Lilly), were initially dissolved in NaOH at 100 mmol/L. The final concentrations (1 mmol/L or less) were achieved with dilutions in aCSF. The potassium channel blocker, 4-AP (Sigma) was diluted with aCSF from a stock solution. The final pH for the 3 compounds was adjusted to 7.4.
In a previous study, microdialysis administration of methylene blue for 1 hour revealed that the dye diffused away from the fiber approximately 5 mm, about the length of 1 spinal lumbar segment.43 To assess the amount of drug diffusion across the semipermeable microdialysis membrane, a microdialysis fiber was placed in a bath containing the drug to be measured. aCSF was infused through the fiber at a rate of 5 μL/min for the duration of the drug treatment. The drug concentration diffusing into the fiber was measured with a spectrophotometer (Beckmann). The percentage of the drug concentration diffusing across the microdialysis fiber into the known fluid volume was calculated to be 11.23% for LY393053 (at the concentration of 1 mmol/L), 2.7 % for AIDA (at the concentration of 1 mmol/L) and 23.7 % for 4-AP (at the concentration of 0.5 mmol/L). Because of the drugs’ molecular weight and lipophilicity and tissue diffusion barriers, the actual tissue concentrations of the drugs are likely to be less than the calculated concentrations in vitro. Therefore, the actual tissue concentration is unknown, but the calculated dose is likely to be the maximal dose to which the tissue might be exposed. The drug concentrations mentioned henceforth refer to the maximal calculated concentrations.
Experimental Protocol
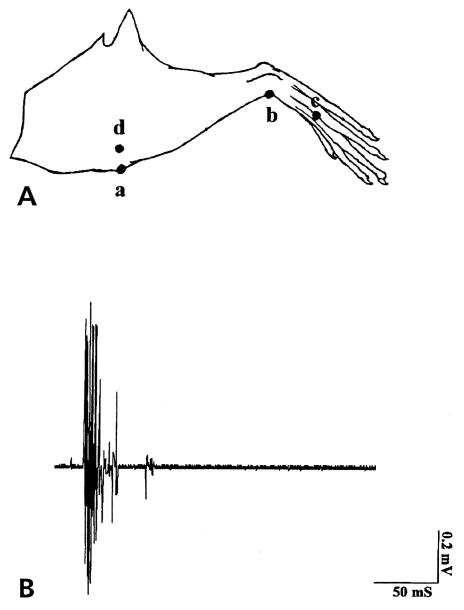
After cutting the nerve adjacent to the inflamed knee joint, the proximal end of the MAN was laid across the electrode as described above. In the arthritic rat, responses to mechanical stimuli were tested at the beginning of the electrophysiological recordings 4 hours after injection of k/c. The threshold for evoking activity in the MAN was established by applying a series of von Frey fibers with decreasing bending forces onto the inflamed leg in each animal. A single von Frey filament that bent at a constant force of 2 N was assessed to establish the use-dependent nature of the recorded activity. Four consecutive stimuli were then delivered in the order of “a” to “d” as illustrated in Fig 1 A. Repeated applications (1/sec for 10 seconds) of a von Frey fiber with a constant bending force of 2 N (a force 3 times the threshold) were applied on each stimulation site. aCSF was infused before addition of drugs for 1 hour. The application of each dose of drugs was given in ascending order for half an hour before stimulation and recordings. Dose response curves were constructed with the DRR spike rate plotted against the maximum calculated concentrations of the drug. In noninflamed control rats in which the microdialysis fiber had been inserted into the spinal cord over 24 hours, all spontaneous action potentials regardless of height or fiber origin were recorded in the whole MAN. The aCSF was infused for 1 hour before addition of compounds. Each dose of each compound tested was infused for about 1 hour before the recordings.
Figure 1.
(A) Stimulation sites on the lower limb where a 2 N bending force of von Frey fiber was used to evoke DRR activity in animals with an acutely inflamed knee joint. Mechanical stimulation was applied in the order of anterior of knee (a), ankle (b), foot (c) and the lateral part of the knee (d). (B) An example of compound action potentials evoked by von Frey fiber application on the inflamed knee joint and recorded in the proximal end of the cut MAN.
Statistical Analysis
All values are expressed as mean ± standard error (S.E.) of 3 to 6 experiments from different animals. Statistical significance between different groups was determined with an analysis of variance (ANOVA) and the Tukey-Kramer multiple comparisons test. The difference between means were considered significant when P ≤ .05
Results
Effects of AIDA and LY393053
As shown previously in our studies, there was no DRR in the MAN in naive animals.6 Four hours after injection of k/c into the rat knee joint, there is a significant increase in antidromic action potentials recorded in the MAN including both spontaneous and evoked activities.
Typically, bursts of action potentials can be recorded in response to mechanical stimulation of the inflamed hindleg (Fig 1B), with a latency of about 50 to 100 milliseconds, an amplitude of 1.4 mV, and a duration of 50 milliseconds. The average threshold for stimulation was a von Frey fiber with a bending force of 0.6 N. Repeated applications of a von Frey fiber (1/sec for 10 seconds) at a constant bending force of 2 N resulted in increased nerve firing. The largest responses were usually recorded following stimulation of the anterior and lateral aspects of the knee joint (Fig 1A parts a and d). With the development of knee inflammation, the receptive field expanded beyond the initial knee area, and eventually the antidromic neuronal activity could be recorded in response to mechanical stimulation applied over the lower limb (Fig 1A parts b [ankle] and c [foot]). No activity could be recorded in response to von Frey fiber stimuli of the contralateral noninflamed knee.
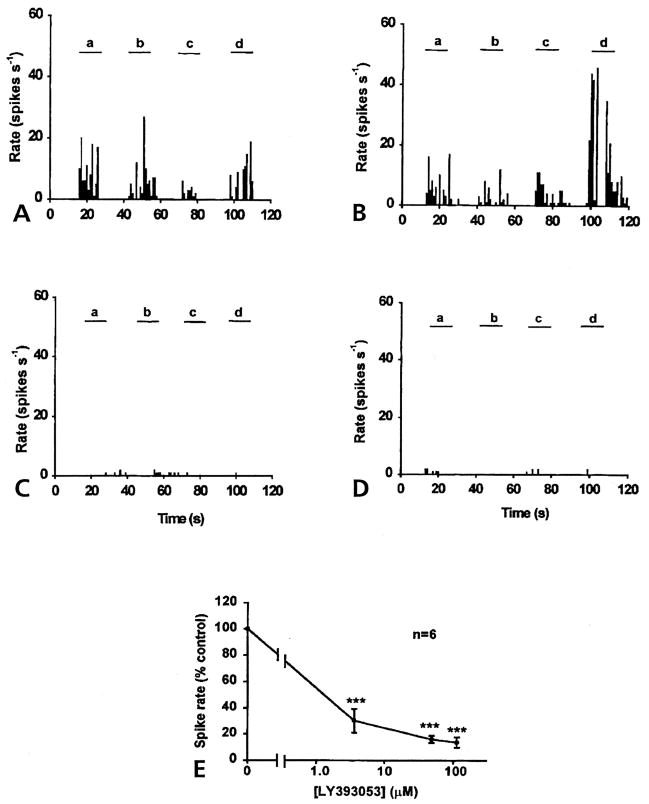
When aCSF was infused through the microdialysis fiber, the DRR activity in arthritic rats was recorded in response to von Frey fiber stimulation of the inflamed lower leg (Figs 2A and 3A). The LY393053 at a given concentration was then infused into the dorsal horn for half an hour before another set of von Frey fiber stimulations was given. Figs 2B, 2C and 2D show that LY393053 had an inhibitory effect on the DRR activity evoked by mechanical stimulation of the inflamed knee (a and d), ankle (b) and foot (c). Although the 3 concentrations (from 3.6 μmol/L to 113 μmol/L) were applied successively, the firing rates in the MAN were significantly reduced. An averaged reduction in the spike rate was 69.8% of control with the dose of 3.6 μmol/L, 83.92% with 48.5μmol/L, and 86.33% with 113 μmol/L. Each data point was significantly different from control (with aCSF infusion) (P < .001, ANOVA, Tukey-Kramer multiple comparisons test) (Fig 2E).
Figure 2.
Inhibitory effects of LY393053 on DRR activity in the MAN 4 hours after injection of a mixture of k/c into the knee joint. (A) Control DRR activity recorded from the MAN was evoked in response to a 2 N bending force von Frey fiber applied on knee (a and d), ankle (b) and foot (c), respectively. Examples of the effects of 3 doses of LY393053, 3.6 μmol/L (B), 48.5 μmol/L (C), and 113 μmol/L (D) are shown with DRR activity in the MAN evoked on 4 sites of the lower limb (a to d), respectively (bin width, 1 second). (E) Dose-response curve shown with the averaged percentage of control DRR activity plotted against the concentration shows the dose-dependent inhibitory effect of LY393053 in arthritic rats (P < .001).
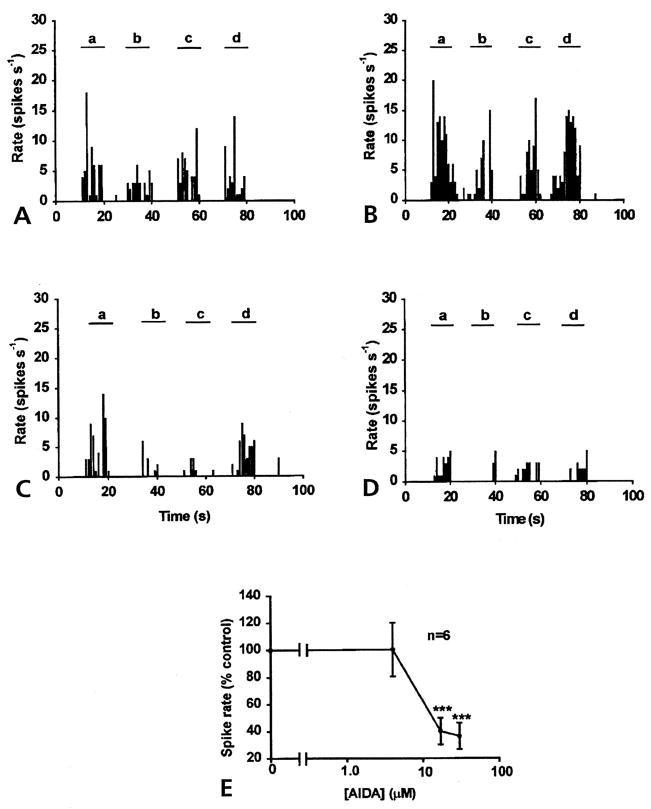
Figure 3.
Inhibitory effect of AIDA on DRR activity 4 hours after injection of k/c into the knee-joint cavity. (A) Control DRR activity recorded from the MAN induced by a 2 N von Frey fiber applied on knee (a and d), ankle (b) and foot (c) of an arthritic animal. Spinal cord application of 4 μmol/L AIDA (B) for half an hour, there was little effect on the DRR activity. The infusion of 17 μmo/L AIDA (C), or 30 μmol/L (D) into the spinal cord for half an hour inhibited DRR activity recorded from the MAN (bin width, 1 second). (E) Dose response curve for AIDA was constructed using the spike rate of DRR activity (percentage of control) plotted against the calculated concentrations applied to the spinal cord through the microdialysis fiber (P < .001).
A similar result was seen with the spinal microdialysis fiber administration of the mGlu1 receptor antagonist, AIDA. There is little effect at the concentration of 4 μmol/L AIDA. Infusion of AIDA at 17 and 30 μmol/L for 30 minutes significantly reduced the DRR recorded in the MAN in response to von Frey fiber stimulation, not only around the knee joint (a and d), but also on the ankle (b) and foot (c) (Fig 3). An averaged reduction in the rate of spike activity of 60.2% ± 10.0 % was observed with 17 μmol/L AIDA and 63.6% ± 9.8% with 30 μmol/L AIDA. Comparing the rate of spike activity at each point with that of aCSF injection (control value) (Fig 3E) showed a significant difference, P < .001 (ANOVA and Tukey-Kramer multiple comparisons test).
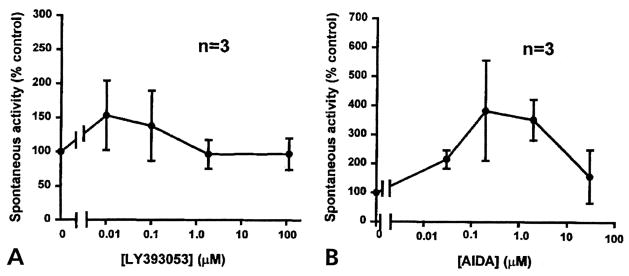
In 6 experiments, the compounds were tested on a noninflamed group of rats to examine any effects on normal synaptic transmission. A low level of spontaneous DRR activity was recorded in the MAN when aCSF was flowing through the microdialysis fiber (Fig 4A). Because no spontaneous DRR activity was observed in naive rats without the microdialysis fiber and in animals with acutely inserted microdialysis fibers (data not shown, see also Rees et al6), the spontaneous DRR activity could be the result of sensitization of dorsal horn neurons when the microdialysis fiber was inserted more than 24 hours before the experiment. Although a low background activity was recorded in this case, it appeared to have no functional relevance, because paw withdrawal latency was not significantly different from baseline level in the same animal preparations in our previous studies.1,44,45 Therefore, this low level of spontaneous DRR activity was taken as the basal control level in the present study. In these control experiments in which 4 concentrations were tested for both LY393053 (Fig 4A) and AIDA (Fig 4B), there were no significant changes in the spontaneous DRR activity recorded in the MAN. The fact that these 2 mGlu1 receptor antagonists had no significant effects on the low level of background DRR activity suggests that they were not affecting functional synaptic transmission in the spinal cord of normal noninflamed animals.
Figure 4.
Effects of LY393053 (A) and AIDA (B) on the background activity in the MAN in noninflamed control rats. No significant effects on the background activity were observed for these 2 compounds after each dose was infused into spinal cord for 1 hour. Dose response curves were drawn with the spike rate plotted against the drug concentrations. Calculated concentrations applied to the spinal cord through the microdialysis fiber for LY393053 (A) were 0.01, 0.1, 1.85, and 113 μmol/L. The concentrations that were applied for AIDA (B) were 0.03, 0.3, 3, and 30 μmol/L.
Effect of AIDA on the Activity Induced by 4-AP
In another set of experiments, the spinal cord neurons were sensitized by 4-AP infusion into the dorsal horn, and the role of mGlu1 receptors in the generation of 4-AP-induced DRR activity was examined. In a group of noninflamed rats when the microdialysis fiber had been in place for 24 hours, there was low-background, spontaneous DRR activity recorded in the MAN, with a mean firing rate of 8/sec (Fig 5A). Administration of 4-AP through the microdialysis fiber into the dorsal horn of these noninflamed rats resulted in an increase of spontaneous discharges recorded in the MAN (Fig 5B). Increases in spontaneous DRR activity started 20 minutes after application of 4-AP and reached the peak at about 30 minutes. The DRR activity was composed of multiple unit action potentials that showed irregular, nonrhythmic patterns. Analysis of the interspike intervals indicates that the most frequent spike interval was at 4 milliseconds (Fig 5B), equivalent to 250 Hz. As the dose increased, the activity became extremely variable in amplitude and frequency. However, in some cases, higher doses of 4-AP caused the discharge to become more organized and rhythmic (data not shown). Fig 6A shows that the frequency of the spike activity in the MAN was increased with application of accumulating doses of 4-AP, although there was increasing variability at the highest concentration of 27.7 μmol/L The mean firing rate at this concentration reached 26/sec, 3.25 times higher than the control rate.
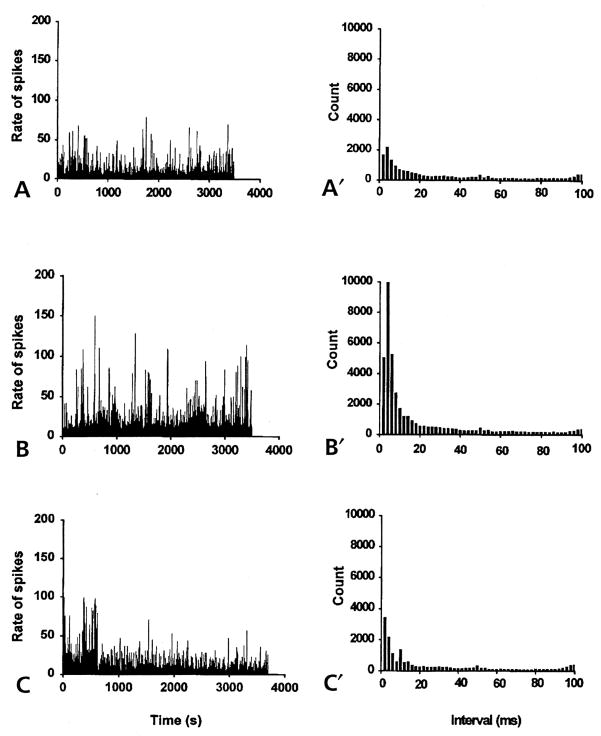
Figure 5.
The spike rate histograms (left) and the interspike interval histograms (right) constructed from spontaneous antidromic action potentials recorded from the MAN of noninflamed rats (bin width, 1 second). (A) Spike-like background activity was recorded from the MAN during microdialysis infusion of aCSF into the spinal cord for about 1 hour. The irregular, antidromic, multi-unit activity had a mean firing rate of 8/sec. Interspike interval histogram (A′) showed the number of counts at each interval with the peak interval at 4 milliseconds. (B) Spinal microdialysis administration of 4-AP (at the concentration of about 27.7 μmol/L for 1 hour) the frequency of spike activity was greatly increased with a mean firing rate of 26/sec. (B′) The peak interspike interval at 4 milliseconds increased in magnitude to about 5 times that of the aCSF control (A′). (C) and (C′) Microdialysis coinfusion of 4-AP and AIDA (17 μmol/L) for about 1 hour reversed the effect of 4-AP. The mean firing rate decreased to the level of the aCSF control (8.29/sec). The interspike interval histogram (C′) returned to the control level.
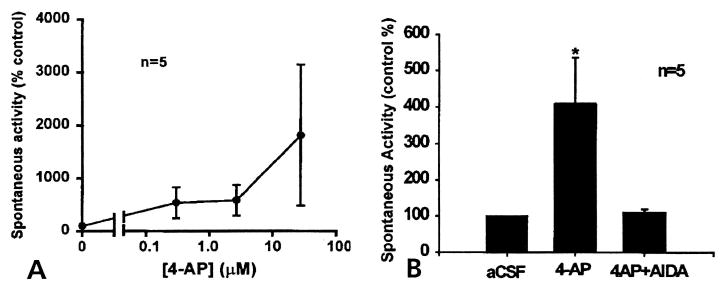
Figure 6.
(A) Dose response curve of the firing rate in the MAN plotted against 4-AP concentrations showing that 4-AP increases the frequency of the spontaneous DRR activity in a dose-dependent manner. (B) A bar chart showing the effects of 4-AP (middle), and the coapplication of 4-AP plus AIDA (right), on the mean firing rate of spontaneous background activity recorded from the MAN. Spinal cord application of 4-AP (for 1 hour) at the concentration of 27.7 μmol/L increased the mean spike rate in DRR recorded from the MAN significantly (P ≤.05). Coinfusion of 4-AP with AIDA (at the concentration of 17 μmol/L) for about 1 hour reversed the increased spike frequency induced by 4-AP back to the control level. Data used in A and B were from different groups of animals. Accumulating doses of 4-AP were applied to the spinal cord in (A), whereas in (B) only 1 dose of 4-AP at 27.7 μmol/L was applied for 1 hour, which was then followed by the coapplication of 4-AP and AIDA.
The effect of spinal application of AIDA at concentrations of 17–32 μmol/L, on 4-AP-induced DRR activity was tested. It was found that AIDA effectively reduced the increased activity to its previous control level (mean firing rate 8.29/sec) (Fig 5C, and averaged data in Fig 6B), suggesting an involvement of mGlu1 receptors in the generation of the spontaneous DRR activity induced by 4-AP.
Discussion
Receptors Contribute to the DRR Activity Evoked by Input From the Inflamed Knee
In the present study, 4 hours after injection of k/c into the rat knee joint, DRR activity in the MAN was evoked in response to mechanical stimulation of the inflamed knee. The area in which mechanical stimulation could evoke DRR activity often continued to expand down the leg to the ankle and foot. Spinal administration of the selective mGlu1 receptor antagonist, AIDA, or the potent but less selective mGlu1 receptor antagonist, LY393053, suppressed DRR activity evoked by stimulation applied to the knee and expanded receptive field. In contrast, the spinal cord administration of LY393053 or AIDA in noninflamed control rats had little effect on background activity.
These results show that blocking spinal mGlu1 receptors in the dorsal horn attenuates DRR activity generated in response to acute knee joint inflammation, but does not affect functional synaptic activity in noninflamed animals. Several group I mGlu receptor antagonists have been observed to inhibit spinal neuronal sensitization during peripheral inflammation, but have no effect on the responses to mechanical stimuli in normal neurons or to innocuous stimuli.13,15,16 Together these findings suggests that group I mGlu receptors are involved in the neuronal sensory processing of hyperexcitable spinal cord neurons impacted by afferent input arising from the inflamed knee joint.
The mechanism by which group I mGlu receptors might contribute to the generation of DRR activity remains unresolved, although their involvement in a number of second-messenger-mediated cellular events have been described. Potentiation of the responses to AMPA and NMDA has been shown both in vitro30,34 and in vivo.31–33 Activation of group I mGlu receptors might initiate protein kinase C activation, which results in the modulation of ion channels, including the inhibition of K+ conductance (IAHP and IM).18 Inhibition of K+ conductance by group I receptor agonists might depolarize central neurons.29,34 Together with the potentiation of iGlu receptor-mediated responses, the activation of group I mGlu receptors might produce a hyperexcitable state of neurons. The long-lasting actions of the intracellular signal transduction systems make group I mGlu receptors strong candidates for sustaining central sensitization in the spinal cord.15–17,26,27,46 Because of the potent action of the selective mGlu1 receptor antagonist AIDA, we can be certain of the involvement of mGlu1 receptors in sustained nociceptive transmission, in agreement with the findings by Young et al.16,17 However, the possible involvement of mGlu5 receptors can not be ruled out, because the group I mGlu receptor antagonist LY393053, which can potently antagonize both of mGlu1 and mGlu5 receptors, also produces a significant inhibitory effect on the evoked DRR activity.
The Generation of DRR Activity is the Result of Central Sensitization of Dorsal Horn Neurons
During development of knee joint inflammation after injection of k/c, the responsiveness of spinal neurons has been found to change in response to stimulation of the inflamed knee.2,5,7,13,47 The responses to innocuous and noxious stimuli are enhanced in wide dynamic range (WDR) neurons. The threshold of noxious-specific (NS) neurons is lowered to the point at which the neuron can be activated by normal innocuous stimuli. The significant changes in the activity of dorsal horn neurons after knee joint inflammation have been postulated to result in the generation of DRR activity in the MAN in our previous studies.6,12 In the present study, activating dorsal horn neurons with 4-AP further shows that this hyperexcitable state generates DRR activity that can be recorded in the MAN. Likewise, the DRR activity recorded from the MAN can be used as a means of monitoring the hyperactivity of spinal dorsal horn neurons.
The origin of centrally generated action potentials recorded from the MAN has been investigated extensively with electrophysiological recordings and using retrograde transport of horseradish peroxidase (HRP) as a tracer. The afferents in the MAN have been found to enter the spinal cord through L5-L6.48–50 Application of lidocaine to the severed nerve proximal to the recording site, or crushing the nerve proximal to the recording electrode abolishing the response to peripheral stimulation, has indicated that the activity is propagated to the periphery from the spinal cord.12 A dorsal rhizotomy between the spinal cord and the dorsal root ganglion was found to abolish the activity recorded from the MAN. All the above evidence indicates that the activity in the MAN does not originate in the dorsal root ganglion, but rather from within the spinal cord.6,12,51
DRR Activity Induced by 4-AP Might be Used as an Experimental Model of Central Sensitization for the Study of the Contribution of mGlu1 Receptors
As a selective voltage–dependent potassium channel blocker, 4-AP blocks K+ flow through the membrane and, hence, depolarizes the membrane potential to increase the excitability of all neurons.40 The 4-AP also enhances synaptic transmission in the spinal cord through a presynaptic action52 and stimulates the release of neurotransmitters such as GABA, glutamate and acetylcholine.53 In the present study, infusion of 4-AP into the spinal dorsal horn would have produced a hyperexcitable state of spinal cord networks. It was interesting to observe the development of DRR activity in the MAN in noninflamed rats after the treatment with 4-AP. The origin of the DRR activity is likely to be the hyperexcitable dorsal horn neurons acted upon by 4-AP. As shown by the development of spontaneous DRR activity in the MAN, the infusion of 4-AP in the dorsal horn could be used as a general experimental model of central sensitization, although further characterization of the model is needed.
In this study, to compliment the discovery of the contribution of mGlu1 receptors in the generation of DRR activity in the MAN in inflamed rats, we have tested the ability of the mGlu1 receptor antagonist AIDA to attenuate the 4-AP-induced DRR activity in the MAN. It was interesting to find that the infusion of AIDA completely suppressed the increased DRR activity, suggesting an important role of mGlu1 receptors in the generation of 4-AP-induced DRR activity.
Thus, the present study showed that antagonism of mGlu1 receptors suppressed the generation of DRR activity both after knee joint inflammation, as well as after the spinal infusion of 4-AP. The results provided additional evidence in support of the view that mGlu1 receptors are activated during the process of central sensitization and are important in the generation of DRR activity.
Acknowledgments
Supported in part by NIH grant R01 32778 and a Sealy endowment.
References
- 1.Sluka KA, Westlund KN. Behavioral and immunohistochemical changes in an experimental arthritis model in rats. Pain. 1993;55(3):367–377. doi: 10.1016/0304-3959(93)90013-F. [DOI] [PubMed] [Google Scholar]
- 2.Schaible HG, Schmidt RF, Willis WD. Response of spinal cord neurons to stimulation of articular afferent fibers in the cat. J Physiol. 1986;372:575–593. doi: 10.1113/jphysiol.1986.sp016026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sluka KA, Westlund KN. Spinal cord amino acid release and content in an arthritis model-the effects of pretreatment with non-NMDA, NMDA and NK1 receptor antagonists. Brain Res. 1993;627:89–103. doi: 10.1016/0006-8993(93)90752-9. [DOI] [PubMed] [Google Scholar]
- 4.Sorkin LS, Westlund KN, Sluka KA, Dougherty PM, Willis WD. Neural changes in acute arthiritis in monkeys IV Time-course of amino acid release into the lumbar dorsal horn. Brain Res Rev. 1992;17:39–50. doi: 10.1016/0165-0173(92)90005-7. [DOI] [PubMed] [Google Scholar]
- 5.Neugebauer V, Schaible HG. Evidence for a central component in the sensitization of spinal neurons with joint input during development of acute arthritis in cat’s knee. J Neurophysiol. 1990;64:299–311. doi: 10.1152/jn.1990.64.1.299. [DOI] [PubMed] [Google Scholar]
- 6.Rees H, Sluka KA, Westlund KN, Willis WD. The role of glutamate and GABA receptors in the generation of dorsal root reflexes by acute arthritis in the anaesthetized rat. J Physiol. 1995;484(2):437–445. doi: 10.1113/jphysiol.1995.sp020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaible HG, Grubb BD. Afferent and spinal mechanism of joint pain. Pain. 1993;55:5–54. doi: 10.1016/0304-3959(93)90183-P. [DOI] [PubMed] [Google Scholar]
- 8.Dougherty PM, Willis WD. Enhanced responses of spinothalamic tract neurons to excitatory amino acids accompany capsaincin-induced sensitization in the monkey. J Neurosci. 1992;12:883–894. doi: 10.1523/JNEUROSCI.12-03-00883.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guibaud G, Iggo A. The effect of lysine acetylsalicylate on joint capsule mechanoreceptors in rat with polyarthritis. Exp Brain Res. 1985;61:164–168. doi: 10.1007/BF00235631. [DOI] [PubMed] [Google Scholar]
- 10.Eccles JC, Kozak W, Magni F. Dorsal root reflexes of muscle group I afferent fibers. J Physiol. 1961;159:128–146. doi: 10.1113/jphysiol.1961.sp006797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis, Duggan A, Felix D, Johnston GAR. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 1971;32:69–96. doi: 10.1016/0006-8993(71)90156-9. [DOI] [PubMed] [Google Scholar]
- 12.Rees H, Sluka KA, Westlund KN, Willis WD. Do dorsal root reflexes augment peripheral inflammation? NeuroReport. 1994;5:821–824. doi: 10.1097/00001756-199403000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Neugebauer V, Lucke T, Schaible HG. Requirement of metabotropic glutamate receptors for the generation of inflammation-evoked hyperexcitability in rat spinal cord neurons. Eur J Neurosci. 1994;6:1179–1186. doi: 10.1111/j.1460-9568.1994.tb00616.x. [DOI] [PubMed] [Google Scholar]
- 14.Young MR, Fleetwood-Walker SM, Mitchell R, Munro FE. Evidence for a role of metabotropic glutamate receptors in sustained nociceptive inputs to rat dorsal horn neurons. Neuropharmacol. 1994;33 (1):141–144. doi: 10.1016/0028-3908(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 15.Young MR, Fleetwood-Walker SM, Mitchell R, Dickinson T. The involvement of metabotropic glutamate receptors and their intracellular signaling pathways in sustained nociceptive transmission in rat dorsal horn neurons. Neuropharmacol. 1995;34 (8):1033–1041. doi: 10.1016/0028-3908(95)00071-d. [DOI] [PubMed] [Google Scholar]
- 16.Young MR, Fleetwood-Walker SM, Dickinson T, Blackburn-Munro G, Sparrow H, Birch PJ, Bountra C. Behavioral and electrophysiological evidence supporting a role for group I metabotropic glutamate receptors in the mediation of nociceptive inputs to the rat spinal cord. Brain Res. 1997;777:161–167. [PubMed] [Google Scholar]
- 17.Young MR, Blackburn-Munro G, Dickinson T, Johnson MJ, Anderson H, Nakalembe I, Fleetwood-Walker SM. Antisense ablation of type I metabotropic glutamate receptor mGluR1 inhibits spinal nociceptive transmission. J Neurosci. 1998;18:10180–10188. doi: 10.1523/JNEUROSCI.18-23-10180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Ann Rev Pharmacol Toxical. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 19.Boxall SJ, Berthele A, Laurie DJ, Sommer B, Zieglgansberger W, Urban L, Tölle TR. Enhanced expression of metabotropic glutamate receptor 3 messager RNA in the rat spinal cord during ultraviolet irradiation induced peripheral inflammation. Neuroscience. 1998;82:591–602. doi: 10.1016/s0306-4522(97)00246-7. [DOI] [PubMed] [Google Scholar]
- 20.Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- 21.Ohishi H, Akazawa C, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for L-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J Comp Neurol. 1995;360:555–570. doi: 10.1002/cne.903600402. [DOI] [PubMed] [Google Scholar]
- 22.Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992;322:121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- 23.Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of metabotropic glutamate receptors, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- 24.Valerio A, Rizzonelli P, Paterlini M, Moretto B, Knoepfel T, Kuhn R, Memo M, Spano PF. mGluR5 immunolocalization in fetal and adult human spinal cord. Neuropharmacol. 1996;35:A33. [Google Scholar]
- 25.Vidnyánszky Z, Hámori J, Négyessy L, Rüegg D, Könpfel T, Kuhn R, Görcs T. Cellular and subcellular localization of the mGluR5a metabotropic glutamate receptor in rat spinal cord. NeuroReport. 1994;6:209–213. doi: 10.1097/00001756-199412300-00053. [DOI] [PubMed] [Google Scholar]
- 26.Fisher K, Coderre TJ. The contribution of metabotropic glutamate receptors to formalin-induced nociception. Pain. 1996;68:255–263. doi: 10.1016/s0304-3959(96)03212-5. [DOI] [PubMed] [Google Scholar]
- 27.Fisher K, Coderre TJ. Comparison of nociceptive effects produced by intrathecal administration of mGluR agonists. NeuroReport. 1996;7:2743–2747. doi: 10.1097/00001756-199611040-00067. [DOI] [PubMed] [Google Scholar]
- 28.Birse EF, Eaton SA, Jane DE, Jones PL, St J, Porter RHP, Pook PC-K, Sunter DC, Udvarhelyi PM, Wharton B, Roberts PJ, Salt TE, Watkins JC. Phenylglycine derivatives as new pharmacological tools for investigating the role of metabotropic glutamate receptors in the central nervous system. Neuroscience. 1993;52:481–488. doi: 10.1016/0306-4522(93)90400-a. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Bacon G, Sher E, Clark BP, Kallmam MJ, Wright RA, Johnson BG, Schoepp DD, Kingston AE. Evaluation of the activity of a novel metabotropic glutamate receptor antagonist (6)-2-amino-2-(3-cis and transcarboxycyclobutyl-3-(9-thioxanthyl) propionic acid) in the in vitro neonatal spinal cord and in an in vivo pain model. Neuroscience. 2000;95(3):787–793. doi: 10.1016/s0306-4522(99)00496-0. [DOI] [PubMed] [Google Scholar]
- 30.Bleakman D, Rusin KI, Chard PS, Glaum SR, Miller RJ. Metabotropic glutamate receptors potentiate ionotropic glutamate responses in the rat dorsal horn. Mol Pharmacol. 1992;42:192–196. [PubMed] [Google Scholar]
- 31.Bond A, Lodge D. Pharmacology of metabotropic glutamate receptor-mediated enhancement of responses to excitatory and inhibitory amino acids on rat spinal neurons in vivo. Neuropharmacol. 1995;34:1015–1023. doi: 10.1016/0028-3908(95)00046-9. [DOI] [PubMed] [Google Scholar]
- 32.Cerne R, Randic M. Modulation of AMPA and NMDA responses in rat spinal dorsal horn neurons by trans-1-aminocyclopentane-1,3-dicarboxylic acid. Neurosci Lett. 1992;144:180–184. doi: 10.1016/0304-3940(92)90745-s. [DOI] [PubMed] [Google Scholar]
- 33.Jones MW, Headley PM. Interactions between metabotropic and ionotropic glutamate receptor agonists in the rat spinal cord in vitro. Neuropharmacol. 1995;34:1025–1031. doi: 10.1016/0028-3908(95)00055-b. [DOI] [PubMed] [Google Scholar]
- 34.Ugolini A, Corsi M, Bordi F. Potentiation of NMDA and AMPA responses by group I mGluR in spinal cord motoneurons. Neuropharmacol. 1997;36:1047–1055. doi: 10.1016/s0028-3908(97)00103-2. [DOI] [PubMed] [Google Scholar]
- 35.Ishida M, Saitoh T, Shimamoto K, Ohfune Y, Shinozaki H. A novel metabotropic glutamate receptor agonist: marked depression of monosynaptic excitation in the newborn rat isolated spinal cord. Br J Pharmacol. 1993;109:1169–1177. doi: 10.1111/j.1476-5381.1993.tb13745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinco M, Lev-Tov A. Synaptic excitation of alpha-motoneurones by dorsal root afferents in the neonatal spinal cord. J Neurophysiol. 1993;70:406–417. doi: 10.1152/jn.1993.70.1.406. [DOI] [PubMed] [Google Scholar]
- 37.Pook PC-K, Sunter DC, Udvarhelyi PM, Watkins JC. Evidence for presynaptic depression of monosynaptic excitation in neonatal rat motoneurones by (1S, 3S)- and (1S, 3R)-ACPD. Exp Physiol. 1992;77:529–532. doi: 10.1113/expphysiol.1992.sp003617. [DOI] [PubMed] [Google Scholar]
- 38.Glaum SR, Miller RJ. Metabotropic glutamate receptors depress afferent excitatory transmission in the rat nucleus tractus solitarii. J Neurophysiol. 1993;70:2669–2672. doi: 10.1152/jn.1993.70.6.2669. [DOI] [PubMed] [Google Scholar]
- 39.Moroni F, Lombrdi G, Thomsen C, Leonardi P, Attucci S, Peruginelli F, Torregrossa AS, Pellegrini-Giampietro DE, Luneia R, Pellicciari R. Pharmacological characterization of 1-aminoindan-1,5-dicarboxylic acid, a potent mGluR1 antagonist. J Pharmacol Exp Ther. 1997;281:721–729. [PubMed] [Google Scholar]
- 40.Bowman WC, Savage AO. Pharmacological actions of aminopyridines and related compounds. Rev Pur Appl Pharmacol Sci. 1981;2:317–371. [PubMed] [Google Scholar]
- 41.Lemeignan M. Analysis of the action of 4-amino pyridine on the cat lumber spinal cord. 1 Modification of the afferent volley, the monosynaptic discharge amplitude and the polysynaptic evoked responses. Neuropharmacol. 1972;11:551–558. doi: 10.1016/0028-3908(72)90010-x. [DOI] [PubMed] [Google Scholar]
- 42.Sluka KA, Westlund KA. An experimental arthritis in rats: dorsal horn aspartate and glutamate increases. Neurosci Lett. 1992;145:141–144. doi: 10.1016/0304-3940(92)90006-s. [DOI] [PubMed] [Google Scholar]
- 43.Sluka KA, Westlund KN. Centrally administered non-NMDA but not NMDA receptor antagonists block peripheral knee joint inflammation. Pain. 1993;55:217–225. doi: 10.1016/0304-3959(93)90150-N. [DOI] [PubMed] [Google Scholar]
- 44.Lu Y, Westlund KN. Gabapentin attenuates nociceptive behaviors in an acute arthritis model in rats. JPET. 1999;290:214–219. [PubMed] [Google Scholar]
- 45.Lawand NB, Lu Y, Westlund KN. Nicotine cholinergic receptors: potential targets for inflammatory pain relief. Pain. 1999;80:291–299. doi: 10.1016/s0304-3959(98)00221-8. [DOI] [PubMed] [Google Scholar]
- 46.Fundytus ME, Fisher K, Drey A, Henry JL, Coderre TJ. In vivo antinociceptice activity of anti-rat mGluR1 and mGluR5 antibodies in rats. NeuroReport. 1998;9:731–735. doi: 10.1097/00001756-199803090-00031. [DOI] [PubMed] [Google Scholar]
- 47.Neugebauer V, Schaible HG. Peripheral and spinal component of the sensitization of spinal neurons during an acute experimental arthritis. Agent actions. 1988;25:234–236. doi: 10.1007/BF01965021. [DOI] [PubMed] [Google Scholar]
- 48.Craig AD, Heppelmann B, Schaible HG. The projection of the medial and posterior articular nerves of the cat’s knee to the spinal cord. J Comp Neurol. 1988;276:279–288. doi: 10.1002/cne.902760210. [DOI] [PubMed] [Google Scholar]
- 49.Gardner E. Conduction rates and dorsal root inflow of sensory fibers from the knee joint of the cat. Am J Physiol. 1948;152:436–445. doi: 10.1152/ajplegacy.1948.152.2.436. [DOI] [PubMed] [Google Scholar]
- 50.Hildebrand C, Öqvist G, Brax L, Tuisku F. Anatomy of the rat knee joint and fiber composition of a major articular nerve. Ana Rec. 1991;229:545–555. doi: 10.1002/ar.1092290415. [DOI] [PubMed] [Google Scholar]
- 51.Sluka KA, Lawand NB, Westlund KN. Joint inflammation is reduced by dorsal rhizotomy and not by sympathectomy or spinal cord transection. Ann Rheum Dis. 1994;53:309–314. doi: 10.1136/ard.53.5.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jankowska E, Lundberg A, Rudomin P, Kykova E. Effect of 4-aminopyridine on synaptic transmission in the cat spinal cord. Brain Res. 1982;240:117–129. doi: 10.1016/0006-8993(82)90649-7. [DOI] [PubMed] [Google Scholar]
- 53.Tapia R, Sitges M. Effect of 4-AP on transmitter release in synaptosomes. Brain Res. 1982;250:291–299. doi: 10.1016/0006-8993(82)90423-1. [DOI] [PubMed] [Google Scholar]