Abstract
Objective
To determine the efficacy of a zoster vaccine on herpes zoster related interference with activities of daily living (ADL) and health-related quality of life (HRQL).
Design
Randomized double-blind placebo controlled trial.
Setting
22 US sites.
Patients
38,546 women and men ≥60 years of age.
Intervention
Zoster vaccine or placebo.
Measurements
Herpes zoster Burden of Interference with ADL and HRQL using ratings from the Zoster Brief Pain Inventory and SF-12 Mental and Physical Component Scores. Vaccine efficacy was calculated for the modified-intention-to-treat trial population and solely among those subjects who developed herpes zoster.
Results
For the modified-intention-to-treat population, the overall zoster vaccine efficacy was 66% (95% CI: 55, 74) for Zoster Brief Pain Inventory ADL Burden of Interference Score and 55% (95% CI: 48, 61) for both the SF-12 Mental and Physical Component Scores. Among subjects who developed herpes zoster, zoster vaccine reduced the Zoster Brief Pain Inventory ADL Burden of Interference Score by 31% (95% CI: 12, 51) respectively, and did not significantly reduce the impact on HRQL.
Conclusions
Zoster vaccine reduced the burden of herpes zoster related interference with ADL in the population of vaccinees and among vaccinees who developed herpes zoster. Zoster vaccine reduced the impact of herpes zoster on HRQL in the population of vaccinees but not among vaccinees who developed herpes zoster.
Keywords: herpes zoster, herpes zoster vaccine, aged, activities of daily living
Introduction
Herpes zoster increases in frequency and severity with advancing age (1). The negative impact of herpes zoster on activities of daily living (ADL) in older adults is due primarily to the effects of herpes zoster related acute and chronic pain and discomfort, although the effects of the rash, of eye involvement and of neurological complications are also important (2). Acute and chronic herpes zoster pain and discomfort reduce physical, emotional and social functioning, lower vitality, impair physical and mental health, and interfere with ADL in older adults (2–4). The magnitude of herpes zoster related interference with ADL increases with increasing pain severity (2). Furthermore, the negative impact of herpes zoster on ADL and quality of life may occur in older individuals who are already experiencing disability from other common age-related diseases or conditions.
In VA Cooperative Study #403: The Shingles Prevention Study, zoster vaccine significantly reduced the incidence of HZ, the incidence of postherpetic neuralgia, and the burden of illness due to herpes zoster pain and discomfort (5). Recognizing the adverse impact of herpes zoster on ADL and the importance of these outcomes in older adults, the Shingles Prevention Study included pre-planned analyses of the effect of zoster vaccine on HZ-related interference with functional status and on generic measures of health-related quality of life (HRQL). This report describes the results of these analyses on ADL and HRQL in all individuals who received vaccine or placebo, as well as in those recipients of vaccine or placebo who developed herpes zoster. An additional objective of this report is to describe the effects of increasing age on these measures of zoster vaccine efficacy.
Methods
Study Design and Population
The Shingles Prevention Study was a randomized, double-blind, placebo-controlled trial of live attenuated Oka/Merck zoster vaccine in 38,546 persons ≥60 years of age conducted at 22 US study sites (5). The study was approved by the IRB at each site. Eligible subjects had a history of varicella or at least 30 years of residence in the Continental United States.
Immunocompromised persons, persons with a prior history of herpes zoster, and persons unable to adhere to protocol-specified assessments were excluded. Subjects were randomized into two age strata (60–69 and ≥70 years of age) at each study site. After receiving the study injection, subjects were educated regarding the signs and symptoms of herpes zoster and urged to contact their study site when experiencing a new rash or unilateral pain syndrome. Active follow-up and case ascertainment were insured by an interactive Automated Telephone Response System (ATRS), which subjects called monthly. Study personnel were available 24/7 to evaluate subjects with suspected herpes zoster.
1308 suspected cases of herpes zoster were serially evaluated for zoster pain and discomfort, rash and impact on ADL and HRQL for at least 182 days, according to a protocol-specified schedule. The evaluating physician offered famciclovir to subjects with clinically diagnosed herpes zoster, together with standard of care treatment for pain. Evaluable cases of HZ were determined by detection of VZV DNA in rash specimens by PCR assay (93.4% of cases) or VZV by culture (0.9%) or, in the absence of a valid laboratory diagnosis, by the clinical diagnosis (5.7%) of a Clinical Evaluation Committee (5). Primary efficacy analyses were performed using a follow-up period that excluded subjects who developed herpes zoster within the first 30 days after vaccination (modified intention-to-treat population, MITT). Of the 1308 suspected cases of herpes zoster, there were 957 evaluable cases of herpes zoster (315 in the vaccine group; 642 in the placebo group) in the primary efficacy analyses.
Outcome Measures
The Zoster Brief Pain Inventory was used to quantify herpes zoster pain and discomfort and measure selected activities of daily living and health (6). The Zoster Brief Pain Inventory was adapted from the Brief Pain Inventory (BPI) to make it a herpes zoster-specific measure of pain severity that captures pain and discomfort (including allodynia and pruritus) caused by herpes zoster (6,7). It uses an 11-point Likert scale (0–10) to rate herpes zoster pain and discomfort for four different dimensions (worst, least, and average during the past 24 hours, and now) and herpes zoster pain and discomfort-related interference with seven items of activities of daily living and health: general activity, mood, walking ability, work, relations with others, sleep, enjoyment of life. The stem instruction for each of these items was: “Circle the one number that describes how much during the past 24 hours, shingles pain has interfered with your:” The stem was followed by the ADL or health item (e.g., “General Activity”) and a printed 0–10 scale with “Does not interfere” under 0 and “Completely interferes” under 10.
The functional and health items in the Zoster Brief Pain Inventory do not include several ADL and measures of HRQL that are important to older people. The Zoster Impact Questionnaire (ZIQ) was developed to rectify this deficiency (6). The ZIQ measures interference with 11 ADL by asking respondents to “circle the one number that best describes how, since your last interview, shingles pain or discomfort has interfered with your ability or desire to: Put on clothing, bathe yourself, eat, groom yourself, travel, do shopping, do housework, prepare meals, get out of the house, participate in leisure activities, concentrate on mental tasks.” As with the Zoster Brief Pain Inventory, under each activity is a printed 0–10 scale with “Does not interfere” under 0 and “Completely interferes” under 10. Because the vaccine efficacy results for analyses using the Zoster Impact Questionnaire were very similar to the results of analyses using the Zoster Brief Pain Inventory, only the results using the Zoster Brief Pain Inventory are presented.
No herpes zoster-specific measure of HRQL was available for the study. Therefore, two generic measures of HRQL were chosen: the EuroQol Visual Analog Scale and SF-12 (8, 9, 10). The EuroQoL is a validated measure of health-related quality of life that consists of 5 questions and a visual analog scale (8). The Visual Analog Scale asks participants to rate their current health state on a scale from 0 (worst imaginable health state) to 100 (best imaginable health state). Since the EuroQoL Visual Analog Scale itself is a validated measure of HRQL, this component of the EuroQoL was used in the Shingles Prevention Study (9). Because the vaccine efficacy results for analyses using the EuroQol Visual Analog Scale were very similar to the results of analyses using the SF-12, only the results using the SF-12 are presented.
The SF-12 items include ratings of 1) general health; 2) limitations in moderate activities; 3) limitations in climbing several flights of stairs; 4) accomplishing less than one would like as a result of physical health; 5) limitations in kind of work or other activities as a result of physical health; 6) accomplishing less than one would like as a result of emotional problems; 7) not doing work or other activities as carefully as usual as a result of emotional problems; 8) how much pain interfered with work; 9) amount of time feeling calm and peaceful; 10) amount of time having a lot of energy; 11) amount of time feeling downhearted and blue; and 12) how physical or emotional health has interfered with social activities. The SF-12 has been validated for use in US populations and is summarized into Mental and Physical Health Summary Scales, providing the Mental Component Scale and the Physical Component Scale scores (10). These summary scales are standardized to have a population mean of 50.
In subjects developing herpes zoster during the study, the ADL and HRQL evaluations were repeated several times during the first 2 weeks after herpes zoster rash onset, weekly for 10 weeks, and then weekly, biweekly or monthly for a total of at least 182 days, according to a protocol-specified schedule.
Statistical Analysis
Zoster Brief Pain Inventory ADL Interference
For the purposes of analysis, the Zoster Brief Pain Inventory ADL interference items were summarized into a single score by taking the mean of the 7 items, using the approach recommended by Cleeland et al. (7). The Zoster Brief Pain Inventory ADL interference summary score is a validated and sensitive measure of the interference by herpes zoster pain and discomfort with patients’ daily lives (11). Using data from a prior validation study, Cronbach’s alpha for the items at baseline was greater than 0.70, indicating that the ADL items were internally consistent (11). Test-retest reliabilities were above the recommended cut point of 0.75. Correlations with the other questionnaires ranged from 0.55 to 0.84 and were statistically significant (p<0.001), demonstrating convergent validity. The measure differentiated between pre-defined pain severity categories (p<0.05), supporting discriminant validity (11). Zoster Brief Pain Inventory interference scores ≥3 were considered clinically significant interference based on correlations with pain and HRQL scores (6).
Zoster Brief Pain Inventory ADL Severity of Interference
For each evaluable case of herpes zoster, the Zoster Brief Pain Inventory interference data were used to calculate an ADL “Severity of Interference Score”, defined as the area under the Zoster Brief Pain Inventory interference summary score-versus-time curve for the 182 day period for a single case of herpes zoster. Higher Severity of Interference Scores indicated increasing functional interference. The full 0–10 interference scale was used to calculate the ADL Severity of Interference Score. The Zoster Brief Pain Inventory Severity of Interference Score was defined as zero for subjects who did not develop an evaluable case of herpes zoster during the study. A Zoster Brief Pain Inventory ADL Severity of Interference Score ≥300 was considered severe because this threshold correlates with markedly reduced quality of life and functional status (6). The SF-12 Mental and Physical Component Scores were employed in a similar fashion to determine an HRQL response-versus-time curve for the 182 day period for each evaluable case of herpes zoster. Higher SF-12 scores indicate better HRQL.
Zoster Brief Pain Inventory ADL Burden of Interference
The Zoster Brief Pain Inventory ADL Burden of Interference Score represents the average Severity of Interference Score (i.e., severity of interference) among subjects in the vaccine and placebo groups; it was calculated as the sum of the Zoster Brief Pain Inventory ADL Severity of Interference Scores of all members of a group divided by the total number of subject-years of follow-up in that group. The observed Zoster Brief Pain Inventory ADL Burden of Interference Score was calculated as a weighted average stratified by age group with weights proportional to the total follow-up time in each age group. Similar calculations were performed with the SF-12 Mental and Physical Component Scores to determine the average rating of HRQL among subjects in the vaccine and placebo groups.
Vaccine Efficacy
Vaccine efficacy for Zoster Brief Pain Inventory Burden of Interference was defined as the relative reduction in Zoster Brief Pain Inventory Burden of Interference in the vaccine group compared with the placebo group. Vaccine efficacy for SF-12 Mental and Physical Component Scores was defined as the relative difference in average area under the curve for the SF-12 Mental and Physical Component Scores in the vaccine group compared with the placebo group. For HRQL analyses, higher area under the curve indicates better HRQL than lower area under the curve.
Vaccine efficacy was calculated separately for the modified intention to treat trial population (excluded subjects who developed herpes zoster within the first 30 days after vaccination) and solely for the evaluable cases of herpes zoster in vaccine and placebo recipients. Vaccine efficacy was also calculated for the intention to treat trial populations (included subjects who developed herpes zoster within the first 30 days after vaccination)
Change in vaccine effect on Zoster Brief Pain Inventory ADL Burden of Interference Score with increasing age was assessed in general linear models including treatment, year of age, and an interaction term for treatment and age to test the significance of the change
Results
Subject Characteristics
Of the 38,546 individuals enrolled in the Shingles Prevention Study, 19,270 received the zoster vaccine and 19,276 received a placebo injection. Approximately 54% of the population was 60–69 years old, 46% of the population was 70 years of age and older, 41% were women, and 95% were white. At the time of enrollment, 90% of participants reported that they had no or mild health limitations on their activity. More than 95% of the subjects were actively followed to the end of the study and completed a closeout interview. Only 0.6% of subjects withdrew or were lost to follow-up. Antiviral medication use in cases of herpes zoster was comparable in vaccine and placebo recipients (87.3% and 85.7%, respectively), and was initiated within 72 hours of herpes zoster rash onset in 64.1% and 65.9%, respectively. The frequency of use of various herpes zoster pain medications was comparable in cases of herpes zoster in the vaccine and placebo recipients, and the average duration and quantity of opioids used per case of herpes zoster were greater in the placebo recipients (5).
Activities of Daily Living Analyses
Frequency of ADL Interference Scores ≥3 Among Evaluable Cases of Herpes Zoster. For descriptive purposes, Table 1 shows the proportion of individuals with HZ who had Zoster Brief Pain Inventory ADL interference scores ≥3 in the zoster vaccine and placebo groups at pre-specified time points following HZ rash onset.
Table 1.
Zoster Brief Pain Inventory (ZBPI) Activities of Daily Living (ADL) Interference Ratings ≥3 Among Evaluable Cases of Herpes Zoster
| Time From Herpes Zoster Rash Onset | Proportion of Subjects with ZBPI ADL Interference Scores ≥3 (%)* | |
|---|---|---|
| Zoster Vaccine (n = 315)† | Placebo (n = 642) | |
| Day 1 | 25.0 | 43.1 |
| Day 2 | 31.9 | 30.4 |
| Day 3 | 30.7 | 29.9 |
| Day 4 or 5 | 29.4 | 32.4 |
| Day 6, 7, or 8 | 28.3 | 28.3 |
| Day 9, 10 or 11 | 18.5 | 23.5 |
| Week 2 | 17.8 | 21.3 |
| Week 3 | 14.3 | 17.1 |
| Week 4 | 12.3 | 13.7 |
| Week 5 | 7.9 | 9.7 |
| Week 6 | 5.7 | 9.1 |
| Week 7 | 4.8 | 5.7 |
| Week 8 | 2.9 | 6.7 |
| Week 10 | 1.5 | 5.0 |
| Week 12 | 0.8 | 4.3 |
| Week 16 | 0.9 | 2.4 |
| Week 20 | 0.0 | 2.0 |
| Week 24 | 0.0 | 1.6 |
| Week 26 | 0.0 | 1.5 |
The ADL Interference score for an individual is calculated as the average of the scores for the 7 interference questions on the Zoster Brief Pain Inventory.
Not all of the 315 patients with herpes zoster who received the zoster vaccine and the 642 patients with herpes zoster who received placebo reported ADL interference data at every visit. For the Zoster Brief Pain Inventory ADL interference, the numbers for zoster vaccine recipients ranged from 28 on Day 1 to 281 at Week 26; for placebo recipients from 58 on Day 1 to 583 at Week 26. The numbers for ZIQ ADL interference were similar.
N/A = not applicable
Vaccine Efficacy for All Subjects
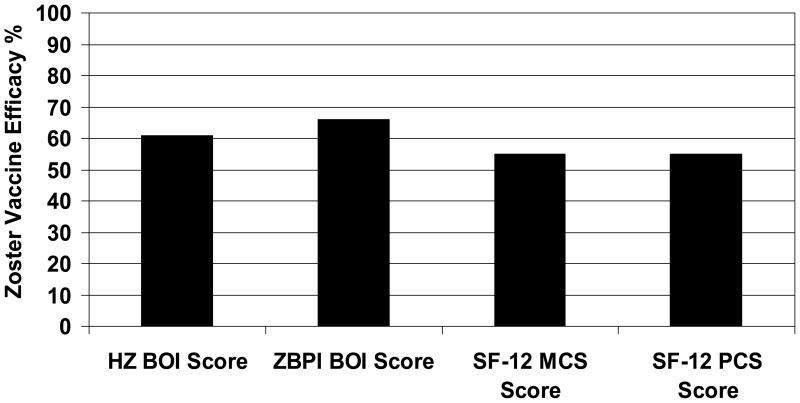
For all subjects from day 0 to182 in the modified intention to treat population, zoster vaccine reduced the Zoster Brief Pain Inventory ADL Burden of Interference Score by 66% (95% CI: 55, 74) (Table 2). In the intention to treat population (all subjects), zoster vaccine reduced the Zoster Brief Pain Inventory ADL Burden of Interference Score by 67% (95% CI: 56, 75). A sensitivity analysis of vaccine efficacy for the Zoster Brief Pain Inventory ADL Burden of Interference Score for all subjects was performed using cutpoints of ≥3 and ≥5 on the 0–10 interference scale. For analyses using a cutpoint of ≥3, vaccine efficacy was 69% (95% CI: 57, 75). For analyses using a cutpoint of ≥5, vaccine efficacy was 64% (95% CI: 52, 72). For comparison, the reduction in the Zoster Brief Pain Inventory ADL Burden of Interference Score was slightly greater than the reduction in Herpes Zoster Pain and Discomfort Burden of Illness Score (61%, 95% CI: 51, 69), the primary endpoint of the Shingles Prevention Study (5)(Figure 1). Figure 1 shows zoster vaccine efficacy for Herpes Zoster Pain and Discomfort Burden of Illness Score and Zoster Brief Pain Inventory ADL Burden of Interference Score among all subjects. In modified intention to treat analyses, vaccine efficacy for the Zoster Brief Pain Inventory ADL Burden of Interference Score diminished with age from 73% (95% CI: 47, 86) for subjects age 60–64 years to 59% (95% CI: 11, 81) for subjects age ≥80 years (Table 2) but this trend was not statistically significant (p = 0.52). The results of intention to treat analyses of vaccine efficacy for the Zoster Brief Pain Inventory ADL Burden of Interference Score by age were very similar to the modified intention to treat analyses (data not shown).
Table 2.
Zoster Vaccine Efficacy for Zoster Brief Pain Inventory (ZBPI) Activities of Daily Living (ADL) Burden of Interference by Age Among All Randomized Subjects (Modified Intention To Treat Population)
| Age Group (Years) | Zoster Vaccine |
Placebo |
Vaccine Efficacy for ZBPI ADL Burden of Interference Point Estimate (95%)§ | ||||
|---|---|---|---|---|---|---|---|
| n* | m† | ZBPI ADL Burden of Interference Score ‡ | n* | m† | ZBPI ADL Burden of Interference Score ‡ | ||
| All ages | 315 | 19254 | 0.89 | 642 | 19247 | 2.64 | 66.2 (55.4, 74.4) |
| 60 to 64 | 54 | 5216 | 0.52 | 153 | 5198 | 1.95 | 73.1 (47.0, 86.3) |
| 65 to 69 | 68 | 5154 | 0.71 | 181 | 5158 | 2.18 | 67.4 (44.1, 81.0) |
| 70 to 74 | 89 | 4545 | 1.16 | 158 | 4560 | 2.96 | 60.8 (35.2, 76.3) |
| 75 to 79 | 67 | 3076 | 1.38 | 103 | 2999 | 3.66 | 62.3 (29.8, 79.7) |
| ≥80 | 37 | 1263 | 2.11 | 47 | 1332 | 5.16 | 59.0 (11.0, 81.1) |
n = Number of evaluable cases of herpes zoster in the Modified Intention To Treat population
m = Number of subjects with follow-up.
ZBPI ADL Burden of Interference Score is calculated as the sum of the ZBPI ADL Interference Scores (i.e., the areas under the ZBPI ADLI Interference score versus time curves during the 6-month period following herpes zoster rash onset) for all subjects in the group divided by the subject years of follow-up. Subjects who did not develop herpes zoster during the study were assigned a ZBPI ADL Interference Score of zero. The figure for all ages is the weighted average of the observed burden of interference of ZBPI ADL stratified by age group with weights proportional to the total follow-up time in each age group.
For all ages, it is calculated as a weighted average of the observed vaccine efficacy stratified by age group with weights proportional to the total follow-up time in each age group. The confidence interval is constructed based on the large sample approximation under the fixed number of events design.
Figure 1.
Zoster Vaccine Efficacy (%) in All Subjects (modified intention to treat analysis, n = 38,501). The figure shows zoster vaccine efficacy for Herpes Zoster Pain and Discomfort Burden of Illness (HZ BOI) Score (61%, 95% CI: 51, 69); Zoster Brief Pain Inventory ADL Burden of Interference Score (ZBPI BOI) (66%, 95% CI: 55, 74); SF-12 Mental Component Scale (MCS) Score (55%, 95% CI: 48, 61), and SF-12 Physical Component Scale (PCS) Score (55%, 95% CI: 48, 61).
Vaccine Efficacy for Subjects with Herpes Zoster
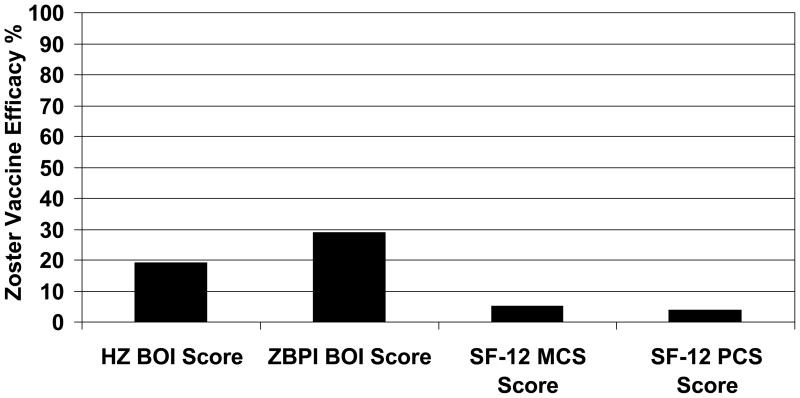
Vaccine efficacy in subjects with an evaluable case of herpes zoster is shown in Table 3. Zoster vaccine reduced the Zoster Brief Pain Inventory ADL Burden of Interference Score by 29% (95% CI: 7, 46). In intention to treat analyses (only herpes zoster cases), zoster vaccine reduced the Zoster Brief Pain Inventory ADL Burden of Interference Score by 30% (95% CI: 8, 47). A sensitivity analysis of vaccine efficacy for the Zoster Brief Pain Inventory ADL Burden of Interference Score for evaluable cases of herpes zoster was performed using cutpoints of ≥3 and ≥5 on the 0–10 interference scale. For analyses using a cutpoint of ≥3, vaccine efficacy was 31% (95% CI: 8, 47). For analyses using a cutpoint of ≥5, vaccine efficacy was 23% (95% CI: −0.5, 41). For comparison, vaccine efficacy for the Herpes Zoster Pain and Discomfort Burden of Illness Score was 19% (95% CI 2, 35) among evaluable cases of herpes zoster (Figure 2). Figure 2 shows the vaccine efficacy for the herpes zoster Pain and Discomfort Burden of Illness Score and the Zoster Brief Pain Inventory ADL Burden of Interference Score among evaluable cases of herpes zoster. Vaccine efficacy for Zoster Brief Pain Inventory ADL Severity of Interference Score increased with age from 23% in subjects age 60–64 years to 51% in subjects age ≥80 years but this trend was not statistically significant (p = 0.15)(Table 3). The results of intention to treat analyses of vaccine efficacy for the Zoster Brief Pain Inventory ADL Burden of Interference Score by age (herpes zoster cases only) were very similar to the modified intention to treat analyses (data not shown).
Table 3.
Zoster Vaccine Efficacy for Zoster Brief Pain Inventory (ZBPI) Activities of Daily Living (ADL) Burden of Interference by Age Among Evaluable Cases of Herpes Zoster.
| Age Group (Years) | Zoster Vaccine |
Placebo |
Zoster Vaccine Efficacy for ZBPI Severity of Interference Point Estimate (95%)‡ | ||
|---|---|---|---|---|---|
| n* | ZBPI ADL Burden of Interference Score † | n* | ZBPI ADL Burden of Interference Score † | ||
| All ages | 315 | 57.8 | 642 | 81.6 | 29.2 (7.0, 46.0) |
| 60 to 64 | 54 | 50.7 | 153 | 66.2 | 23.4 (−50.8, 61.1) |
| 65 to 69 | 68 | 53.8 | 181 | 62.0 | 13.3 (−48.6, 49.4) |
| 70 to 74 | 89 | 59.2 | 158 | 85.3 | 30.6 (−14.7, 58.0) |
| 75 to 79 | 67 | 63.4 | 103 | 106.6 | 40.5 (−10 7, 68.1) |
| ≥80 | 37 | 72.1 | 47 | 146.1 | 50.7 (−7.2, 77.3) |
n = Number of evaluable cases of herpes zoster in the Modified Intention To Treat population
ZBPI ADL Burden of Interference Score is calculated as the sum of the ZBPI ADL Interference Scores (i.e., the areas under the ZBPI ADLI Interference score versus time curves during the 6-month period following herpes zoster rash onset) for all subjects in the group with herpes zoster divided by number of subject in the group with herpes zoster
For all ages, it is calculated as a weighted average of the observed vaccine efficacy
Figure 2.
Zoster Vaccine Efficacy (%) in Evaluable Cases of Herpes Zoster (n = 857). The figure shows vaccine efficacy for Herpes Zoster Pain and Discomfort Burden of Illness (HZ BOI) Score (19%, 95% CI: 2, 35); Zoster Brief Pain Inventory ADL Burden of Interference (ZBPI BOI) Score (29%, 95% CI: 7, 46), SF-12 Mental Component Scale (MCS) Score (5.2%, 95% CI: −9.4, 17.8), and SF-12 Physical Component Scale (PCS) Score (3.9%, 95% CI: −11, 16).
Vaccine Efficacy for Severe ADL Interference
The number of herpes zoster cases with Zoster Brief Pain Inventory ADL Severity of Interference Scores ≥300 was lower in vaccine vs. placebo recipients (n=13, 4.1% vs. n=42, 6.5%; relative reduction 69%, 95%CI 42, 85).
Health-Related Quality of Life Analyses
Vaccine Efficacy for All Subjects
In the modified intention to treat population, zoster vaccine reduced the impact on physical HRQL as measured by the SF-12 Physical Component Score by 55% (95% CI: 48, 61), and the impact on mental HRQL as measured by the SF-12 Mental Component Score by 55% (95% CI: 48, 61) (Figure 1). In the intention to treat population, zoster vaccine reduced the impact on physical HRQL as measured by the SF-12 Physical Component Score by 56% (95% CI: 48, 62), and the impact on mental HRQL as measured by the SF-12 Mental Component Score by 56% (95% CI: 49, 62). For comparison, vaccine efficacy for these parameters was slightly lower than vaccine efficacy for the Herpes Zoster Pain and Discomfort Burden of Illness Score and for the Zoster Brief Pain Inventory ADL Burden of Interference Score (Figure 1).
Vaccine Efficacy for Subjects with Herpes Zoster
Among subjects with herpes zoster, zoster vaccine had minimal effects on the impact of herpes zoster on HRQL measured by the SF-12 Physical Component Score (vaccine efficacy 3.9%, 95% CI: −1.1, 16), and by the SF-12 Mental Component Score (vaccine efficacy 5.2%, 95% CI: −9.4, 18) (Figure 2). The results of the intention to treat analyses were nearly identical for the SF-12 Physical Component Score (vaccine efficacy 3.9% (95% CI: −1.1, 17), and the SF-12 Mental Component Score (vaccine efficacy 5.1% (95% CI: 9.4, 18). For comparison, these effects of zoster vaccine were less than the vaccine efficacy for the Herpes Zoster Pain and Discomfort Burden of Illness Score and for the Zoster Brief Pain Inventory ADL Burden of Interference Score (Figure 2).
Discussion
The Shingles Prevention Study provides unique longitudinal data on the impact of herpes zoster on ADL and HRQL in a large number subjects with herpes zoster who exhibited a wide range of herpes zoster severity. These data show that the proportion of patients with herpes zoster who had ADL interference ratings of 3 or greater on a 0–10 point Likert scale, which was our threshold for a clinically meaningful impact on ADL, was slightly higher in placebo versus vaccine recipients at most time points after rash onset. The proportion of patients with clinically meaningful ADL interference ratings appears to be lower in the Shingles Prevention Study than in studies of outpatients with herpes zoster, although direct comparisons are difficult because other studies employed different measures of impact on ADL. However, in a study of the impact of acute herpes zoster neuralgia on ADL in outpatients with herpes zoster, which employed the same measures, the proportion of patients with Zoster Brief Pain Inventory ADL interference ratings of 5 or greater at 14 days after rash onset was 66% (2), substantially higher than observed in the Shingles Prevention Study. The lower proportion of subjects with herpes zoster with clinically significant ADL interference ratings reported here is likely the result of differences the population of subjects studied. The Shingles Prevention Study employed active surveillance and case finding, which almost certainly resulted in the inclusion of subjects with mild cases of herpes zoster who ordinarily would not have sought medical care, whereas the studies of herpes zoster outpatients only enrolled persons with herpes zoster of sufficient severity to cause them to seek medical attention. Furthermore, in the Shingles Prevention Study, 86–87% of subjects with herpes zoster received antiviral treatment, primarily with famciclovir, and 64–66% of subjects with herpes zoster were treated within 72 hours of rash onset, which may be better than usual care and have reduced the impact of herpes zoster on ADL and HRQL.
Mean ratings or proportions of individuals at any one point in time do not take into account the severity and duration of interference with ADL or impact on HRQL. To address this deficiency we employed a severity-by-duration area-under-the-curve measure, the Severity of Interference Score for each individual with herpes zoster which in turn was used to determine the Burden of Interference Score for a group of individuals (e.g vaccine or placebo recipients). In the whole population, zoster vaccine reduced the Zoster Brief Pain Inventory ADL Burden of Interference Score by 68% and reduced the herpes zoster related decrement in HRQL by 55% compared to placebo, indicating beneficial effects on a population basis. These effects of zoster vaccine on ADL and HRQL were similar in magnitude to the effect of zoster vaccine on the Herpes Zoster Pain and Discomfort Burden of Illness Score in the Shingles Prevention Study (Figure 1). Much of the reduction was related to the ability of the vaccine to prevent herpes zoster.
Zoster vaccine also reduced the severity of the disease in those vaccine recipients who developed herpes zoster. In vaccinated subjects who developed herpes zoster, zoster vaccine reduced the impact of herpes zoster on ADL compared to the placebo. Among subjects with herpes zoster, zoster vaccine reduced Zoster Brief Pain Inventory ADL Burden of Interference Score by 29%. Zoster vaccine reduced severe interference with ADL by 69%, although only 4% of subjects with herpes zoster suffered severe interference. These results suggest a beneficial effect of zoster vaccine on herpes zoster related interference with ADL beyond the vaccine’s ability to prevent herpes zoster. Zoster vaccine had only a minimal effect on the impact of herpes zoster on HRQL, which was measured with generic instruments, including the EuroQol VAS and SF-12 (a 5–10% reduction, which was not statistically significant). The relatively small difference between groups in the proportions of subjects with scores of 3 or more at a given time point in Table 1 and the larger difference between groups in Zoster Brief Pain Inventory ADL Burden of Interference Score is probably due to the fact that the Burden of Interference measure includes the full range of severity of interference scores and the duration of interference, making it a more sensitive measure than proportions.
The more robust reduction in the Zoster Brief Pain Inventory ADL Burden of Interference compared with the herpes zoster induced decline in HRQL among all zoster vaccine recipients and those who developed herpes zoster may relate to the herpes zoster -specificity of the measures. The Zoster Brief Pain Inventory is herpes zoster specific and therefore more sensitive to the impact of a vaccine against herpes zoster, whereas the SF-12 and EuroQol Visual Analog Scale are generic measures that include non- herpes zoster influences on HRQL in the older adult population studied. As expected, these generic measures are less sensitive to the impact of a zoster vaccine.
Study limitations are worth noting. The clinical interpretation of severity by duration area-under-the-curve numbers for a summary measure of ADL is challenging. The measurement of both duration and severity of a clinical problem (e.g., fever, pain, disability) is an important and well recognized aspect of clinical care but the clinical meaning of absolute differences in the interference severity by duration area-under-the-curve is not clear. One way to understand the clinical meaning of a difference in score is to examine an individual example. Vaccine efficacy of 29% can change an individual who experiences a significant average interference score of 7 for 30 days (score = 210) to one who experiences a less severe average interference score of 5 for 30 days (score = 150). In addition, decreasing Zoster Brief Pain Inventory ADL Burden of Interference correlates with decreasing Herpes Zoster Pain and Discomfort Burden of Illness and better HRQL scores (2, 6). Another potential limitation is that area-under-the-curve is a novel approach to measuring zoster-related impact on ADL which may be very sensitive to the impact of zoster and possibly magnify the benefit of vaccine with regards to function. Also, area-under-the-curve does not discriminate between persons with severe interference for a short time and persons with mild interference for a long time (e.g., interference score of 8 for 10 days would have the same area-under-the curve as an interference score of 1 for 80 days). In addition, the Zoster Brief Pain Inventory summary measure is not as well recognized a measure of function in older adults as basic ADL and instrumental ADL. However, the Zoster Brief Pain Inventory ADL Burden of Interference Score is a validated and sensitive measure of the interference of zoster pain and discomfort with patients’ daily lives (11). The study results suggest that future studies of herpes zoster that employ HRQL measures should use herpes zoster-specific rather than generic measures.
Finally, the benefits of the vaccine should be weighed against the risks. In the Shingles Prevention Study, the rates of serious adverse events were similar in people who received zoster vaccine (1.4%) compared to those who received the placebo (1.4%)(4). Only two of the SAEs were considered vaccine related; neither subject was 80 years or older (4). The number and rates of serious adverse events in persons 80 years and older was 27 in 1220 (2.2%) participants who received zoster vaccine versus 21 in 1289 (1.6%) participants who received placebo (relative risk 1.36, 95% confidence interval (0.78, 2.37)(12). In a smaller adverse event monitoring substudy of the Shingles Prevention Study, the number and rates of serious adverse events in persons 80 years and older was 11 in 217 (5.1%) participants who received zoster vaccine versus 4 in 173 (2.3%) participants who received placebo (relative risk 2.19, 95% confidence interval (0.75, 6.45)(12). The FDA has concluded that the available data do not establish that these events are related to the vaccine (13). A detailed analysis of zoster vaccine safety data was recently published by our group including an analysis for serious adverse events that occurred during the 42 days after inoculation in all participants 80 years or older (14). The overall rate of serious adverse events was not statistically significantly different in zoster vaccine versus placebo group in participants 80 years or older (risk difference, 0.6 percentage points [95% CI, −0.5 percentage points to 1.7 percentage points]), and there were no statistically significant differences between groups for any body system (COSTART) or Physiologic Diagnostic Category classification (14). The risk of serious adverse events in persons over 80 years old has been examined in a recent, separate safety study of the zoster vaccine, the results of which are pending publication (15).
In conclusion, in the ≥60 year old adults evaluated in the Shingles Prevention Study, zoster vaccine reduced the herpes zoster-related Burden of Interference with ADL by two-thirds and the herpes zoster-related impact on HRQL by about half. Most of this reduction was due to the vaccine’s efficacy in preventing herpes zoster. However, among subjects who developed herpes zoster, the impact of herpes zoster on ADL was reduced by about one-third in vaccine recipients compared with placebo recipients. This confirmed that, in addition to being able to prevent herpes zoster, zoster vaccine also reduces the severity of herpes zoster in those who develop the disease.
Acknowledgments
Support: This study was conducted by the Cooperative Studies Program of the Department of Veterans Affairs in collaboration with the NIAID, National Institutes of Health and Merck & Co. Inc. Funding was provided by the Cooperative Studies Program of the Department of Veterans Affairs, Office of Research and Development, by a grant from Merck & Co., Inc. to the VA Cooperative Studies Program, by the NIA Duke Pepper OAIC 5P30AG028716-04 (Dr. Schmader), and by the James R. and Jesse V. Scott Fund for Shingles Research (RHM, MN0).
The authors wish to acknowledge Dr. Charles Cleeland who developed the Brief Pain Inventory and holds the copyright of the ZBPI and Josephine Norquist, Alex Nikas, Kristi McNaughton, and Paul Coplan for their work on the validation of the ADL items of the ZBPI and ZIQ.
Sponsor’s Role:
The VA Cooperative Studies Program carried out the Shingles Prevention Study in collaboration with the NIAID (NIH), and Merck & Co., Inc. (the NDA holder and producer of the zoster vaccine). The Study Protocol was designed and written by a VA Cooperative Studies Program Planning/Executive Committee. Merck & Co., Inc. (Merck) contributed to the planning process through their non-voting Committee members. The conduct and management of the Study, including the acquisition, custody, analysis, and interpretation of the Study data, were the responsibility of the Executive Committee in accordance with VA Cooperative Studies Program policies and procedures. The Study data were held, verified and analyzed by the VA Cooperative Studies Program Coordinating Center in West Haven, Connecticut. Merck & Co., Inc. personnel contributed ideas and suggestions from the planning stage through manuscript revision. The Executive Committee approved all decisions regarding data analysis and interpretation, manuscript content, and manuscript submission.
The Shingles Prevention Study was planned and/or administered by a Planning/Executive Committee: Michael N. Oxman (Chair), Robert Arbeit, Patricia Barry, Chris Beisel,, Kathy D. Boardman, Cindy L. Colling, Larry Davis, Lawrence Gelb, Anne A. Gershon, Anthony R. Hayward, Michael R. Irwin, Gary R. Johnson, Myron J. Levin, Peter N. Peduzzi, Kenneth Schmader, Michael S. Simberkoff, Stephen E. Straus, Adriana Weinberg, Heather M. Williams, Jeffrey L. Silber, Paula Annunziato, Christina Y. Chan, Ivan S.F. Chan.
The VA Cooperative Studies Program Shingles Prevention Study Investigators include: LE. Davis (Albuquerque, NM), CA Kauffman (Ann Arbor, MI), SK Keay (Baltimore, MD), AR Marques, NE Soto, P Brunell (Bethesda), JW Gnann (Birmingham, AL), R Serrao, DJ Cotton, RP Goodman, RD Arbeit (Boston, MA), CT Pachucki (Hines, IL), MJ Levin (Denver), KE Schmader (Durham), WA Keitel (Houston, TX), RN Greenberg (Lexington), VA Morrison (Minneapolis, MN), PF Wright, MR Griffin (Nashville, TN), MS Simberkoff (New York, NY), SS Yeh, Z Lobo (Northport, NY), M Holodniy, J Loutit (Palo Alto, CA), RF Betts (Rochester, NY), LD. Gelb (St. Louis), GE Crawford (San Antonio, TX), J Guatelli, PA Brooks (San Diego, CA), KM Neuzil (Seattle, WA), and JF Toney (Tampa, FL)
Footnotes
Kenneth E. Schmader: Grants, including Shingles Prevention Study, consultant
Gary R. Johnson: No conflicts
Patricia Saddier: Employee
Maria Ciarleglio: No conflicts
William W.B. Wang: Employee
Jane H. Zhang: No conflicts
Ivan S.F. Chan: Employee
Shing-Shing Yeh: No conflicts, Shingles Prevention Study only
Myron J. Levin: Grants including Shingles Prevention Study, consultant, patent
Ruth M. Harbecke: No conflicts; Shingles Prevention Study only
Michael N. Oxman: No conflicts; Shingles Prevention Study only
Author Contributions:
Kenneth E. Schmader: study concept and design, acquisition of subjects and data, interpretation of data, and preparation of manuscript; Gary R. Johnson: study concept and design, acquisition of data, analysis and interpretation of data, and preparation of manuscript; Patricia Saddier: study concept and design, interpretation of data, and preparation of manuscript; Maria Ciarleglio: study design, analysis and interpretation of data, and preparation of manuscript; William W.B. Wang: study design, analysis and interpretation of data, and preparation of manuscript; Jane H. Zhang: study design, analysis and interpretation of data, and preparation of manuscript; Ivan S.F. Chan: study design, analysis and interpretation of data, and preparation of manuscript; Shing-Shing Yeh: study design, acquisition of subjects and data, interpretation of data, and preparation of manuscript; Myron J. Levin: study concept and design, acquisition of subjects and data, interpretation of data, and preparation of manuscript; Ruth M. Harbecke: interpretation of data, and preparation of manuscript; Michael N. Oxman: study concept and design, acquisition of subjects and data, interpretation of data, and preparation of manuscript. All authors approved the final version.
Conflict of Interest:
This study was conducted by the Cooperative Studies Program of the Department of Veterans Affairs in collaboration with the NIAID, National Institutes of Health and Merck & Co. Inc. Funding was provided by the Cooperative Studies Program of the Department of Veterans Affairs, Office of Research and Development and by a grant from Merck & Co., Inc. to the VA Cooperative Studies Program.
References
- 1.Hope-Simpson RE. The nature of herpes zoster: A long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. [PMC free article] [PubMed] [Google Scholar]
- 2.Schmader KE, Sloane R, Pieper C, et al. The impact of acute herpes zoster pain and discomfort on functional status and quality of life in older adults. Clin J Pain. 2007;23:490–497. doi: 10.1097/AJP.0b013e318065b6c9. [DOI] [PubMed] [Google Scholar]
- 3.Chidiac C, Bruxelle J, Daures J-P, et al. Clin Infect Dis. 2001;33:62–69. doi: 10.1086/320884. [DOI] [PubMed] [Google Scholar]
- 4.Katz J, Cooper EM, Walther RR, et al. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis. 2004;39:342–48. doi: 10.1086/421942. [DOI] [PubMed] [Google Scholar]
- 5.Oxman MN, Levin MJ, Johnson GR, Schmader, et al. the Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 6.Coplan PM, Schmader K, Nikas A, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: Adaptation of the brief pain inventory. J Pain. 2004;5:344–356. doi: 10.1016/j.jpain.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Cleeland CS, Ryan KM. Pain Assessment: Global use of the Brief Pain Inventory. Annals Academy of Medicine. 1994;23:129–138. [PubMed] [Google Scholar]
- 8.Brazier J, Jones N, Kind P. Testing the validity of the Euroqol and comparing it with the SF-36 health survey questionnaire. Qual Life Res. 1993;2:169–180. doi: 10.1007/BF00435221. [DOI] [PubMed] [Google Scholar]
- 9.Gudex C, Dolan P, Kind P, et al. Health state valuations from the general public using the visual analogue scale. Qual Life Res. 1996;5:521–531. doi: 10.1007/BF00439226. [DOI] [PubMed] [Google Scholar]
- 10.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Norquist J, Nikas A, Saddier P, et al. Measuring aspects of daily life in patients with herpes zoster. Abstract. International Society for Quality of Life Research; 12th Annual Conference; San Francisco, CA. October, 2005. [Google Scholar]
- 12.Food and Drug Administration. Zostavax Package Insert. http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM132831.pdf.
- 13.Food and Drug Administration. [accessed March 14, 2010.];Zostavax (Herpes Zoster Vaccine) Questions and Answers. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/QuestionsaboutVaccines/ucm070418.htm.
- 14.Simberkoff MS, Arbeit RD, Johnson GR, et al. Safety of zoster vaccine in the Shingles Prevention Study: A Randomized Trial. Ann Intern Med. 2010;152:545–554. doi: 10.7326/0003-4819-152-9-201005040-00004. [DOI] [PubMed] [Google Scholar]
- 15.Murray AV, Lawless JF, Reisinger KS, et al. Safety and tolerability of zoster vaccine in adults ≥60 years old. J Am Geriatr Soc. 2010;58:S21–22. [Google Scholar]