Abstract
Glucocorticoid hormones are secreted in response to stimuli that activate the hypothalamo-pituitary-adrenocortical (HPA) axis and self-regulate through negative feedback. Negative feedback that occurs on a rapid time scale is thought to act through nongenomic mechanisms. In these studies, we investigated fast feedback inhibition of HPA axis stress responses by direct glucocorticoid action at the paraventricular nucleus of the hypothalamus (PVN). Local infusion of dexamethasone or a membrane-impermeant dexamethasone-BSA conjugate into the PVN rapidly inhibits restraint-induced ACTH and corticosterone release in a manner consistent with feedback actions at the cell membrane. The dexamethasone fast feedback response is blocked by the cannabinoid CB1 receptor antagonist AM-251, suggesting that fast feedback requires local release of endocannabinoids. Hypothalamic tissue content of the endocannabinoid 2-arachidonoyl glycerol is elevated by restraint stress, consistent with endocannabinoid action on feedback processes. These data support the hypothesis that glucocorticoid-induced fast feedback inhibition of the HPA axis is mediated by a nongenomic signaling mechanism that involves endocannabinoid signaling at the level of the PVN.
Rapid feedback inhibition of the HPA axis response to restraint stress by glucocorticoids is mediated by cannabinoid CB1 receptor signaling.
Hypothalamic-pituitary-adrenal (HPA) axis stress responses are regulated in part by glucocorticoid negative feedback, which occurs on fast and slow time frames (1). Fast feedback occurs with glucocorticoid exposures of less than about 10–15 min and is assumed to be mediated by nongenomic signaling pathways because it can occur independently of protein and mRNA synthesis (2,3). Rapid glucocorticoid effects are distinct from traditional genomic glucocorticoid signaling, which is mediated by ligand-activated transcription factors (glucocorticoid receptors or mineralocorticoid receptors) (4).
It was demonstrated in the 1940s that products of the adrenal glands inhibit the biosynthetic activity of the adrenal glands by acting on extraadrenal tissue (5). This inhibition was seen within 30 min, a timeline that may be consistent with fast nongenomic feedback inhibition of the HPA axis. Subsequent studies revealed that exposure of an animal to a second stressor as early as 2 min after a prior stress exposure profoundly inhibits CRH secretion (6). Glucocorticoids appear sufficient to account for rapid inhibition of the HPA axis, because exogenous glucocorticoid administration before stress exposure significantly dampens the corticosterone response (7). Early in vitro studies suggested that fast feedback may occur at the level of the hypothalamus (8, 9), independent of gene expression. Recent electrophysiological studies showed that fast feedback can occur through the inhibition of glutamate release onto CRH-containing cells in the paraventricular nucleus of the hypothalamus (PVN) via membrane glucocorticoid receptor-mediated endocannabinoid synthesis and retrograde activation of presynaptic cannabinoid receptors (10,11).
Cannabinoid receptors are activated in vivo by endogenous ligands known as endocannabinoids (12,13). The major endocannabinoids are arachidonoyl ethanolamide (AEA, also known as anandamide) and 2-arachidonoyl glycerol (2-AG). In general, the endocannabinoids act as retrograde synaptic signaling molecules that reduce the release of neurotransmitter from presynaptic terminals and have important roles in certain types of synaptic plasticity (14,15).
Endocannabinoid signaling through cannabinoid CB1 receptors appears to be important for HPA axis regulation (16). Antagonism of the CB1 receptor in vivo leads to an enhanced HPA axis response to restraint stress (17,18), whereas facilitation of endocannabinoid signaling blunts restraint-induced corticosterone secretion in mice (17). Consistent with these results, the HPA axis response to novelty stress is enhanced in CB1 receptor knockout mice (19). These data suggest that stress and/or glucocorticoids increase endocannabinoid signaling, which in turn constrains activation of the HPA axis. In hypothalamic slices, rapid glucocorticoid-mediated inhibition of parvocellular neurons is mediated by CB1 receptor signaling (10), suggesting that this receptor may mediate fast negative feedback in vivo. Interestingly, repeated restraint stress leads to elevations in 2-AG levels in the amygdala and forebrain in mice (20). In these studies, however, there was no effect of a single episode of restraint on 2-AG levels, and to our knowledge, there has not been any report of elevated brain endocannabinoid levels in response to acute restraint stress, although decreased levels of hypothalamic 2-AG immediately after restraint stress have been reported in mice (17). Cannabinoid CB1 receptors are expressed in the PVN (21), suggesting a role for endocannabinoid signaling in this nucleus. However, a role for endocannabinoid signaling in fast negative feedback has yet to be confirmed.
We undertook the current studies to test the hypothesis that glucocorticoid-mediated fast feedback inhibition of the HPA axis occurs at the membrane level in the PVN and to confirm the causal role of local endocannabinoid actions in the PVN in fast glucocorticoid feedback signaling.
Materials and Methods
Animals
Adult male Sprague Dawley rats (Harlan, Indianapolis, IN) weighing 275–350 g were used for all the described studies. Animals were singly housed and kept in temperature- and humidity-controlled rooms with a 12-h light, 12-h dark cycle (lights on at 0600 h). Animals were given ad libitum access to water and standard rat chow. After arrival in the lab, rats were allowed to adapt to the new surroundings for at least 1 wk before surgery or other experiments were performed. All procedures were done in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Cannula surgeries
Rats were anesthetized using ketamine (90 mg/kg) and xylazine (10 mg/kg) and given butorphanol as a preemptive analgesic. Under anesthesia, they were implanted with 26-gauge bilateral guide cannulas (1.0 mm separation between sides, center to center; Plastics One, Roanoke, VA). Stereotactic coordinates used were 1.9 mm posterior from bregma, centered over the sagittal sinus, and 6.3 mm ventral to the dura mater (final depth for the injector was 7.3 mm). After surgery, the animals were allowed to recover for at least 5–7 d before restraint challenge. Beginning 2 d after surgery, the cannulas were handled daily to maintain patency of the guide and to accustom the rats to handling. After the stress challenges, cannula placement was verified using a cresyl violet Nissl stain.
Drugs
The synthetic glucocorticoid dexamethasone 21-phosphate was purchased from Sigma Chemical Co. (St. Louis, MO), dissolved in 0.9% saline, and then diluted into vehicle [saline or 0.4% dimethylsulfoxide (DMSO) in saline] before injection. Dexamethasone covalently bound to BSA (dex:BSA) was purchased from Steraloids (Newport, RI) and dissolved in 0.9% saline. Dex:BSA as supplied from the vendor is extensively dialyzed, and thus free of unbound dexamethasone, and stable to hydrolysis (personal communication from Steraloids chemist; also see Refs. 22 and 23). The CB1 receptor antagonist AM-251 was purchased from Tocris (Ellisville, MO), dissolved in DMSO to make a stock solution, and then diluted into vehicle (0.4% DMSO in saline).
Restraint challenge
Restraint challenges were performed in the morning, between 0800 and 1200 h (during which time circadian HPA activity is low). Each animal was brought from the animal housing room into the procedure room immediately before beginning the restraint challenge. Rats were given intra-PVN injections immediately before a 30-min restraint. Intra-PVN injections (0.5 μl per side, bilaterally) were given over 1 min, using a PHD 2000 syringe pump (Harvard Instruments, Holliston, MA) at 500 nl/min injection speed. Restraint stress was performed by placing animals in transparent Plexiglas tubes. Blood samples (∼300 μl) were taken by tail clip at the indicated times. At the end of the restraint challenge, rats were given lethal injections of Fatal Plus and then perfused with 3.7% formaldehyde in potassium phosphate buffer (KPB) (50 mm potassium phosphate, pH 7.4), after which brains were collected for immunohistochemistry. Alternatively, animals were rapidly decapitated, and trunk blood and brains were collected. In either case, brains were immediately postfixed in 3.7% formaldehyde in KPB (24 h for perfused animals and 5 d for rapidly decapitated animals), except for biochemical analysis of the endocannabinoid system, in which tissue was rapidly frozen after decapitation and dissection (see below).
Radioimmunoassay
Plasma corticosterone was measured using a commercial RIA kit (MP Biomedicals, Solon, OH). ACTH was measured by 125I RIA, as described (24). The ACTH antibody used was a generous gift of W. Engeland.
Endocannabinoid extraction and analysis
For biochemical studies, animals were randomly assigned to either basal (no stress), stress (immediately after a 30 min restraint stress), or stress recovery (1 h after cessation of stress) conditions. After restraint challenge, the hypothalamus was dissected as previously described (25), frozen in liquid nitrogen within 5 min of decapitation, and stored at −80 C until analysis. Hypothalami were then subjected to a lipid extraction process as described previously (26). Briefly, tissue samples were weighed and placed into borosilicate glass culture tubes containing 2 ml of acetonitrile with 84 pmol [2H8]AEA and 186 pmol [2H8]2-AG for extraction. Tissue was homogenized with a glass rod and sonicated for 30 min. Samples were incubated overnight at −20 C to precipitate proteins and then centrifuged at 1500 × g to remove particulates. The supernatants were removed to a new glass tube and evaporated to dryness under N2 gas. The samples were resuspended in 300 μl methanol to recapture any lipids adhering to the glass tube and dried again under N2 gas. Finally, lipid extracts were suspended in 20 μl methanol and stored at −80 C until analysis. The contents of AEA and 2-AG were determined using isotope-dilution liquid chromatography-mass spectrometry as described previously (27).
Membrane preparation
Brain sections were homogenized in 10 vol TME buffer [50 mm Tris HCl (pH 7.4), 1 mm EDTA, and 3 mm MgCl2]. Homogenates were centrifuged at 18,000 × g for 20 min, and the resulting pellet, which constitutes the membrane fraction, was resuspended in 10 vol TME buffer. Protein concentrations were determined by the Bradford method (Bio-Rad, Hercules, CA).
CB1 receptor binding assay
CB1 receptor binding assays were performed using a multiscreen filtration system with Durapore 1.2-μm filters (Millipore, Bedford, MA) as described previously (28). Incubations (total volume = 0.2 ml) were carried out using TME buffer containing 1 mg/ml BSA (TME/BSA). Membranes (10 μg protein per incubate) were added to the wells containing 0.1, 0.25, 0.5, 1.0, 1.5, or 2.5 nm [3H]CP 55,940, a cannabinoid CB1 receptor agonist. Δ9-Tetrahydrocannabinol (10 μm) was used to determine nonspecific binding. Dissociation constant (Kd) and binding maximum (Bmax) values were determined by nonlinear curve fitting to the single-site binding equation using Prism (GraphPad, San Diego, CA).
Immunohistochemistry
After postfixation, brains were placed in 30% sucrose in KPB until saturated and then sectioned on a sliding microtome (Leica, Bannockburn, IL). Sections were placed in cryoprotectant [30% sucrose, wt/vol; 1% polyvinylpyrrolidone (PVP-40), wt/vol; 30% ethylene glycol, vol/vol; in 50 mm sodium phosphate buffer (pH 7.4)] and stored at −20 C until staining. Immunohistochemistry for c-Fos protein expression and for BSA was performed as previously described (29). Primary antibodies were rabbit anti-c-Fos used at 1:20,000 dilution (Calbiochem, La Jolla, CA), and rabbit anti-BSA (Rockland Immunochemicals, Gilbertsville, PA), used at 1:1000 dilution. Secondary antibody was biotinylated goat antirabbit Ig, used at 1:500 dilution. Sections were developed using 3,3′-diaminobenzidine.
Imaging
Stained sections were photographed using an Axioplan 2 microscope (Zeiss, Thornwood, NY). For c-Fos-stained slides, immunoreactive cells were counted using the ImageJ software package (National Institutes of Health, Bethesda, MD). Micrographs were cropped, and brightness and contrast were adjusted when needed, but no other manipulations were performed on digital images.
Statistics
Nonlinear regression analysis for the RIAs was performed using AssayZap (Biosoft, Cambridge, UK) and Prism (GraphPad, San Diego, CA) software packages. Plasma hormone data were analyzed by two- or three-way ANOVA, with repeated measures for the time-course data. Area under the curve (AUC) calculations were made using the trapezoidal method and analyzed by ANOVA. Hormone levels were square root or log10 transformed as necessary to obtain homogeneity of variance, and significant main effects and interactions were further examined using Fisher’s least significant difference post hoc tests. Because our intention was only to compare the treatments against each other, we decided a priori to compare treatments only within each time point. c-Fos data were also analyzed by ANOVA. Outliers were identified using Grubb’s test (30) and excluded from analyses. Significance in all cases was set at P < 0.05. Statistical analysis was performed using GBStat (Dynamic Microsystems, Silver Spring, MD) and Prism.
Results
Cannula placement
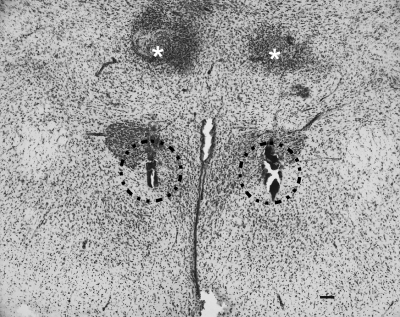
After each of the experiments described, cannula placement was confirmed by Nissl stain (Fig. 1). Criteria for a correct placement included visualization of the internal cannula track in the medial parvocellular PVN without any obvious tissue damage to the structure. The hit rate for PVN injections was approximately 90%. Only animals with correctly placed cannulas were included in the analyses of data in these studies.
Figure 1.
Bilateral cannula placement in the PVN. Cannula placement was verified using Nissl staining. The injection locations are outlined by dashed lines, and the end of the guide cannulas are indicated with asterisks. Scale bar, 100 μm.
Intra-PVN dexamethasone rapidly inhibits the HPA axis
Published data suggest that glutamate-mediated excitation of the CRH-containing parvocellular neurons in the PVN is rapidly reduced by local glucocorticoid actions (10). To test the hypothesis that glucocorticoids act within the PVN to induce fast negative feedback inhibition of the HPA axis response to stress, we injected dexamethasone (10 ng per side) or an equimolar dose of a membrane-impermeant dex:BSA (1.25 μg per side) bilaterally into the PVN (n = 9 per group). As illustrated in Fig. 2, animals treated with dexamethasone or dex:BSA exhibited significantly lower ACTH [main effect of treatment, F(2,24) = 3.79; P < 0.05] and corticosterone [main effect of treatment, F(2,24) = 12.87; P < 0.001] responses to restraint. There was a trend toward reduced ACTH AUC [F(2,22) = 2.95; P = 0.07] and a significant reduction in corticosterone AUC [F(2,23) = 4.785; P < 0.05].
Figure 2.
Intra-PVN administration of dexamethasone causes rapid inhibition of the HPA axis response to restraint. Animals were given bilateral intra-PVN injections of dexamethasone (10 ng per side) or an equimolar amount of dex:BSA (1.25 μg per side), and plasma hormone responses to 25 min of restraint stress were measured. A, Time course of ACTH response to restraint. Dexamethasone and dex:BSA treatment both attenuated the ACTH response to restraint within 15 min of injection. B, Integrated ACTH response to restraint stress (AUC). The magnitude of the integrated ACTH response to restraint was not significantly decreased by either dexamethasone or dex:BSA. C, Time course of corticosterone (CORT) response to restraint. Dexamethasone and dex:BSA both significantly attenuated the corticosterone response to restraint within 60 min of the injection. D, Integrated corticosterone response to restraint. Dexamethasone and dex:BSA both decreased the magnitude of the integrated corticosterone response to restraint. *, P < 0.05 vs. vehicle-treated animals.
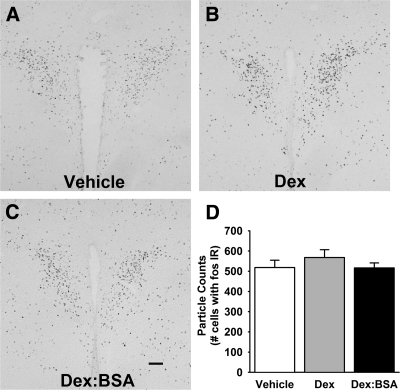
In addition, because manipulations affecting HPA axis activity are known to alter expression of the immediate-early gene c-fos (31), c-Fos protein expression was analyzed using immunohistochemistry. There was no significant effect of dexamethasone treatment on c-Fos expression in the PVN (Fig. 3).
Figure 3.
Dexamethasone-induced fast feedback does not alter c-Fos expression in the PVN. To test whether dexamethasone treatment was altering the activation of paraventricular neurons, the expression of c-Fos protein was assessed by immunohistochemistry. A–C, Representative photomicrographs are shown from animals treated with vehicle (A), dexamethasone (B), and dex:BSA (C). Scale bar, 100 μm. D, There was no significant difference in the number of c-Fos-immunoreactive cells (fos IR) in the PVN among the three treatment groups.
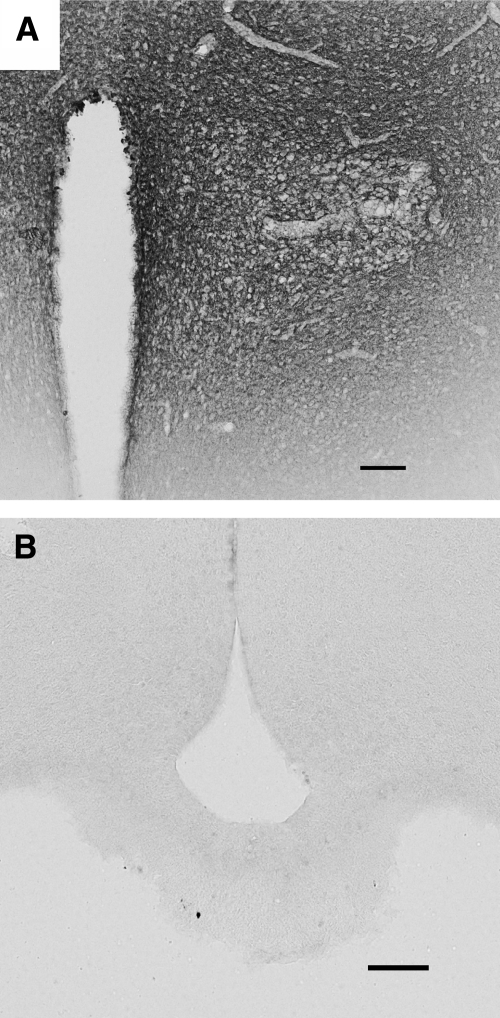
The use of dex:BSA in this experiment also allowed us to assess the local spread of the drug after injection. We performed immunohistochemistry against BSA in these animals and found that dex:BSA diffused throughout the PVN, indicating that most cells in the nucleus were exposed to the drug (Fig. 4). Second, the spread of immunoreactivity was limited to the region of the PVN and peri-PVN, suggesting that the effects of the compound were limited to local actions in the PVN or peri-PVN.
Figure 4.
BSA immunohistochemistry of brains of dex:BSA-injected animals. Immunohistochemistry was performed on brain slices from animals treated with dex:BSA to determine the extent of diffusion of the injection. A, Low-power photomicrograph showing the spread of BSA immunoreactivity in the region of the PVN; B, photomicrograph from the area of the median eminence, showing a lack of immunoreactivity either along the edge of the third ventricle or at the median eminence. These data suggest that injections at the PVN covered the PVN but did not diffuse to distant areas, including the pituitary gland. In addition, the immunoreactivity against BSA shown does not appear to be inside the cell bodies. This supports the model for fast feedback effects occurring at the membrane, rather than at receptors inside the cell body. Scale bars, 100 μm.
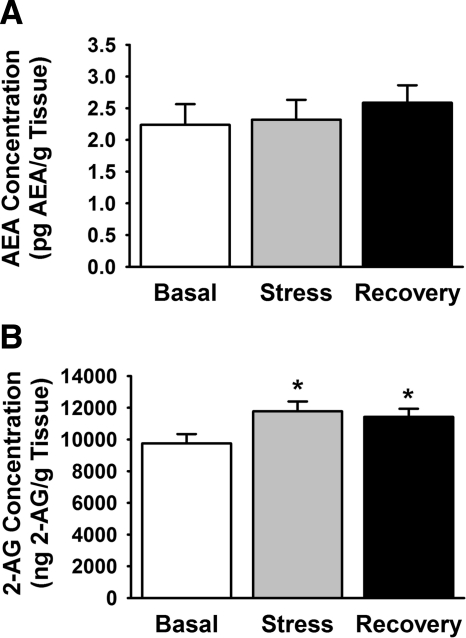
Restraint stress increases 2-AG content of hypothalamic tissue
To test the hypothesis that stress is sufficient to increase endocannabinoid production in the PVN region, we assessed 2-AG and AEA levels using liquid chromatography-mass spectrometry (Fig. 5). Restraint stress increased regional 2-AG levels [F(2,23) = 3.61; P < 0.05] immediately after stress exposure and 60 min after stress cessation (n = 9–10 per group). The stress-induced increase in hypothalamic endocannabinoid levels was specific for 2-AG, because AEA levels were not affected by the restraint stress. To determine whether restraint stress rapidly changed the CB1 receptor binding capacity in the hypothalamus, we also measured CB1 receptor binding characteristics using a radiolabeled CB1 agonist, [3H]CP 55,940. There was no significant effect of stress on the Bmax or Kd values of the CB1 receptor in hypothalamic tissue (data not shown).
Figure 5.
Restraint stress leads to elevated 2-AG but not AEA content of hypothalamic tissue. To determine whether restraint per se increases endocannabinoid synthesis in the PVN, AEA, and 2-AG levels were measured in the hypothalamus of rats subjected to 30 min restraint stress. A, AEA levels in control, restrained, and recovered animal groups; B, 2-AG levels. 2-AG levels were significantly elevated in restrained and recovery animals compared with unstressed controls. AEA levels were not altered. *, P < 0.05 vs. basal levels.
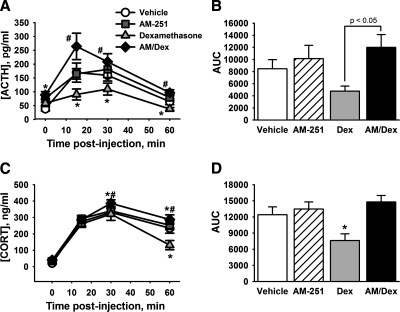
Intra-PVN infusion of AM-251 blocks fast feedback
Because endocannabinoids have been shown to mediate the fast glucocorticoid effects on PVN excitability in vitro, we tested the impact of CB1 receptor blockade in vivo using a CB1 antagonist (AM-251) concomitantly with dexamethasone treatment locally in the PVN (Fig. 6). We mixed the effective doses of dexamethasone (10 ng per side) and AM-251 (20 pmol per side) and injected the cocktail immediately before the onset of restraint stress (n = 7–9 animals per group). Controls received equivalent doses of AM-251 only, dexamethasone only, or vehicle only. AM-251 treatment alone had no effect on either the ACTH or corticosterone response to restraint. As expected, dexamethasone alone caused a significant inhibition of the ACTH response to restraint [main effect of dexamethasone treatment, F(1,27) = 10.16; P < 0.01] as well as the AUC of the ACTH response [main effect of dexamethasone treatment F(1,27) = 5.929, P < 0.05). There was also a dexamethasone × AM-251 interaction, F(1,27) = 6.11; P < 0.05]. Similarly, there was a main effect of dexamethasone treatment on the time course of corticosterone secretion [F(1,28) = 7.328; P < 0.05] and a dexamethasone × AM-251 × time interaction [F(3,84) = 3.60; P < 0.05] as well as a dexamethasone × AM-251 interaction on the integrated corticosterone stress response (AUC) [F(1,28) = 5.326; P < 0.05]. AM-251 completely blocked the dexamethasone-induced inhibition of both ACTH and corticosterone responses to restraint.
Figure 6.
CB1 receptor signaling is necessary for fast feedback. Animals were treated with dexamethasone, the CB1 receptor antagonist AM-251, or both and then subjected to 30 min restraint. Plasma ACTH and corticosterone responses were measured for 60 min after the injection. A, Plasma ACTH response to restraint. Dexamethasone treatment rapidly reduced the magnitude of the ACTH response, whereas AM-251 alone did not affect the stress-induced elevation of ACTH. AM-251 also completely reversed the dexamethasone-induced inhibition of HPA axis activity. B, Total magnitude of ACTH response, expressed as AUC. The magnitude of the integrated ACTH response was lower in dexamethasone-treated animals compared with dexamethasone and AM-251 cotreatment. C, Plasma corticosterone (CORT) response to restraint. Again, dexamethasone decreased the corticosterone response to restraint at 60 min after injection, whereas AM-251 treatment had no effect on the response. AM-251 also completely reversed the dexamethasone-induced inhibition of the corticosterone response. D, AUC of corticosterone secretion. Dexamethasone treatment decreased the corticosterone response relative to all other groups. *, P < 0.05 vs. vehicle; #, P < 0.05 vs. dexamethasone.
Discussion
The current studies demonstrate that glucocorticoid signaling at the PVN leads to CB1 receptor-mediated fast feedback inhibition of the HPA axis response to restraint stress. Both dexamethasone and the membrane-impermeant dex:BSA conjugate effectively blunt HPA axis responses to acute stress when administered directly into the PVN. Dexamethasone-mediated suppression of the HPA axis response is reversed by coadministration of a CB1 receptor antagonist; the antagonist did not affect HPA axis activation per se. These data indicate that CB1 receptor-mediated signaling is required for glucocorticoid negative feedback but not for the initial HPA response to restraint. Restraint elevated 2-AG content in rat hypothalamic tissues, indicating that stress promotes local synthesis of endocannabinoids. Our data are consistent with the hypothesis that fast feedback inhibition of the HPA axis is mediated at least in part by glucocorticoid actions at a membrane glucocorticoid receptor in the PVN and that CB1 receptor signaling is required for the effect, as originally proposed by Tasker and colleagues (10).
In these studies, administration of dexamethasone directly into the PVN led to a rapid inhibition of the HPA axis response to restraint. This effect was completely reversed by concomitant local administration of the CB1-selective antagonist AM-251, suggesting that CB1 receptor signaling is necessary for this fast feedback pathway to be activated. This is consistent with the model of fast feedback signaling suggested by Di et al. (10). In this proposed model, nongenomic glucocorticoid actions at CRH-containing neurons in the PVN lead to activation of endocannabinoid signaling, thus initiating a retrograde inhibition of glutamate release onto CRH-containing parvocellular neurons in the PVN (see Fig. 7). The current studies build upon those reported by Di et al. (10) by showing that glucocorticoid treatment of the PVN in vivo leads to decreased activity of the HPA axis, as predicted by the decreased glutamatergic signaling caused by glucocorticoid administration to hypothalamic slices in vitro. This model of endocannabinoid signaling at the PVN in HPA axis responses to restraint is consistent with known actions of endocannabinoids in reducing neurotransmitter release from axon terminals (32).
Figure 7.
Model for cannabinoid-mediated actions of the PVN on the HPA axis. The results of these studies are consistent with the model for fast feedback actions at the PVN proposed by Di et al. (10). A, Glucocorticoids in the vicinity of the parvocellular neuron bind to a putative membrane-localized glucocorticoid receptor. Binding of glucocorticoid to this receptor leads to synthesis of endocannabinoids, which are released into the synapse. After diffusion in a retrograde manner across the synapse, the endocannabinoids initiate CB1 receptor signaling, which leads to decreased glutamate release onto the CRH-containing neuron. This leads to decreased CRH release from the cell. B, Blocking CB1 receptors leads to normalization of the glutamate release and, thus, normalization of the CRH output of the cells. Parvo, Parvocellular neuron; PLC, phospholipase C; DAG, diacyl glycerol. Adapted from Di et al. (10).
Restraint stress led to a rapid increase in the levels of 2-AG in the hypothalamus, further supporting a role for endocannabinoid signaling in fast feedback. Previous work has shown that restraint stress leads to increased plasma corticosterone in rats within 5 min (33). Rapidly rising corticosterone levels could be expected to induce fast feedback inhibition of the HPA axis. Indeed, one of the proposed physiological actions of fast feedback is to rapidly reduce the magnitude of the HPA axis response to a stressor (1). Furthermore, it has recently been demonstrated that acute administration of corticosterone produces a rapid elevation in 2-AG content within the hypothalamus in vivo (34). These data corroborate earlier in vitro data showing an increase in 2-AG and AEA in the PVN within 10 min of exposure of hypothalamic slices to dexamethasone (11). Thus, the 2-AG response to restraint may represent the endocannabinoid signal generated by endogenous glucocorticoids acting at the PVN to mediate endogenous fast feedback responses to restraint stress (see Fig. 7).
Local administration of AM-251 alone did not increase the corticosterone or ACTH levels in the current studies, which increase might be expected if fast feedback is limiting the HPA axis response to restraint. Interestingly, AM-251 administered peripherally 2 min before restraint stress leads to a potentiation of the ACTH response to restraint (18), consistent with the proposed role for endocannabinoid signaling in endogenous glucocorticoid-mediated fast feedback. However, AM-251 administered peripherally at the same dose 15 min before restraint onset does not alter the ACTH response (35), suggesting that there is an effect of the timing of AM-251 administration on this effect. It is unclear why this is the case, but these results may explain why AM-251 administered with dexamethasone just before restraint inhibits the negative feedback induced by dexamethasone but not that presumably mediated by the endogenous rise in corticosterone, which would likely occur in the hypothalamus approximately 20–30 min after the application of dexamethasone in these studies (36).
Interestingly, although restraint rapidly led to an increase in 2-AG levels in the hypothalamus, AEA levels were not affected in these studies. This may be because the fast feedback mechanism is mediated specifically by 2-AG. On the other hand, it is also possible that local perisynaptic AEA levels are elevated sufficiently to allow for modulation of specific synapses in the PVN during fast feedback events, whereas the average content of the region dissected for AEA quantification remains grossly unchanged. A previous in vitro study in hypothalamic slices trimmed to include only the PVN showed dexamethasone-induced increases in both 2-AG and AEA release (11), suggesting that the latter may be the case. However, in mice, decreases in 2-AG in response to restraint have been reported (17). This discrepancy with the current results may reflect species differences, or subtle differences in the area of hypothalamus dissected, due to the smaller size of the mouse brain. Our methods are not sensitive enough to detect localized changes in endocannabinoid content, so we cannot definitively rule out a role for AEA in fast glucocorticoid feedback. Future studies will be needed to define the degree of endocannabinoid release at synapses involved in fast feedback in the HPA axis at the PVN.
Previous studies indicate that fast feedback inhibition of the HPA axis by glucocorticoid signaling is mediated by nongenomic signaling (1,10,37). This is because 1) the effects occur quickly, 2) the effects can be initiated by treatment with membrane-impermeant BSA conjugates of glucocorticoids, 3) in vitro studies have demonstrated glucocorticoid fast feedback effects in the presence of inhibitors of transcription or translation (2) (38) or in the absence of cell nuclei (8), and 4) rapid glucocorticoid actions are blocked by G protein and cell signaling blockers (11). Our current results lend further support for a nongenomic mechanism of action for glucocorticoid-mediated fast feedback, due to the rapid onset of negative feedback (<15 min) and the ability of the membrane-impermeant dex:BSA to mimic the actions of dexamethasone. Thus, our data suggest that fast feedback is mediated by a membrane-bound receptor for glucocorticoids in the PVN and that fast feedback signaling is likely nongenomic.
CB1 receptors are located almost exclusively in the presynaptic terminals of central nervous system neurons (15). Thus, it is very likely that CB1 mediates effects on the HPA axis through a presynaptic mechanism. However, presynaptic regulation of the HPA axis by CB1 raises the question of where the CB1-containing projections to the PVN originate. There are a number of areas that are known to project to the PVN, including the dorsomedial hypothalamus, ventromedial hypothalamus, bed nucleus of the stria terminalis, and lateral hypothalamic area (39). If fast feedback is mediated by endocannabinoid actions at glutamatergic synapses, it is likely that the neurons supplying the CB1 innervation also express vesicular glutamate transporters. Some areas that supply vesicular glutamate transporter-expressing fibers to the PVN and that may thus be targets for fast glucocorticoid actions include the lateral hypothalamus, bed nucleus of the stria terminalis, ventromedial hypothalamus, and dorsomedial hypothalamus (40,41). All of these are known or suspected to play a role in regulating or modulating the HPA axis response to stress (42,43,44,45). The lateral hypothalamus, bed nucleus of the stria terminalis, and PVN also express CB1 (46) and so are candidates for further study.
The current studies support our hypothesis that the fast feedback effects of dexamethasone occur at least in part at the PVN. The PVN is the most important integration point for relaying central stress-response signaling to the pituitary-adrenal axis (47). However, the size of our injection would lead to diffusion of the drugs beyond the PVN proper. Thus, it is possible that these fast feedback responses have a location of action other than the PVN. Based on the spread of BSA, as measured by anti-BSA immunohistochemistry, the site of action of dexamethasone in these studies must be close to the PVN.
In conclusion, we present evidence that glucocorticoid signaling within the PVN leads to fast feedback inhibition of the HPA axis response to restraint stress in a time frame that is consistent with nongenomic glucocorticoid signaling. CB1 receptor-mediated signaling is necessary for this fast feedback to occur, and the fact that restraint stress elevates tissue levels of endocannabinoids supports a regulatory role for these molecules in regulating HPA axis responses. The fast feedback signaling pathway also appears to involve a membrane-localized form of receptor for glucocorticoids.
Acknowledgments
We thank Dennis Choi, Mark Dolgas, Jonathan Flak, Amy Furay, Amanda Jones, Kenny Jones, Michelle Ostrander, Ben Packard, Ingrid Thomas, Yvonne Ulrich-Lai, and Rong Zhang for assistance with the restraint challenge experiments.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants MH069725 (to J.P.H.), NS007453 (to N.K.E.), MH066958 (to J.G.T.), DA022439 (to C.J.H.), and DA09155 (to C.J.H.) and by a postdoctoral fellowship from the Canadian Institute of Health Research (CIHR) (to M.N.H.).
Disclosure Summary: Apart from funding from the NIH and CIHR, as outlined in the manuscript, the authors have nothing to disclose.
First Published Online August 11, 2010
Abbreviations: AEA, Arachidonoyl ethanolamide; 2-AG, 2-arachidonoyl glycerol; AUC, area under the curve; dex:BSA, dexamethasone covalently bound to BSA; DMSO, dimethylsulfoxide; HPA, hypothalamic-pituitary-adrenal; KPB, potassium phosphate buffer; PVN, paraventricular nucleus of the hypothalamus.
References
- Keller-Wood ME, Dallman MF 1984 Corticosteroid inhibition of ACTH secretion. Endocr Rev 5:1–24 [DOI] [PubMed] [Google Scholar]
- Abou-Samra AB, Catt KJ, Aguilera G 1986 Biphasic inhibition of adrenocorticotropin release by corticosterone in cultured anterior pituitary cells. Endocrinology 119:972–977 [DOI] [PubMed] [Google Scholar]
- Hinz B, Hirschelmann R 2000 Rapid non-genomic feedback effects of glucocorticoids on CRF-induced ACTH secretion in rats. Pharm Res 17:1273–1277 [DOI] [PubMed] [Google Scholar]
- Heitzer MD, Wolf IM, Sanchez ER, Witchel SF, DeFranco DB 2007 Glucocorticoid receptor physiology. Rev Endocr Metab Disord 8:321–330 [DOI] [PubMed] [Google Scholar]
- Sayers G, Sayers MA 1947 Regulation of pituitary adrenocorticotrophic activity during the response of the rat to acute stress. Endocrinology 40:265–273 [DOI] [PubMed] [Google Scholar]
- Sakakura M, Saito Y, Takebe K, Ishii K 1976 Studies on fast feedback mechanisms by endogenous glucocorticoids. Endocrinology 98:954–957 [DOI] [PubMed] [Google Scholar]
- Yates FE, Leeman SE, Glenister DW, Dallman MF 1961 Interaction between plasma corticosterone concentration and adrenocorticotropin-releasing stimuli in the rat: evidence for the reset of an endocrine feedback control. Endocrinology 69:67–80 [DOI] [PubMed] [Google Scholar]
- Edwardson JA, Bennett GW 1974 Modulation of corticotrophin-releasing factor release from hypothalamic synaptosomes. Nature 251:425–427 [DOI] [PubMed] [Google Scholar]
- Jones MT, Hillhouse EW 1976 Structure-activity relationship and the mode of action of corticosteroid feedback on the secretion of corticotrophin-releasing factor (corticoliberin). J Steroid Biochem 7:1189–1202 [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG 2003 Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci 23:4850–4857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, Tasker JG 2006 Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci 26:6643–6650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ 2000 Biochemistry and pharmacology of the endocannabinoids arachidonylethanolamide and 2-arachidonylglycerol. Prostaglandins Other Lipid Mediat 61:3–18 [DOI] [PubMed] [Google Scholar]
- Di Marzo V 2009 The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res 60:77–84 [DOI] [PubMed] [Google Scholar]
- Mackie K 2008 Signaling via CNS cannabinoid receptors. Mol Cell Endocrinol 286:S60–S65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D 2003 Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83:1017–1066 [DOI] [PubMed] [Google Scholar]
- Hill MN, McEwen BS 2010 Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry 34:791–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ 2004 Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology 145:5431–5438 [DOI] [PubMed] [Google Scholar]
- Ginsberg AB, Pecoraro NC, Warne JP, Horneman HF, Dallman MF 2010 Rapid alteration of stress-induced hypothalamic-pituitary-adrenal hormone secretion in the rat: a comparison of glucocorticoids and cannabinoids. Stress 13:248–257 [DOI] [PubMed] [Google Scholar]
- Barna I, Zelena D, Arszovszki AC, Ledent C 2004 The role of endogenous cannabinoids in the hypothalamo-pituitary-adrenal axis regulation: in vivo and in vitro studies in CB1 receptor knockout mice. Life Sci 75:2959–2970 [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ 2005 Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci 21:1057–1069 [DOI] [PubMed] [Google Scholar]
- Moldrich G, Wenger T 2000 Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides 21:1735–1742 [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ 2003 Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci 23:9529–9540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore PF, Neulen J, Lattanzio F, Beebe SJ 1991 Cell surface-binding sites for progesterone mediate calcium uptake in human sperm. J Biol Chem 266:18655–18659 [PubMed] [Google Scholar]
- Engeland WC, Miller P, Gann DS 1989 Dissociation between changes in plasma bioactive and immunoreactive adrenocorticotropin after hemorrhage in awake dogs. Endocrinology 124:2978–2985 [DOI] [PubMed] [Google Scholar]
- Hill MN, Ho WS, Sinopoli KJ, Viau V, Hillard CJ, Gorzalka BB 2006 Involvement of the endocannabinoid system in the ability of long-term tricyclic antidepressant treatment to suppress stress-induced activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology 31:2591–2599 [DOI] [PubMed] [Google Scholar]
- Patel S, Wohlfeil ER, Rademacher DJ, Carrier EJ, Perry LJ, Kundu A, Falck JR, Nithipatikom K, Campbell WB, Hillard CJ 2003 The general anesthetic propofol increases brain N-arachidonylethanolamine (anandamide) content and inhibits fatty acid amide hydrolase. Br J Pharmacol 139:1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Carrier EJ, Ho WS, Rademacher DJ, Cunningham S, Reddy DS, Falck JR, Cravatt BF, Hillard CJ 2005 The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res 46:342–349 [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Edgemond WS, Campbell WB 1995 Characterization of ligand binding to the cannabinoid receptor of rat brain membranes using a novel method: application to anandamide. J Neurochem 64:677–683 [DOI] [PubMed] [Google Scholar]
- Evanson NK, Van Hooren DC, Herman JP 2009 GluR5-mediated glutamate signaling regulates hypothalamo-pituitary-adrenocortical stress responses at the paraventricular nucleus and median eminence. Psychoneuroendocrinology 34:1370–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett V, Lewis T 1994 Outliers in statistical data. 3rd ed. New York: John Wiley, Sons [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ 1995 Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64:477–505 [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR 2006 Man-made marijuana: Endocannabinoid modulation of synaptic transmission and implications for the regulation of synaptic plasticity. In: Onaivi ES, Sugiura T, Di Marzo V, eds. Endocannabinoids: the brain and body’s marijuana and beyond. Boca Raton, FL: Taylor & Francis; 229–248 [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D'Alessio DA, Herman JP 2005 Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab 289:E823–E828 [DOI] [PubMed] [Google Scholar]
- Hill MN, Karatsoreos IN, Hillard CJ, McEwen BS 2010 Rapid elevation of limbic endocannabinoid tissue content by glucocorticoid hormones in vivo. Psychoneuroendocrinology 10.1016/j.psyneuen.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanson NK, Ulrich-Lai YM, Furay AR, Tasker JG, Herman JP, Hypothalamic paraventricular cannabinoid receptor signaling in fast feedback inhibition of the hypothalamus-pituitary-adrenal axis response to acute restraint stress. Program of the 37th Annual Meeting of the Society for Neuroscience, San Diego, CA, 2007 (Abstract 197.5) [Google Scholar]
- Droste SK, de Groote L, Atkinson HC, Lightman SL, Reul JM, Linthorst AC 2008 Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology 149:3244–3253 [DOI] [PubMed] [Google Scholar]
- Buckingham JC, John CD, Solito E, Tierney T, Flower RJ, Christian H, Morris J 2006 Annexin 1, glucocorticoids, and the neuroendocrine-immune interface. Ann NY Acad Sci 1088:396–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmaier EP, Dallman MF 1984 The effects of corticotropin-releasing factor on adrenocorticotropin secretion from perifused pituitaries in vitro: rapid inhibition by glucocorticoids. Endocrinology 115:2368–2374 [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Hoffman DL, Zimmerman EA 1981 The descending afferent connections of the paraventricular nucleus of the hypothalamus (PVN). Brain Res Bull 6:47–61 [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H 2002 Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol 444:39–62 [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Cullinan WE, Herman JP 2002 Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J Comp Neurol 448:217–229 [DOI] [PubMed] [Google Scholar]
- Suemaru S, Darlington DN, Akana SF, Cascio CS, Dallman MF 1995 Ventromedial hypothalamic lesions inhibit corticosteroid feedback regulation of basal ACTH during the trough of the circadian rhythm. Neuroendocrinology 61:453–463 [DOI] [PubMed] [Google Scholar]
- Thrivikraman KV, Nemeroff CB, Plotsky PM 2000 Sensitivity to glucocorticoid-mediated fast-feedback regulation of the hypothalamic-pituitary-adrenal axis is dependent upon stressor specific neurocircuitry. Brain Res 870:87–101 [DOI] [PubMed] [Google Scholar]
- Evans SB, Wilkinson CW, Gronbeck P, Bennett JL, Zavosh A, Taborsky Jr GJ, Figlewicz DP 2004 Inactivation of the DMH selectively inhibits the ACTH and corticosterone responses to hypoglycemia. Am J Physiol Regul Integr Comp Physiol 286:R123–R128 [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP 2007 Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci 27:2025–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM 1998 Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83:393–411 [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Ziegler DR, Tasker JG 2002 Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci 16:381–385 [DOI] [PubMed] [Google Scholar]