Abstract
Sexual differentiation of the rodent brain occurs during a perinatal critical period when androgen production from the male testis is locally converted to estradiol in neurons, resulting in masculinization of adult sexual behavior. Adult brain responses to hormones are programmed developmentally by estradiol exposure, but the mechanism(s) by which these changes are permanently organized remains poorly understood. Activation of steroid receptors plays a major role in organization of the brain, and we hypothesized that estradiol-induced alteration of steroid-receptor gene methylation is a critical component to this process. Estrogen receptor (ER)-α and ER-β and progesterone receptor are expressed at high levels within the preoptic area (POA) and the mediobasal hypothalamus, two brain regions critical for the expression of male and female sexual behavior. The percent methylation on the ER-α promoter increased markedly across development. During the critical period of sexual differentiation, females had significantly increased methylation than males or females masculinized with estradiol at two CpG sites. By adulthood, the neonatal sex difference and hormonal modulation of methylation were replaced with a new pattern at a different CpG site on the ER-α promoter. In contrast, the percent methylation on the progesterone receptor and ER-β promoter did not change developmentally but was modulated by hormones and exhibited only late emerging transient sex differences. These data indicate that sex differences in the methylation pattern of genes important for sexual behavior are epigenetically modified during development, but the specific changes observed do not endure and are not necessarily temporally associated with neonatal hormone exposure.
CpG islands in the promoter regions of ER-α, ER-β, and progesterone receptor are methylated in a manner that is dependent on brain region, age, sex, and neonatal hormone exposure.
Both profound and subtle sex differences in the brain are the presumed basis of sex differences in physiology and behavior (for reviews see Refs. 1 and 2). In rats and mice, many of these sex differences are determined during a critical period of development when testosterone synthesized by the developing male testes gains access to the brain and is locally converted to estradiol (3,4). This early estradiol exposure permanently differentiates the male from the female by masculinizing and defeminizing specific brain regions important for sexual behavior, including the mediobasal hypothalamus (MBH) and the preoptic area (POA). These sex differences are organized during development but are activated in adulthood in a sexually differentiated manner via exposure to circulating hormones. One target of circulating hormone is their own receptors, leading to sex differences in the levels of estrogen receptor (ER) and progesterone receptor (PR) in key brain areas. In the neonatal POA, ERα mRNA and protein are higher in males than females (5,6) due to down-regulation by higher estradiol in the male brain. Conversely, ER-β expression is higher in the male POA and hypothalamus during early development (7), but there are conflicting reports on sex differences in ERβ expression in these regions in adulthood (8,9,10). Levels of PR within the POA and MBH are clearly higher in males during development, and this sex difference is dependent on ER-α expression (11,12). This sex difference diminishes into adulthood (13), wherein estradiol treatment more potently induces PR expression in females (14) and differentially regulates PRA and PRB isoform expression within the female but not male POA and MBH (15). We sought to determine whether there is a hormonally induced sex difference in the DNA methylation pattern of three genes important for the expression of sex behavior, ER-α, ER-β, and PR and whether any sex differences observed are the result of hormonally mediated sexually differentiation by estradiol during the postnatal sensitive period.
DNA methylation refers to the addition of a methyl group to the five-carbon of a cytosine in a CpG dinucleotide, a process that predominantly decreases gene transcription directly by influencing the binding of methyl-sensitive DNA-binding proteins and indirectly by influencing regional chromatin conformation. CpG islands are regions of the genome that contain a high level of CpG dinucleotides (>60%) and are generally localized to the 5′ promoter region of genes (16). Although nearly 90% of CpG dinucleotides outside a CpG island are methylated, CpGs within a CpG island are predominantly unmethylated, allowing for differential methylation and subsequently differential expression of the gene (17). We hypothesized that neonatal estradiol establishes sex differences in the methylation levels of the ER-α, ER-β, and PR gene promoter regions in neurons in the POA and MBH and that these sex differences are maintained into adulthood, presumably thereby altering expression of the gene and influencing adult brain hormonal sensitivity to regulate behavior. To test this hypothesis, we examined methylation patterns in male, female, and estradiol-treated females at postnatal day (PN) 1, during the sensitive period; PN20, after the sensitive period yet before the onset of puberty; and PN60 when adult levels of endogenous hormones are circulating. Contrary to expectation, we found that sex differences and hormonal modulation of DNA methylation are dynamic across development, and as such, methylation levels do not appear to be directly linked to changes in receptor expression.
Materials and Methods
Animals and neonatal treatment
Female Sprague Dawley rats (Harlan Laboratories, Frederick, MD) maintained on a reverse 12-h light, 12-h dark cycle and provided ad libitum food and water were mated in the University of Maryland School of Medicine animal facility and pregnancy confirmed by presence of sperm in a vaginal smear. Pregnant females were isolated and allowed to deliver normally. Cages were checked regularly for the presence of pups to determine the time and day of birth, designated as PN0. On PN0 male and female pups were collected and separated into the following treatment groups: 1) males treated sc with 0.1 cc of sesame oil (vehicle), 2) females treated sc with 0.1 cc of oil (vehicle), and 3) females treated sc with 100 μg estradiol benzoate (Sigma-Aldrich, St. Louis, MO) in 0.1 cc oil, a standard dose for reliably producing behavioral masculinization. Animals were euthanized, and the MBH and POA collected at PN1 (24 h after treatment), PN20, or PN60. Brains were removed and placed in a Zivic Miller brain mold and sectioned 1 mm just rostral to the optic tract for the POA. The POA was then microdissected from the 1-mm-thick section using the perimeter of the anterior commissure for both the dorsal and lateral boundaries. For dissection of the MBH at PN1 and PN20, a 2-mm section was taken directly caudal to the optic tract. On PN60, a 2-mm section was taken 2 mm caudal to the optic tract and the MBH dissected from this section, 1 mm lateral from the third ventricle in both directions with the dorsal boundary being the tip of the third ventricle. Estrous cycles of all PN60 females were recorded using vaginal smears for 6 d before brain collection. There were no observable disparities in methylation of ER-α, ER-β, or PR across the estrous cycle. Persistent estrus was confirmed in all estradiol benzoate-treated females to validate neonatal defeminization/masculinization.
DNA isolation and bisulfite conversion
Genomic DNA was isolated from the MBH and POA using the Wizard Genomic DNA purification kit (Promega, Madison, WI). Briefly, tissue was homogenized in a nuclei lysis buffer, 400 μl for the POA and 600 μl for the MBH, followed by a 30-min incubation at 65 C. Ribonuclease solution was added to each sample, and each sample was incubated at 37 C for 30 min. After the samples were cooled to room temperature, 200 μl of a protein precipitation solution was added and the sample vortexed for 20 sec before being chilled on ice for 10 min. The samples were centrifuged at 16,000 × g for 4 min and the supernatant transferred to a clean tube containing 600 μl isopropanol. The extracted DNA was centrifuged for 1 min at 16,000 × g and the resultant DNA pellets washed two times with 70% ethanol and allowed to dry completely before rehydration in 100 μl of DNA rehydration solution.
Bisulfite conversion was completed using the EZ DNA methylation gold kit (Zymo Research, Orange, CA) according to the manufacturer’s directions on 1000 ng of isolated DNA from each sample. After bisulfite treatment, the nonmethylated cytosines were converted to uracils. The recovered DNA is typically A, U, and T rich, and the original base pairing no longer exists. Instead, it is a single strand with limited nonspecific base pairing at room temperature. As a result, the absorption coefficient at 260 nm wavelength resembles that of RNA (40 ng/ml for Ab260 = 1.0), and all the resulting concentration calculations were made based on this coefficient.
Determination of DNA methylation using pyrosequencing
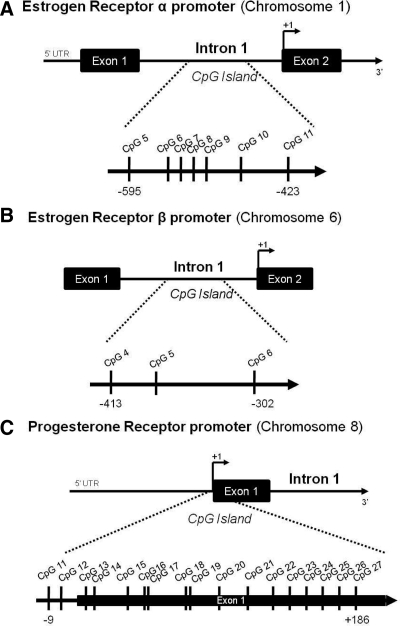
To determine the methylation status of CpG sequences in the PR, ER-α, and ER-β gene promoter, pyrosequencing was performed at EpigenDX (Worcester, MA). Pyrosequencing for allele quantification (PSQ H96A; Biotage, Uppsala, Sweden) is a real-time sequencing-by-synthesis method that quantitatively monitors the real-time incorporation of nucleotides through the enzymatic conversion of released pyrophosphate into a proportional light signal. Pyrosequencing is valued for its high degree of accuracy combined with its ability to quantify a large number of DNA strands rapidly and economically. The bioluminometric response is highly linear (R2 > 0.99) for all nucleotides. Pyrosequencing is used in a number of applications including genotyping of polyploidy samples, determination of single-nucleotide polymorphism allele frequencies in pooled samples, quantification of copy number, and others (reviewed in Ref. 18). The pyrosequencing technology has been adapted for precise quantification of the degree of methylation of individual CpG sites located close together, a so-called CpG island (18). The ER-α methylation assay covered seven CG dinucleotides in intron 1 ranging from −595 to −423 in reference to the translational start site (start codon). The ER-β methylation assay covered three CpG sites within intron 1 of the ER-β promoter, ranging from −302 to −413 of the start codon. The PR methylation assay covered two CG dinucleotides in the promoter region and 15 CG dinucleotides in the exon 1 region ranging from −9 to +186 in reference to the start codon. Methylation assays of the ER-α, -β, and PR promoters were designed by EpigenDx. Site analysis was based on the ability to generate primers located around CpG islands and that meet the requirements required for pyrosequencing of each site accurately. All primer sequences are propriety and owned by EpigenDx.
The promoter sequences of ER-α, ER-β, and PR were PCR amplified from 1 μl of bisulfite-modified DNA (∼50 ng) from each sample and 200 μm of each deoxyribonucleotide triphosphate, 1× PCR buffer, 1.5 mm MgCl2, and 0.2 μm biotinylated PCR primers designed by EpigenDX. The PCR conditions for PR gene amplification were as follows: 15 min at 95 C, 45 cycles of 30 sec at 95 C, 30 sec at 60 C, and 30 sec at 72 C, followed by a final extension step for 5 min at 72 C. The PCR conditions for ER-α were as follows: 15 min at 95 C, 45 cycles of 30 sec at 95 C, 30 sec at 62 C, and 30 sec at 72 C, followed by a final extension step for 5 min at 72 C. Finally, the PCR conditions for ER-β were: 15 min at 95 C, 45 cycles of 30 sec at 95 C, 30 sec at 54 C, and 30 sec at 72 C, followed by a final extension step for 5 min at 72 C. PCR was performed with biotinylated primers to convert the PCR product to single-stranded DNA templates. Pyrosequencing primers were designed by EpigenDX to determine the CpG dinucleotide methylation status. The PCR products (each 10 μl) were sequenced by Pyrosequencing PSQ96 HS system (Biotage) following the manufacturer’s instructions (Biotage, Kungsgatan, Sweden). The methylation status of each locus was analyzed individually as a T/C single-nucleotide polymorphism using QCpG software (Biotage).
Analyzed data are presented as the percent methylation at each individual CpG site in each promoter region. First, the overall percent methylation for each promoter was compared between groups separately for each brain region and time point by one-way ANOVA. Second, the mean percent methylation was calculated at each CpG site for all animals within a treatment group and analyzed using a one-way ANOVA followed by a Tukey’s post hoc test to determine pair-wise significance when appropriate. All statistical tests used α < 0.05 as the criterion for significance. Data presented in graphical form represent the mean ± sem.
Results
Methylation patterns of ER-α, ER-β, and PR in the POA across development
ER-α methylation
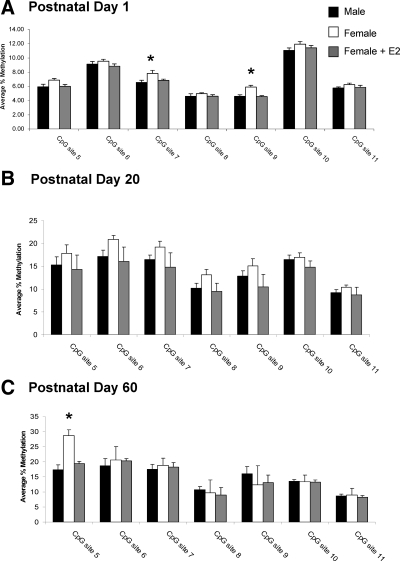
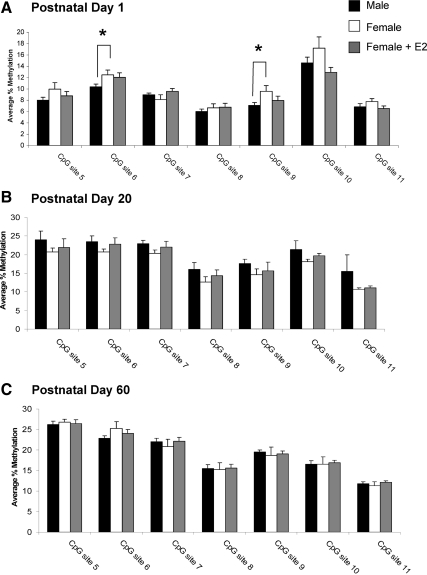
In the POA, the major brain region controlling male sexual behavior, seven CpG sites in the ER-α promoter were selected for analysis (Fig. 1A). On PN1, the average methylation for these sites ranged from 5 to 13%. There was a main effect of group at two CpG sites, site 7 (F2,19 = 2.53; P < 0.05) and site 9 (F2,19 = 12.08; P < 0.001; vehicle, n = 6; male, n = 9, female + estradiol (E2), n = 7; Fig. 2A). Specifically, females had significantly higher levels of methylation than males and treatment of females with estradiol 24 h before tissue collection reduced the methylation levels at these same CpG sites to levels seen in the male (P < 0.05 for both comparisons). A one-way ANOVA on percent methylation collapsed across all sites confirmed females had overall higher levels of methylation than males or females treated with estradiol (F2,19 = 5.91, P < 0.02). This was not true for any other time points, brain regions or promoters.
Figure 1.
Regions of methylation analysis along the ER-α, ER-β, and PR promoters. A, Analysis of methylation on the ER-α promoter included seven CG dinucleotides in intron 1 ranging from −595 to −423 in reference to the translational start site, or start codon (+1). B, The region of analysis in the ERβ promoter consisted of three CG dinucleotides in exon 1 ranging from −301 to −413 in relation to the translational start codon (+1). C, Analysis of methylation on the progesterone receptor promoter covered two CG dinucleotides in the 5′UTR of the promoter region and 15 CG dinucleotides in the exon 1 region ranging from −9 to +186 in reference to the start codon (+1).
Figure 2.
Percent methylation of CpG islands in the ER-α promoter in the POA. A, Methylation analysis of seven CpG sites along the CpG island of the ER-α promoter at PN1 reveals an estradiol (E2)-mediated sex difference in the methylation of two CpG sites, site 7 and site 9, with females having significantly greater levels of methylation than either males or females masculinized by neonatal estradiol treatment (ANOVA; *, P < 0.05 compared with males and females + E2; n = 6–9 per group). B, Analysis of the same sites at PN20 indicates no effect of sex or neonatal estradiol treatment on methylation levels at any CpG site (n = 5–8 per group). C, Analyses at PN60 reveals a sex difference at CpG site 5 with females having significantly higher percent methylation than either males or females masculinized by neonatal estradiol treatment (ANOVA; *, P < 0.05 compared with males and females + E2; n = 4–5 per group).
To determine whether the neonatal estradiol-induced sex difference in methylation within the ER-α promoter is maintained into adulthood, we analyzed the POA at PN20 and PN60 after treatment with estradiol at birth. Independent of sex or hormone treatment, the percent methylation at many CpG sites within the ER-α promoter at PN20 and PN60 was nearly double that seen at PN1 (Fig. 2, B and C), revealing a developmental up-regulation of ER-α methylation. At PN20, the estradiol-mediated sex difference in CpG methylation at site 7 and site 9 was no longer present (female, n = 8; male, n = 8; female + E2 n = 5; Fig. 2B), and there were no group differences at any other CpG sites analyzed. By PN60 the effect of sex and hormone on CpG methylation of ER-α reemerged (F2,11 = 13.4; P < 0.01; female, n = 4; male, n = 5; female + E2, n = 5; Fig. 2C), although at a different CpG site, CpG site 5, with females having significantly greater methylation than males (P < 0.05), and neonatal estradiol treatment of females significantly decreasing methylation at this site to levels seen in adult males (P < 0.05 compared with females).
ER-β methylation
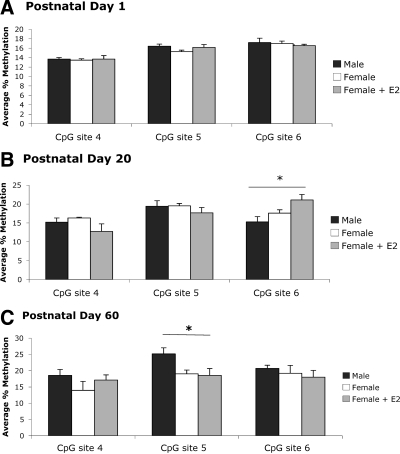
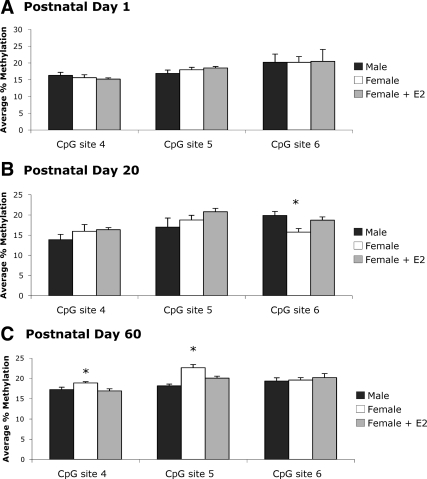
Three CpG sites within the ER-β promoter were analyzed in the POA (Fig. 1B). There were small, marginally significant increases in methylation on the ER-β promoter across developmental time points (F2,6 = 4.98, P = 0.053; Fig. 3, A–C). Despite reported sex differences in ER-β expression in the neonatal POA (5), we found no measurable differences between groups in CpG methylation at any site on PN1. However, by PN20 we detected group differences at CpG site 6 on the ER-β promoter in the POA (F2,16 = 4.90, P < 0.05; Fig. 3B). Specifically, methylation levels at this site were significantly greater in females treated neonatally with estradiol than males (P < 0.01), with no significant differences in methylation between estradiol- and vehicle-treated females (P = 0.07). Interestingly, the estradiol-induced increase in methylation seen at PN20 was transient, and by PN60 there were no observable differences at site 6. However, group differences emerged at site 5 in the PN60 POA (F2,11 = 4.19, P < 0.05; Fig. 3C). At this site, adult males had significantly higher levels of CpG methylation than both vehicle-treated (P < 0.05) and estradiol-treated (P < 0.05) females. Thus, neonatal estradiol treatment of females impacted the methylation status of the ER-β gene; however, the effect did not manifest until later developmental time points and did not mimic the pattern observed in males.
Figure 3.
Percent methylation of CpG islands in the ER-β promoter in the POA. A, Methylation analysis of three CpG sites in the ERβ promoter indicated no significant sex- or estradiol-induced differences at PN1 (n = 6–8 per group). B, At PN20, neonatal estradiol treatment in females resulted in a significant increase in methylation levels above males (ANOVA; *, P < 0.01); however, methylation levels at this site were not significantly different from vehicle-treated females (P = 0.077; n = 5–7 per group). C, Although the estradiol-induced difference in methylation was not present at site 6 in the PN60 POA, males had significantly higher levels of CpG methylation compared with both vehicle-treated (ANOVA; *, P < 0.05) and estradiol-treated (P < 0.05; n = 4–5 per group) females at site 5.
PR methylation
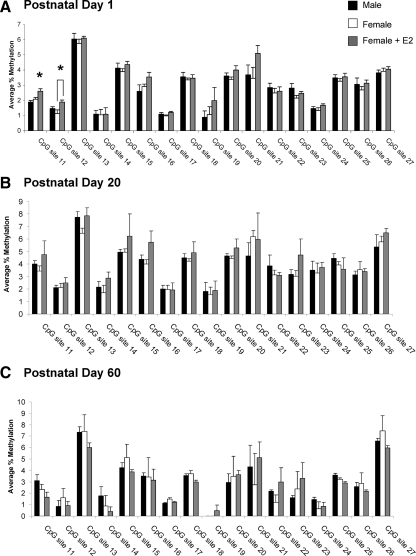
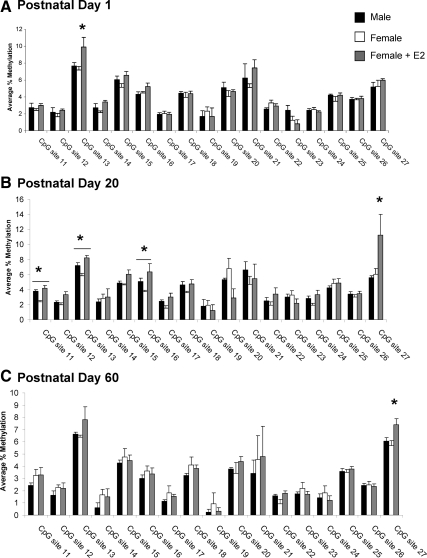
Seventeen CpG sites were selected for analysis in the PR promoter (Fig. 1C). In contrast to ER-α, the level and pattern of CpG methylation within the PR promoter remained similar throughout development and into adulthood, indicating little or no developmental regulation, and there was no sex difference in the methylation pattern of PR during the neonatal period (female, n = 6; male, n = 8; female + E2, n = 7), adolescence (female, n = 8; male, n = 8; female + E2, n = 5), or adulthood (female, n = 4; male, n = 5; female + E2, n = 5; Fig. 4, A–C). However, we did find an effect of group at PN1 at CpG site 11 (F2,18 = 8.25; P < 0.01) and site 12 (F2,18 = 5.32; P < 0.05; Fig. 4A), although not at any other time point. Specifically, estradiol treatment of females on PN0 increased methylation at site 11 to levels significantly greater than control males or females (P < 0.05) and at site 12 to levels significantly greater than control females (P < 0.05).
Figure 4.
Percent methylation of CpG islands in the PR promoter in the POA. A, Methylation analysis of CpG sites in the PR promoter on PN1 indicates that treatment of females with estradiol significantly increased the methylation at CpG site 11 over that of males and control females (ANOVA; *, P < 0.01) and at site 12 over that of control females (ANOVA; *, P < 0.05; n = 4–8 per group). B, Analysis of the same sites on PN20 reveals no effect of sex or neonatal estradiol treatment on the methylation levels at any CpG site within the POA (n = 6–8 per group). C, There was also no sex difference or effect of estradiol treatment at these same sites at PN60 (n = 5–6 per group).
Methylation patterns of ER-α, ER-β, and PR in the MBH across development
ER-α methylation
In the MBH, the major brain region controlling female sexual behavior, all seven CpG sites exhibited some degree of methylation with an average range of 6–16%. As seen in the POA, there were group differences in the methylation levels at CpG site 9 in the ER-α promoter on PN1, with females having a higher average percent methylation than males (t13 = 2.25; P < 0.05). Treatment of females with estradiol only partially reduced the percent methylation (F2,19 = 2.59; P = 0.10), resulting in intermediate levels not significantly different from either control males (males vs. females + E2; P = 0.16) or control females (females vs. females + E2; P = 0.44). A similar pattern was observed for site 6 (F2,19 = 2.54, P = 0.10; males vs. females, t13 = 2.17; P < 0.05; female, n = 7; male, n = 8; female + E2, n = 7; Fig. 5A).
Figure 5.
Percent methylation of CpG islands in the ER-α promoter in the MBH. A, The same seven CpG sites along the ER-α promoter analyzed in the POA were also assessed in the MBH at PN1, revealing a sex difference in the methylation of two CpG sites, site 6 and site 9 (*, P < 0.05 compared with males; n = 7–8 per group). Treatment of neonatal females with estradiol increased levels of methylation at these same sites to intermediate levels not significantly different from either males or females. B, By PN20 there was no effect of sex or neonatal estradiol treatment on percent methylation of CpG islands in the ER-α promoter in the MBH (n = 5–8 per group). C, This pattern persisted through PN60 (n = 5–6 per group).
As seen in the POA, there was a 2-fold increase in the level of methylation at most CpG sites on the ERα gene at PN20 and PN60 when compared with PN1 (Fig. 5, B and C), suggesting an important developmental regulation of ERα that is not dependent on sex, hormones, or brain region. There was no effect of sex or neonatal hormone exposure on the methylation pattern of ERα at PN20 within the MBH (female, n = 8; male, n = 8; female + E2, n = 5) or PN60 (female, n = 5; male, n = 6; female + E2, n = 5).
ER-β methylation
There were no detectable differences in ERβ promoter methylation in the MBH at PN1 (Fig. 6A). As seen in the POA, significant differences emerged at site 6 by PN20 (F2,18 = 5.98, P < 0.05; Fig. 6B). Specifically, males (P < 0.01) and estradiol-treated females (P < 0.05) had significantly higher levels of methylation compared with females, providing further evidence that hormonal regulation of this site is dependent on developmental time point. There were no group differences in methylation at sites 4 or 5 in the MBH at PN20. As previously seen in the POA, the group differences in methylation that were detected at CpG site 6 in the MBH were gone by PN60. However, as in the POA, a new pattern of ER-β promoter methylation emerged in the MBH in adult animals (F2,13 = 3.99, P < 0.05; Fig. 6C). At site 4, adult females had significantly higher methylation compared with males (P < 0.05) and females treated neonatally with estradiol (P < 0.05). Similarly, group differences in methylation appeared at site 5 in adulthood (F2,13 = 15.64, P < 0.001), which were not present at PN1 or PN20. Estradiol-treated females (P < 0.01) and males (P < 0.0001) had significantly lower methylation on the ER-β promoter compared with vehicle-treated females. In addition, males had lower methylation levels at this site compared with estradiol-treated females (P < 0.05).
Figure 6.
Percent methylation of CpG islands in the ER-β promoter in the MBH. A, The same three CpG sites of the ER-β promoter analyzed in the POA were analyzed in the MBH at PN1. As previously seen in the POA, there were no significant sex- or estradiol-induced differences in methylation at any site analyzed (n = 5–6 per group). B, Analysis of these sites at PN20 revealed both sex- and estradiol-induced differences in methylation at CpG site 6, wherein estradiol-treated females (ANOVA; *, P < 0.05) and males (*, P < 0.01; n = 5–8 per group) showed significantly higher levels of methylation compared with vehicle-treated females. C, By PN60 sex- and estradiol-induced differences in methylation emerged at site 4 and site 5 in the MBH. Adult females treated neonatally with vehicle had significantly higher levels of CpG methylation at site 4 compared with estradiol-treated females (ANOVA; *, P < 0.05) and males (*, P < 0.05; n = 5–6 per group). There was a similar pattern at site 5 in the adult MBH, with vehicle-treated females having significantly higher methylation than estradiol-treated females (*, P < 0.01) and males (*, P < 0.0001). In addition, males had significantly lower methylation at this site compared with estradiol-treated females (*, P < 0.05; n = 5–6 per group).
PR methylation
Similar to the POA, there were no sex differences in the level of methylation within the PR promoter in the MBH at PN1. At CpG site 13, there was an overall group effect on methylation levels (F2,15 = 4.91, P < 0.05; female, n = 6; male, n = 8; female + E2, n = 4; Fig. 7A); specifically estradiol increased the percent methylation at this CpG site by PN1. In contrast, analysis of the PR promoter region at PN20 revealed significant group differences in the methylation levels at three different CpG sites, site 11 (F2,17 = 9.52, P < 0.005), site 13 (F2,17 = 10.09; P < 0.005), and site 17 (F2,17 = 4.95; P < 0.05; female, n = 7; male, n = 8; female + E2, n = 6; Fig. 7B), with males having significantly higher levels of methylation at all three CpG sites than females (P < 0.05). Neonatal treatment of females with estradiol increased the methylation levels at these same sites to levels seen in males, consistent with a hormonally induced sex difference. At CpG site 27 on the PR promoter, there was an overall group difference in methylation levels (F2,17 = 4.09; P < 0.05) because neonatal estradiol treatment significantly increased the methylation levels assessed at PN20. However, there was no sex difference at this site, suggesting some hormonally induced changes in methylation are not related to the sexual differentiation of the brain. There were no sex differences in methylation detected at any CpG site at PN60 (female, n = 5; male, n = 6; female + E2, n = 5; Fig. 7C), indicating the organizational sex difference detected at PN20 is either eliminated in the mature adult or may be altered by the circulating hormones of the adult. Interestingly, the neonatal estradiol-induced increase in the methylation levels at CpG site 27 seen at PN20 is maintained at PN60 (F2,13 = 5.59; P < 0.05).
Figure 7.
Percent methylation of CpG islands in the PR promoter in the MBH. A, The same sites analyzed for the POA on the PR promoter were also assessed in the MBH at PN1. Neonatal estradiol (E2) treatment significantly increased methylation at one CpG site, site 13 (ANOVA; *, P < 0.05 compared with males and females; n = 4–8 per group). B, By PN20 there was a sex difference in the methylation levels at three different CpG sites, site 11, site 13, and site 17 (ANOVA; *, P < 0.05 compared with control females). Males had significantly greater levels of methylation compared with females and neonatal treatment of females with estradiol (E2) significantly increased the methylation at these same three sites to the levels seen in males and increased the methylation at CpG site 27 to levels significantly greater than males and control females (ANOVA; *, P < 0.05; n = 6–8 per group). C, Analyses of the same sites at PN60 found no effect of sex on the methylation levels at any CpG site. Neonatal treatment of females with estradiol significantly increased the methylation at CpG site 27 to levels greater than both males and females (*, P < 0.05; n = 5–6 per group).
Discussion
The cellular processes whereby sex differences in the brain are organized by sex steroids during development and subsequently maintained into adulthood remain poorly understood. The current results indicate that a component of sexual differentiation of the brain includes sex differences in and hormonal modulation of the methylation at individual CpG sites in the promoters of three genes, ER-α, ER-β, and PR, which are critical transducers of endocrine information for the regulation of brain development and adult sexual behavior. ER-α, ER-β, and PR are expressed at high levels in the developing and adult POA and MBH, key brain areas in the regulation of male and female sexual behavior (19,20,21), as well as maternal behavior, body temperature control, and ingestive behavior (22,23,24,25). We report here that CpG islands in the promoter regions of the ER-α, ER-β, and PR genes are differentially methylated across the life span in a manner specific to the brain region, age, sex, and neonatal hormone exposure of the animal.
The discovery process for epigenetic contributions to sexual differentiation of the brain is in its earliest stages and questions naturally emerge regarding reliability, robustness, and relevance. Whereas CpG dinucleotides outside a CpG island are predominantly methylated (∼90%), CpG sites within a CpG island are predominantly unmethylated (6–8%), allowing for the regulation of methylation and presumably gene expression. Thus, relatively small changes in the percent methylation (2–3%) at CpG sites within a CpG island can have profound effects on gene expression (17) but do not necessarily do so (26). The use of pyrosequencing provides a highly quantitative measure of the percent methylation at a single CpG site in one animal, allowing reliable detection of very small changes. Notably, the se associated with mean levels of percent methylation varies considerably across CpG sites, being consistently small at some sites (i.e. ∼1% at site 9 for ER-α) and consistently high at others (i.e. ∼2.5% at site 21 for PR). This suggests that some sites are tightly regulated, whereas others are less so. As one might predict, the relative importance of methylation at each CpG site to gene expression is not equal, with some sites being profoundly important to gene expression and others largely irrelevant (27).
Views on the function of CpG methylation are changing. In the early 1990s, it was already apparent that promoters with low CpG content did not show a clear relationship between methylation level and expression level (28,29). More recently, analysis of 16,000 promoters in the human epigenome reveals that when CpG density is high there is a greater degree of methylation and this reasonably predicts gene expression. However, when CpG content is low, there is no clear relationship between methylation and expression (30). From the same data set, it is apparent that, whereas DNA methylation is sufficient to inactivate CpG-rich gene promoters, it is not necessary because many hypomethylated CpG-rich promoters are inactive. The associated chromatin structure is implicated as an additional regulatory variable in such circumstances and highlights the important interplay between DNA methylation and histone modifications. Additional proposed functions of CpG methylation include monoallelic expression, parasitic element silencing (28), or changes to the associated chromatin (31). Moreover, some have suggested that many reported instances of a correlation between CpG methylation and expression patterns reflect a consequence of gene transcription on the epigenome and not vice versa (26).
Given the dynamic profile of CpG methylation we observed across development and the changing view of the significance these epigenetic tags to gene expression patterns, we did not attempt to correlate the percent change in CpG methylation with the expression levels of ER or PR. We cannot rule out the possibility that epigenetic changes at other promoters on ER or PR mediate sex differences in expression if and when they occur. Previous reports have found little or no sex differences in amount of ER within the hypothalamus at birth (6,32), whereas a sex difference in ER appears after the close of the critical period for sexual differentiation (PN10) that is maintained into adulthood (PN35) (32). Within the POA, females have significantly more ER-α than males by PN4 (32), and this is maintained into adulthood (P35) (33,34). In contrast, PR is profoundly sexually dimorphic in expression during the sensitive period but not later (11,12,13). It has also been demonstrated that the distribution of ER mRNA within brain regions such as the POA is even across multiple cell types, whereas the levels of ER protein within the same region is heterogeneous, some cells showing high levels and others showing lower levels. Data such as these indicate that whereas gene expression is one mechanism by which protein levels can be modified; there may be many other mechanisms by which the cell controls protein levels (34). Interestingly, previously reported sex differences in ER or PR expression do not correspond with the direction or the timing of the sex differences in methylation observed here. For example, in the POA we see higher levels of methylation in the ER-α promoter of new born females, which would be predicted to lead to reduced expression, but consistent reports in the literature indicate both mRNA and protein levels are higher in females at this time point (5,6). Therefore, we conclude on the basis of the current observation that at least some portion of developmental and hormone-induced epigenetic changes are not directly tied to gene expression but may instead serve some other yet-to-be-identified function.
We have identified two distinct effects of sex and neonatal estradiol on gene methylation levels: 1) sex differences in gene methylation that are induced and immediately evident during the critical period of sexual differentiation, which may be relevant to the differentiation process per se, and 2) sex differences in gene methylation that are determined during the critical period but not apparent until adulthood. Both the POA and the MBH exhibited a consistent sex difference in methylation at CpG site 9 in particular, which may indicate either a brain-wide sex difference in ER-α promoter methylation during development or a consistent methylation mark specific to brain regions undergoing estradiol-induced sexual differentiation during the critical neonatal period.
The direction and magnitude of the sex difference in methylation along the ER-α promoter that was present within the POA at PN1 was still present in adulthood (PN60) although at a different CpG site, long after the neonatal exposure to estradiol had occurred. That this sex difference is the result of estradiol exposure in neonatal males is supported by the observation that treatment of females with a masculinizing dose of estradiol during the critical period of sexual differentiation induced a methylation pattern identical with that of males, even in adulthood. These adult-manifested changes were apparent in the POA at CpG site 5 on the ER-α promoter, despite no sex difference or effect of estradiol at this site during development. Thus, it does not seem likely that the function of the differential methylation observed here is to regulate sex differences in total ER-α levels within the POA or MBH. Instead, the decreased methylation of the ER-α promoter in males or females treated with estradiol could serve the purpose of allowing ongoing transcription and replenishment of ER-α, which is ubiquitinated and rapidly degraded after activation by estradiol (35,36). In this way, the sensitivity of the male brain to estradiol would be maintained during the critical period of sexual differentiation. The later developmental increase in ER-α promoter methylation would reduce sensitivity to estradiol and thus prevent inappropriate changes in gene expression outside the critical period. Interestingly, developmental increases in methylation along the ER-α promoter have also been identified in the cortex (37). These data, and our own data here, indicate that a brain-wide decrease in estradiol sensitivity may occur after the critical period of sexual differentiation. As reported by Westberry et al. (37), developmental increases in ER-α promoter methylation within the cortex were correlated with increased expression of the methyl-binding protein MeCP2 and a developmental decrease in ER-α expression. Others have also reported sex differences in MeCP2 in the hypothalamus during the critical period, but not thereafter, with no observable differences in the POA (38). These data suggest that the process by which sex or hormones affect the epigenome involves differential methylation and also a broader orchestration of methyl binding proteins.
ER-α promoter methylation within the developing POA is also altered by maternal licking and grooming in a sex-specific manner. Simulated maternal grooming masculinizes ER-α promoter methylation in the POA and decreases ER-α expression by the end of the critical period, implicating early-life experiences as a component of sexual differentiation of the brain, through epigenetic mechanisms (33). Conversely, others have reported the opposite effect of maternal care in the POA, reporting that licking and grooming decreases ER-α methylation and increases the receptors expression (39,40). Although these methylation changes were observed at different developmental time points, the disparities in the ability of the environment to regulate ER-α promoter methylation highlight the complexity of epigenetic regulation of hormone receptors during development.
Analysis of the ER-β and PR promoters indicate that developmentally organized epigenetic modifications may not emerge until later in life. There were no observable group differences in ER-β promoter methylation in either brain region at birth, but by PN20 there were hormonally induced increases in methylation at CpG site 6 in both regions. This modification was transient because by PN60 there were no differences between groups in either region at site 6, yet new differences emerged at different sites in adult animals.
Analysis of the PR promoter region within the MBH determined that there was a profound sex difference in methylation at three CpG sites that emerged only later in life. The percent methylation level at these three CpG sites was significantly greater in males than females. During the critical period, there were no detectable sex differences in the methylation levels at these three sites or at any site along the PR promoter, but exposure to estradiol at this time significantly increased the methylation at the same three CpG sites at which a sex difference emerges by PN20. These data indicate that similar to CpG site 5 on ER-α, the sex difference at these three sites on PR is established during development, although it is not manifest until later in life. The mechanism by which a late emerging sex difference in methylation can be determined by neonatal exposure to estradiol during the critical period of sexual differentiation remains an intriguing question.
Epigenetics is emerging as a critical component of the mechanism by which early life experiences endure and impact adult physiology and behavior. Changes in DNA methylation are in particular considered to be some of the more profound and lasting epigenetic changes by robustly modulating the pattern of gene expression. This prevailing view is beginning to change in the face of new evidence that even DNA methylation patterns are dynamic and contribute to neuronal plasticity (41,42), a process that by definition is not permanent. Further research is required to expand our understanding of the enduring consequences of early hormone exposure on the epigenome of the brain.
Footnotes
This work was supported by Grant R01MH052716 from the National Institute of Mental Health (to M.M.M.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online August 11, 2010
Abbreviations: ER, Estrogen receptor; MBH, mediobasal hypothalamus; PN, postnatal day; POA, preoptic area; PR, progesterone receptor.
References
- McCarthy MM 2008 Estradiol and the developing brain. Physiol Rev 88:91–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries GJ, Södersten P 2009 Sex differences in the brain: The relation between structure and function. Horm Behav 55:589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Lieberburg I, Chaptal C, Krey LC 1977 Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav 9:249–263 [DOI] [PubMed] [Google Scholar]
- Naftolin F, MacLusky N 1984 Aromatization hypothesis revisited. In: Serio M, Motta M, Zanisi M, Martini L, eds. Sexual differentiation: basic and clinical aspects aromatization hypothesis revisited. New York: Raven Press; 79 [Google Scholar]
- Yokosuka M, Okamura H, Hayashi S 1997 Postnatal development and sex difference in neurons containing estrogen receptor-α immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol 389:81–93 [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, McAbee M, Ramer-Quinn DS, Stancik DM 1995 Estrogen receptor mRNA levels in the preoptic area of neonatal rats are responsive to hormone manipulation. Brain Res Dev Brain Res 84:253–260 [DOI] [PubMed] [Google Scholar]
- Karolczak M, Beyer C 1998 Developmental sex differences in estrogen receptor-β mRNA expression in the mouse hypothalamus/preoptic region. Neuroendocrinology 68:229–234 [DOI] [PubMed] [Google Scholar]
- Zhang JQ, Cai WQ, Zhou DS, Su BY 2002 Distribution and differences of estrogen receptor β immunoreactivity in the brain of adult male and female rats. Brain Res 935:73–80 [DOI] [PubMed] [Google Scholar]
- Orikasa C, Kondo Y, Hayashi S, McEwen BS, Sakuma Y 2002 Sexually dimorphic expression of estrogen receptor β in the anteroventral periventricular nucleus of the rat preoptic area: implication in luteinizing hormone surge. Proc Natl Acad Sci USA 99:3306–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orikasa C, Sakuma Y 2004 Sex and region-specific regulation of oestrogen receptor β in the rat hypothalamus. J Neuroendocrinol 16:964–969 [DOI] [PubMed] [Google Scholar]
- Quadros PS, Goldstein AY, De Vries GJ, Wagner CK 2002 Regulation of sex differences in progesterone receptor expression in the medial preoptic nucleus of postnatal rats. J Neuroendocrinol 14:761–767 [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Goldstein AY, De Vries GJ, Wagner CK 2002 Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology 143:3727–3739 [DOI] [PubMed] [Google Scholar]
- Lauber AH, Romano GJ, Pfaff DW 1991 Gene expression for estrogen and progesterone receptor mRNAs in rat brain and possible relations to sexually dimorphic functions. J Steroid Biochem Mol Biol 40:53–62 [DOI] [PubMed] [Google Scholar]
- Brown TJ, MacLusky NJ, Shanabrough M, Naftolin F 1990 Comparison of age- and sex-related changes in cell nuclear estrogen-binding capacity and progestin receptor induction in the rat brain. Endocrinology 126:2965–2972 [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Coyoy-Salgado A, Camacho-Arroyo I 2002 Sex differences in the regulation of progesterone receptor isoforms expression in the rat brain. Brain Res Bull 59:105–109 [DOI] [PubMed] [Google Scholar]
- Caiafa P, Zampieri M 2005 DNA methylation and chromatin structure: the puzzling CpG islands. J Cell Biochem 94:257–265 [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Bird A 2003 Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33:245–254 [DOI] [PubMed] [Google Scholar]
- Tost J, Gut IG 2007 DNA methylation analysis by pyrosequencing. Nat Protoc 2:2265–2275 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I 1997 Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol 388:507–525 [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y 1979 Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol (Lond) 288:189–202 [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD, Feder HH 1979 Cytoplasmic progestin-receptors in guinea pig brain: characteristics and relationship to the induction of sexual behavior. Brain Res 169:481–497 [DOI] [PubMed] [Google Scholar]
- Numan M 1974 Medial preoptic area and maternal behavior in the female rat. J Comp Physiol Psychol 87:746–759 [DOI] [PubMed] [Google Scholar]
- Szymusiak R, Satinoff E 1982 Acute thermoregulatory effects of unilateral electrolytic lesions of the medial and lateral preoptic area in rats. Physiol Behav 28:161–170 [DOI] [PubMed] [Google Scholar]
- Jolicoeur FB, Bouali SM, Fournier A, St-Pierre S 1995 Mapping of hypothalamic sites involved in the effects of NPY on body temperature and food intake. Brain Res Bull 36:125–129 [DOI] [PubMed] [Google Scholar]
- Dube MG, Kalra SP, Kalra PS 1999 Food intake elicited by central administration of orexins/hypocretins: identification of hypothalamic sites of action. Brain Res 842:473–477 [DOI] [PubMed] [Google Scholar]
- Walsh CP, Bestor TH 1999 Cytosine methylation and mammalian development. Genes Dev 13:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ 2009 Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12:342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes J, Bird A 1992 Repression of genes by DNA methylation depends on CpG density and promoter strength: evidence for involvement of a methyl-CpG binding protein. EMBO J 11:327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL 1994 Dependence of transcriptional repression on CpG methylation density. Mol Cell Biol 14:5487–5494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Hellmann I, Stadler MB, Ramos L, Pääbo S, Rebhan M, Schubeler D 2007 Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet 39:457–466 [DOI] [PubMed] [Google Scholar]
- Martinowich K, Hattoir D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE 2003 DNA methylation-related chromatin remodeling in activity-dependent bdnf gene regulation. Science 302:890–893 [DOI] [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, McEwen BS 1982 Sex differences in rat brain oestrogen and progestin receptors. Nature 300:648–649 [DOI] [PubMed] [Google Scholar]
- Kurian JR, Olesen KM, Auger AP 2010 Sex differences in epigenetic regulation of the estrogen receptor-α within the developing preoptic area. Endocrinology 151:2297–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, Yuri K, Morita N, Kawata M 1997 Differential expression of estrogen receptor mRNA and protein in the female rat preoptic area. Neurosci Lett 239:81–84 [DOI] [PubMed] [Google Scholar]
- Nawaz Z, Lonard DM, Dennis AP, Smith CL, O'Malley BW 1999 Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci USA 96:1858–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonard DM, Nawaz Z, Smith CL, O'Malley BW 2000 The 26S proteasome is required for estrogen receptor-α and coactivator turnover and for efficient estrogen receptor-α transactivation. Mol Cell 5:939–948 [DOI] [PubMed] [Google Scholar]
- Westberry JM, Trout AL, Wilson ME 2010 Epigenetic regulation of estrogen receptor α gene expression in the mouse cortex during early postnatal development. Endocrinology 151:731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian JR, Bychowski ME, Forbes-Lorman RM, Auger CJ, Auger AP 2008 Mecp2 organizes juvenile social behavior in a sex-specific manner. J Neurosci 28:7137–7142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA 2008 Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol 29:386–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Curley JP 2008 Maternal regulation of estrogen receptor α methylation. Curr Opin Pharmacol 8:735–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, Malone LM, Sweatt JD 2006 Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem 281:15763–15773 [DOI] [PubMed] [Google Scholar]
- Miller CA, Campbell SL, Sweatt JD 2008 DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem 89:599–603 [DOI] [PMC free article] [PubMed] [Google Scholar]