Abstract
The hypothalamus is a key region of the central nervous system involved in the control of homeostasis, including energy and core body temperature (Tc). 17β-Estradiol (E2) regulates Tc, in part, via actions in the basal hypothalamus and preoptic area. E2 primarily controls hypothalamic functions via the nuclear steroid receptors, estrogen receptor α/β. However, we have previously described an E2-responsive, Gq-coupled membrane receptor that reduces the postsynaptic inhibitory γ-aminobutyric acid-ergic tone and attenuates postovariectomy body weight gain in female guinea pigs through the administration of a selective Gq-mER ligand, STX. To determine the role of Gq-mER in regulating Tc, energy and bone homeostasis, ovariectomized female guinea pigs, implanted ip with temperature probes, were treated with STX or E2 for 7–8 wk. Tc was recorded for 4 wk, whereas food intake and body weight were monitored daily. Bone density and fat accumulation were determined postmortem. Both E2 and STX significantly reduced Tc in the females compared with controls. STX, similar to E2, reduced food intake and fat accumulation and increased tibial bone density. Therefore, a Gq-mER-coupled signaling pathway appears to be involved in maintaining homeostatic functions and may constitute a novel therapeutic target for treatment of hypoestrogenic symptoms.
A Gq-coupled membrane estrogen receptor regulates multiple hypothalamic homeostatic functions including core body temperature, energy intake, and bone remodeling.
Control of core body temperature (Tc) is a hypothalamic homeostatic function that is directly regulated by the sex steroids, 17β-estradiol (E2) and progesterone. Depending upon the model, estrogens and progesterone may have synergistic or antagonistic effects on Tc. Mice, which have elevated Tc during the dark cycle, exhibit subtle but significant strain differences in the regulation of Tc, but within each strain, there is no difference in Tc during the estrous cycle (1). In rats, Tc is suppressed during the light phase of proestrus (2) and has a different daily rhythm during proestrus, when estrogens are elevated before ovulation (3). Guinea pig females, a rodent species with a true luteal phase and a similar hormonal profile compared with primates, exhibit no change in Tc (deep rectal temperature) during the preovulatory phase of their 14- to 18-d estrous cycle before ovulation (4). Furthermore, gastrointestinal Tc measured in human females during the menstrual cycle, via swallowed telemetry pills, is lowest in the late follicular (preovulatory) and highest in the luteal phase with the early follicular phase in between (5).
One major concern of women experiencing surgical or natural menopausal states is the disregulation of the Tc that results in hot flushes. Hot flushes affect 75–85% of perimenopausal and postmenopausal women (6). Hot flushes are characterized by periods of sweating and peripheral vasodilation and are often associated with increased environmental temperature (7). Therefore, a hot flush can be defined as an exaggerated heat dissipation response. Although the underlying mechanism(s) is not known, repeated observations have found that the majority of hot flushes are preceded by elevation in the Tc independent of peripheral vasoconstriction or elevated metabolic rate (8,9). Therefore, it has been postulated that elevated Tc may serve as one of the triggers of menopausal hot flushes (7,10). Of importance is the reduction in rectal temperature in individuals receiving estrogens compared with the menopausal women without estrogens (11,12,13). Therefore, a small Tc elevation within a constricted thermoneutral zone may be the most important factor in triggering hot flushes in symptomatic menopausal women. In addition, there is compelling experimental evidence that the incidence of hot flushes is decreased in E2-treated females (10,11,12). This has led to the hypothesis that E2 widens the thermoneutral window by elevating the sweating threshold in women (10). However, the specific mechanism(s) of E2’s action is unknown.
Tc is controlled by hypothalamic preoptic neurons (14,15,16), and it is thought that direct (postsynaptic) actions of E2 on these neurons are at least, in part, responsible for maintaining Tc (11,12). In addition, there is a number of monoaminergic systems involved in temperature regulation, one of the most prominent being the ascending serotonergic neurons emanating primarily from the dorsal and median raphe nuclei (17,18). Specifically, the 5HT2A/2C receptors, which are metabotropic Gq-coupled receptors, are critically involved. Indeed, serotonin reuptake inhibitors, which elevate endogenous serotonin levels, are effective for treatment of hot flushes (19). These same receptors are also involved in the serotonergic regulation of energy and bone homeostasis (20,21). We have characterized a Gq-coupled, membrane-initiated E2-responsive pathway (Gq-mER) that is involved in mediating the anorectic effects of E2 in hypoestrogenic females and developed a diphenylacrylamide compound, called STX, that activates the same pathway in hypothalamic neurons (22,23,24). Furthermore, E2, known to attenuate the osteoporotic effects of hypoestrogenic states, has membrane-initiated effects on the cells involved in bone remodeling (25). Therefore, we hypothesized that E2 could regulate Tc through activation of a Gq-mER signaling pathway in hypothalamic neurons in addition to other indices of energy and bone homeostasis, such as food intake, fat accumulation, and tibial bone density. Here, we have developed a thermoregulatory rodent model using intact and ovariectomized adult female guinea pigs implanted ip with internal temperature probes to determine whether activation of a Gq-mER with a selective ligand (STX) controls Tc similarly to E2.
Materials and Methods
Tc recordings in intact and ovariectomized, female guinea pigs
All animal procedures described in this study are in accordance with institutional guidelines based on National Institutes of Health standards and approved by the Oregon Health and Science University Institutional Animal Care and Use Committee. Young, adult female multicolor guinea pigs (Elm Hill Breeding Labs, Chelmsford, MA) were used in these experiments. The guinea pigs were maintained under constant temperature (21 C) and light (on between 0630 and 2030 h). Animals were group housed with food and water provided ad libitum. In experiment 1, the baseline temperature in intact, cycling females was determined using two intact females implanted ip with dataloggers (SubCue Dataloggers; Canadian Analytical Technologies, Calgary, Alberta, Canada) to record Tc for 36 d with recordings every 40 min. To determine the effects of E2 benzoate (EB) on Tc, 12 females were ovariectomized under ketamine-xylazine anesthesia (33 and 6 mg/kg, respectively, sc) 7 d before experimentation and implanted with dataloggers programmed to record Tc every 20 min for 4 wk. Females were divided into groups of six and sc injected with either sesame oil or EB (8 μg/kg) every Monday, Wednesday, and Friday (M-W-F) at 1000 h for 4 wk. At the completion of the experiment, the animals were anesthetized with ketamine (33 mg/kg), decapitated, and dissected. Temperature data from the dataloggers were immediately downloaded and calibrated according to the manufacturer’s calibration equation. Three temperature recordings from each hour of each day were averaged and then sorted according to day and time. The uterus from each female was removed and weighed.
In experiment 2, STX [2-(4-hydroxyphenyl)-3-phenylpent-2-enoic acid [4-(2-dimethylaminoethoxy)phenyl]amide, E-enantiomer], a Gq-mER selective ligand, was injected into a subset of ovariectomized females to determine whether the STX-activated Gq-mER also regulated Tc. Fourteen female guinea pigs were ovariectomized, implanted with dataloggers, and allowed to recover for 1 wk. As described above, the females were sc injected with either vehicle [propylene glycol (PPG; STX does not dissolve in sesame oil), n = 5], EB in PPG (8 μg/kg, n = 5), or STX in PPG (6 mg/kg, n = 4) every M-W-F at 1000 h for 30 d. At the end of the experiment, the females were treated as above for euthanasia and tissue collection.
STX was produced by AAPharmaSyn, LLC (Ann Arbor, MI) under contract with the authors with the synthetic protocol for STX published in Tobias et al. (26). The potency of STX activating the Gq-mER signaling pathway was approximately 18-fold greater than E2 (23), and 10 nm STX was just as efficacious as 100 nm E2 in attenuating the γ-aminobutyric acid (GABA)B response in proopiomelanocortin (POMC) neurons (22). Furthermore, the relative binding affinities of STX for estrogen receptor (ER)α/β was previously reported to be approximately 1,000,000-fold less than E2, 4-hydroxytamoxifen, and raloxifene (22). Also, STX activates the Gq-phospholipase C (PLC)-protein kinase C (PKC)-protein kinase A (PKA) pathway in αER knockout (KO) (Chambon), βERKO (Korach), α/βERKO (Chambon), and GPR30 KO mice and has a different pharmacological profile than other nonclassical ERs [ERX and G-protein receptor 30 (GPR30)] (23,27,28). Finally, STX does not bind to and activate any of the aminergic Gq-coupled GPCRs known to control energy homeostasis and thermoregulation (Roth, B., personal communication; University of North Carolina at Chapel Hill, NC).
In experiment 3, another 20 female guinea pigs were ovariectomized and implanted with dataloggers to determine whether there is a dose effect of STX on Tc. However, due to the diminished battery life of the first set of dataloggers, another set was purchased and initially tested in four intact, cycling female guinea pigs for comparison with the previous datalogger set (see figure 3A). All temperature probes were calibrated according to the manufacturer’s instructions and were secondarily tested in house using a water bath set to a range of temperature from 33 to 42 C with no significant differences between individual dataloggers. In the intact females, the second set of dataloggers recorded a higher Tc (+0.6 C) than the first set (see Results). The difference in Tc recorded between these readings is most likely due to the use of different calibrating thermometers by the manufacturer (Bauce, L., personal communication; Canadian Analytical Technologies). Several probes from the second set have also been used in ovariectomized female primates (Rhesus Macaque), wherein they measured a typical Tc range (37.0–37.9 C) for primates.
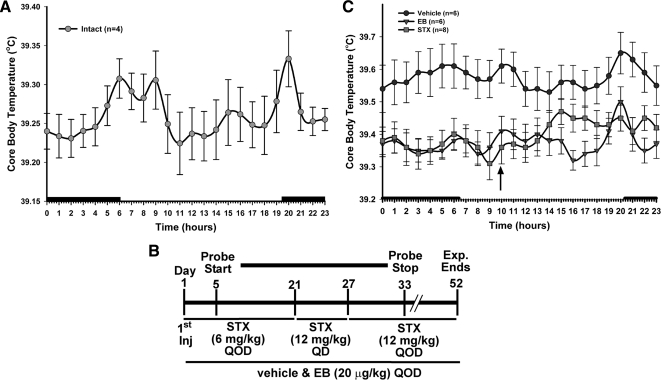
Figure 3.
Examination of the dose effect of STX on Tc. A, A new set of dataloggers was implanted into four intact females to record Tc for 63 d (45-min recording intervals). Dark bars above the x-axis represent lights off. B, To determine a dose effect of STX, ovariectomized female guinea pigs, implanted with temperature probes, were divided into three groups (vehicle, EB, or STX) and injected on d 1 with either vehicle (PPG, n = 6), EB (20 μg/kg in oil, n = 6), or STX (6 mg/kg in PPG, n = 8) QOD at 1000 h. Vehicle- and EB-treated females were injected as described for the entire 52-d experiment. On d 5, the probes began recording Tc and continued recording until d 33. On d 21, the STX-treated females were injected QD for 1 wk with 12 mg/kg. On d 27 (no injection d 28), the regimen frequency was reduced to 12 mg/kg QOD for the remainder of the experiments until d 52. C, STX (6–12 mg/kg QOD, n = 8) and EB (20 μg/kg, n = 6) significantly decreased the Tc compared with the vehicle (n = 6). Data are presented as the mean ± sem for each hour of the day averaged over the last 21 d of the probe recordings for vehicle- and EB-treated females and the first 14 (6 mg/kg QOD) and the last 7 d (12 mg/kg QOD) for STX-treated females. An arrow indicates time of injection. The data were analyzed using a two-way ANOVA (P < 0.05, F = 5.202, df = 2) with post hoc Newman-Keuls multiple comparison test. All STX and EB data points were significantly different from vehicle with a P < 0.01 with the exception of the late evening hours in the STX-treated females (P < 0.05).
In experiment 3, females were divided into three groups (vehicle, EB, and STX) 1 wk after ovariectomy and injected with either vehicle (PPG, n = 6) or EB (20 μg/kg in sesame oil, n = 6) or STX (6 mg/kg in PPG, n = 8) every other day (QOD) at 1000 h. Vehicle- and EB-treated females were injected as described for the entire 52-d experiment. Sesame oil was used for EB, because there was no difference in Tc between oil-treated animals from experiment 1 and PPG-treated animals in experiment 2 (see Figs. 1 and 2). On d 5, the probes began recording Tc and continued to record Tc until d 33 (for a timeline illustrating the experimental protocol, please see Fig. 3B). On d 21, to determine whether STX had a dose effect on Tc, the STX-treated females were injected every day (QD) for 1 wk with 12 mg/kg. On d 27 (no injection on d 28), the frequency of administration was reduced to 12 mg/kg QOD for the remainder of the experiments until completion on d 52. On each injection day, body weight and food weight were measured. At the end of the experiment, the females were treated as above for euthanasia and tissue collection. In addition, the dorsal abdominal and the periuterine fat pads were removed and weighed. For bone density analysis, the tibia from each hind leg was removed, cleaned, wrapped in saline-soaked gauze, and stored at −20 C until analyzed for bone density. Daily hourly averages were calculated for each animal and averaged within treatments. Furthermore, the daily hourly averages for Tc were determined during the respective dosage treatment periods to determine the dose effect of STX on Tc.
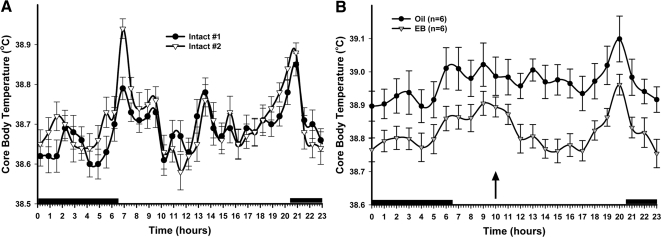
Figure 1.
The Tc of intact and ovariectomized female guinea pigs treated with either vehicle or EB. A, Dataloggers were implanted into two intact females to record Tc for 36 d with recordings every 40 min. Both females exhibited a crepuscular rhythm in Tc, where the peaks were associated with increases in activity at dawn and dusk. The dark bars above the x-axis represent lights off or nighttime. B, EB (8 μg/kg M-W-F, n = 6) significantly decreased the Tc compared with the vehicle (n = 6). The data are presented as the mean ± sem for each hour of the day averaged over the last 21 d of the temperature probe recordings. An arrow indicates time of injection. The data were analyzed using a two-way ANOVA (P < 0.05, F = 5.773, df = 1) with post hoc Newman-Keuls multiple comparison test. All data points in EB-treated females were significantly different from vehicle controls (P < 0.01).
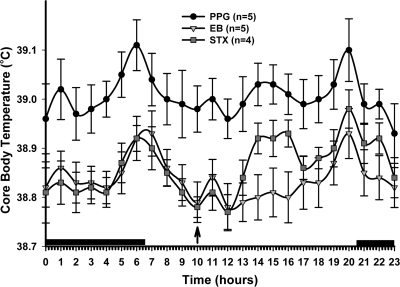
Figure 2.
The Tc of ovariectomized female guinea pigs treated with either vehicle, EB, or STX. The Gq-mER-selective ligand STX (6 mg/kg M-W-F, n = 4) significantly decreased the Tc compared with the PPG vehicle (n = 5) but was not significantly different from the EB group (8 μg/kg M-W-F, n = 5). The data are presented as the mean ± sem for each hour of the day averaged over the last 21 d of the temperature probe recordings. An arrow indicates time of injection. The data were analyzed using a two-way ANOVA (P < 0.05, F = 4.787, df = 2) with post hoc Newman-Keuls multiple comparison test. All data points for both STX and EB were significantly different from vehicle with a P < 0.01 with the exception of the STX 1400- to 1600-h time points (P < 0.05). The dark bars above the x-axis represent lights off or nighttime.
Measurement of food intake and meal pattern analysis
The feeding protocol used to evaluate the effects of STX and EB have been described in detail elsewhere (29). Briefly, a Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH) was used to monitor daily and hourly food intake, as well as meal frequency, size, and duration, in ovariectomized, female guinea pigs. The animals were acclimated to their respective CLAMS chambers for 3 d to adjust to their new environments, as well as the daily handling and weighing procedures. After the acclimation period, the animals were subject to 24-h monitoring of the feeding parameters for 7 d under ad libitum conditions. Each morning at 0800 h, the animals were weighed and injected with either STX (6 mg/kg, sc) or its PPG vehicle (150 μl, sc) and immediately returned to their respective chambers to continue analysis of feeding behavior. For the comparative studies designed to assess the effects of EB on food intake and meal pattern, EB (20 μg/kg, sc) or sesame oil (100 μl, sc) was administered QOD for 7 d (i.e. on d 1, 3, 5, and 7 of the monitoring period).
Measurement of tibia bone density
Peripheral quantitative computed tomography was performed at the proximal metaphysis of the tibia using the STRATEC XCT 4.50 equipment (Stratec, Inc., Pforzheim, Germany) (30). The scanner was positioned at the tibial metaphysis, and a coronal computed radiograph (scout view) in distal direction was carried out. The scout view was used to position the scanner at the site of measurement. Three tomographic slices were recorded 3.75, 4.25, and 15 mm distal of the reference line.
Image acquisition, processing, and calculation of the results were performed using the software package (XCT 5.40; Stratec, Inc.). Total density (mg/cm3) and total area (mm2) were defined as the means of the first and second cross sectional slices of measurement. Cancellous density and area in the cross section were calculated by acquiring data within the default threshold of 280–710 mg/cm3. Tissue with a density above 710 mg/cm3 was regarded to be cortical bone (30). Cortical area and cortical density were calculated from the third slice.
Statistical analyses
Comparisons between treatment groups in the Tc experiments, group-housed food intake, and meal pattern analysis (meal size, frequency, duration, and total amount) were performed using a two-way ANOVA (repeated measures) with post hoc Newman-Keuls multiple comparison test. The differences in group-housed food intake and daily food intake from the microstructural analysis were analyzed using a one-way ANOVA with a Newman-Keuls multiple comparison test after the repeated measures ANOVA. A one-way ANOVA with a Newman-Keuls multiple comparison test was used for fat pads, bone density, and uterine weight. Differences were considered statistically significant if the probability of error was less than 5%.
Results
Experiment 1: Regulation of Tc by EB
The Tc of intact, female guinea pigs exhibits a crepuscular circadian rhythm with two major peaks associated with the increased activity that occurs at dawn and dusk (Fig. 1A). Furthermore, the daily average Tc (over each 24-h time period) exhibits fluctuations across the 14- to 18-d estrous cycle of the guinea pigs (data not shown). The daily Tc fluctuated over a range of 0.6 C for each female with the highest peaks in daily Tc significantly higher than the nadirs (P < 0.01) (data not shown).
As hypothesized, ovariectomy resulted in an elevated Tc (Fig. 1B). Ovariectomy increased the daily average Tc compared with the two intact females (intact, 38.69 ± 0.07 C; ovariectomized, 38.97 ± 0.05 C). Systemic treatment with EB in ovariectomized females suppressed the average daily Tc by 0.20 C in experiment 1 and by an average of 0.19 ± 0.01 C over all experiments. The crepuscular rhythm was maintained in both the oil- and EB-treated females, although there was a smaller peak at dawn.
Experiment 2: Regulation of Tc by EB and STX
In the first STX experiment (Fig. 2), STX suppressed the ovariectomy-induced increase in Tc similarly to EB except for the afternoon hours (1400–1600 h) where there was a significant increase in Tc by STX compared with EB-treated females (P < 0.05), although these same points were also significantly lower than in the vehicle-treated females (P < 0.05). Except for the midafternoon increase in Tc, the STX-treated females maintained a normal crepuscular rhythm in daily Tc regulation. STX suppressed the average daily Tc by 0.14 ± 0.03 C (n = 4) compared with the vehicle. The afternoon increase in Tc after STX injection suggests that STX has an acute thermogenic effect. This effect may be due to interactions between the STX formulation and inflammatory cytokines, which are known to increase Tc (31). Finally, although Tc is primarily centrally regulated, questions remain as to the STX binding sites and the bioavailability of STX to these sites. We have recently begun studies to identify the specific binding sites of STX in the hypothalamus using membrane preparations and to assess the bioavailability of STX in the hypothalamus.
Experiment 3: The dose effect of STX on Tc
Due to the limited battery life of the dataloggers (see Materials and Methods), a new set of dataloggers was used for this experiment. To test these new dataloggers, four intact females were implanted with temperature probes programmed to record Tc every 45 min for 63 d. The same crepuscular circadian rhythm was observed as described above (experiment 1). However, in contrast to the first set of probes, the Tc of the second group of intact females averaged approximately 0.6 C higher than in the previous intact animals (38.69 ± 0.07 C vs. 39.26 ± 0.03 C) (Fig. 3A).
To determine the dose effects of STX on Tc, females were subjected to three dosing regimens during the course of the experiment, which included a 6 mg/kg QOD, 12 mg/kg QOD, and a 12 mg/kg QD regimen (Fig. 3B). As in experiment 2, STX (6 and 12 mg/kg QOD; STX data series includes 14 and 7 d from both regimens, respectively) suppressed Tc similarly to EB treatment except for the afternoon thermogenic response observed in experiment 2 (Fig. 3C). These time points remained significantly different compared with vehicle (P < 0.05). Similar to experiment 2, STX suppressed the average daily Tc by 0.18 ± 0.04 C (n = 8) compared with vehicle. The 6 and 12 mg/kg QOD regimens were combined in the presented graph because there was no statistical difference between the two regimens (average daily temperature, 6 mg/kg = 39.41 ± 0.04 C; 12 mg/kg = 39.37 ± 0.06 C). We used the last 21 d of the vehicle and EB-treated animals for a balanced number of days.
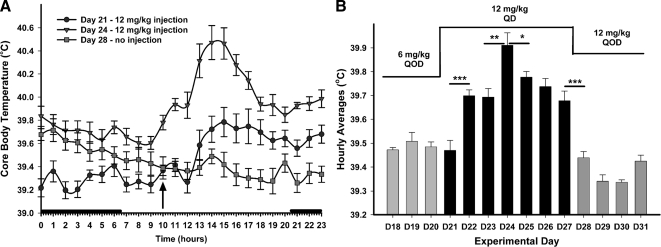
Escalating the frequency and dose of STX to 12 mg/kg QD for 7 d (d 21–27) significantly increased the afternoon thermogenic response, as well as the baseline Tc of the circadian rhythm (Fig. 4A). The daily average Tc peaked on d 24 after four daily injections of 12 mg/kg STX and decreased significantly in the 3 d after the peak Tc, while remaining significantly higher than the lower dose days (P < 0.05) (Fig. 4B). STX daily injections also increased the daily average Tc from a baseline of 39.49 ± 0.01 C (d 18–20) to a 7-d average of 39.71 ± 0.05 C with a peak of 39.91 ± 0.05 C on d 24. The average daily Tc and the peak were significantly higher than the average daily Tc for the vehicle-treated females during that same time period (vehicle, 39.57 ± 0.06 C). After the 7 d of daily treatment, the STX administration frequency was reduced to 12 mg/kg QOD starting on d 27 (no injection on d 28). The daily average Tc immediately decreased to 39.39 ± 0.03 C (d 28–31). The Tc increase in response to a higher daily dose of STX may be a result of tachyphylaxis due to desensitization of the GPCR by STX.
Figure 4.
High doses of STX increase Tc. A, On d 21, the frequency of STX treatment was increased to 12 mg/kg QD for 7 d. The higher dose of STX increased the baseline hourly temperatures and the afternoon peak immediately and continued to increase these parameters reaching a maximum effect on the fourth day (d 24). The increase in Tc was eliminated immediately upon cessation of the daily injections (d 28). Each symbol represents the mean ± sem of the eight STX-treated females. An arrow indicates time of injection. A two-way ANOVA reported a significant difference between d 21, 24, and 28 (P < 0.0001, F = 14.01, df = 2). An analysis of STX serum levels using liquid chromatography-mass spectrometry assay (Debarber, A., E. A. Rick, T. S. Scanlan, O. K. Rønnekleiv, and M. J. Kelly, unpublished data) showed that STX levels are 2.5-fold higher 24 h after injection with a 12 mg/kg dose vs. the 6 mg/kg dose. B, The higher dose of STX increased the average hourly temperatures for each day and continued to increase these parameters reaching a maximum effect on the fourth day (d 24). The increase in Tc was abrogated immediately upon cessation of the daily injections (d 28). Each bar represents the hourly mean for each day ± sem of the eight STX-treated females (*, P < 0.05; **, P < 0.01; ***, P < 0.001; Newman-Keuls pairwise comparison). A two-way ANOVA reported a significant difference between doses (P < 0.001, F = 13.3, df = 2). The 3 d before the increase are a representative of the first 16 d of the temperature recordings (d 5–20). The average daily Tc for the first 16 d was 39.41 ± 0.02 C.
Regulation of feeding behavior and fat accumulation by EB and STX
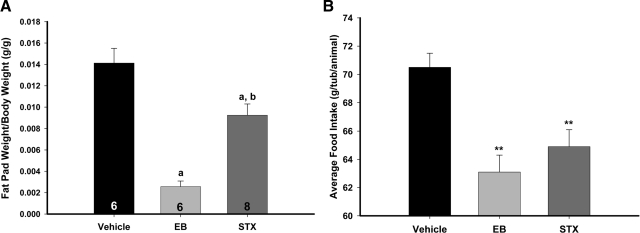
Another effect of E2 on energy balance is the control of peripheral fat accumulation (32). In our model, EB reduced the dorsal abdominal and periuterine fat pad by about 5-fold (P < 0.001), whereas STX reduced the fat pad by about 45% (P < 0.01) (Fig. 5A). Because the effects of E2 on fat accumulation are thought to be direct effect of E2 on adipose tissue (32), the effects of STX treatment on fat pad accumulation may be a result of the central control of food intake or another peripheral metabolic process, such as glucose homeostasis. It is thought that E2 inhibits food intake in mice through an ERα-dependent mechanism (33). Indeed, EB and STX both inhibited food intake during long-term treatment (Fig. 5B). The average food intake in g/tub/animal for the PPG-treated animals was 70.5 ± 1.0 g/tub/animal, and for EB and STX, 63.1 ± 1.2 and 64.9 ± 1.2 g/tub/animal, respectively (P < 0.001).
Figure 5.
STX and EB decrease food intake and peripheral fat accumulation in ovariectomized female guinea pigs. A, STX and EB reduced the dorsal abdominal and periuterine fat pads. The female guinea pigs were ovariectomized and administered QOD sc injections of vehicle (n = 6), EB (20 μg/kg, n = 6), or STX (6–12 mg/kg, n = 8). Bars represent the mean ± sem for each treatment in fat (g)/body weight (g). A one-way ANOVA (P < 0.0001, F = 153.5, df = 2) followed by a post hoc Newman-Keuls multiple comparison test was used to compare significance between each treatment. a, P < 0.001, vehicle and STX vs. EB and; b, P < 0.01, vehicle vs. STX. B, Both STX and EB significantly attenuated food intake in female guinea pigs compared with vehicle. A one-way ANOVA (P < 0.0001, F = 24.96, df = 2) followed by a post hoc Newman-Keuls multiple comparison test revealed a significant effect of EB and STX compared with vehicle (**, P < 0.01). Bars represent the mean ± sem of the 48 d of the food intake measurements.
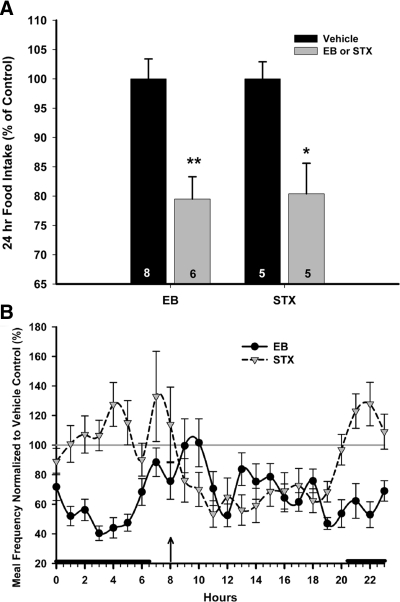
For a thorough investigation of the effects of STX (6 mg/kg) on food intake, including the microstructural parameters of food intake (hourly intake, meal size, meal frequency, and meal duration), 10 ovariectomized female guinea pigs were divided into two equal groups and treated with injections of PPG or STX. For comparison, 14 ovariectomized female guinea pigs were treated with either sesame oil (n = 8) or EB (20 μg/kg, n = 6) and tested for meal pattern analysis. Both EB and STX significantly decreased the daily (24 h) food intake in ovariectomized females by approximately 20% of the control (P < 0.01 EB, P < 0.05 STX) (Fig. 6A). EB and STX both decreased hourly food intake compared with vehicle treatment (P < 0.0001) (data not shown). The EB-induced decrease in hourly food intake was associated with a highly significant reduction in meal frequency (P < 0.0001) (Fig. 6B), although EB treatment also increased meal duration (P < 0.001) that resulted in an elevated meal size (P < 0.01). The increase in meal duration, and subsequently meal size, was not sufficiently long to overcome the decrease in meal frequency. In contrast, although STX also reduced meal frequency (P < 0.001) (Fig. 6B), it did not affect meal duration, thereby resulting in a significant diminution in meal size (P < 0.05). The reduction in meal frequency occurred during the 12 h after injection.
Figure 6.
STX decreases hourly food intake in ovariectomized female guinea pigs. A, A CLAMS unit was used to monitor daily and hourly food intake, as well as meal frequency, size, and duration, in ovariectomized, female guinea pigs. After an acclimation period, the animals were subject to 24-h monitoring of the aforementioned feeding parameters for 7 d under ad libitum conditions. Each morning at 0800 h, the animals were weighed and injected with either PPG (150 μl; sc, n = 5) or STX (6 mg/kg; sc in PPG, n = 5) QOD for 7 d (i.e. on d 1, 3, 5, and 7 of the monitoring period.). For EB treatment, EB (20 μg/kg; sc, n = 6) or the sesame oil vehicle (100 μl; sc, n = 8) was administered. The bars represent means ± sem of the daily food intake that was normalized to values obtained from the vehicle-treated control animals. The daily food intake was analyzed using a one-way ANOVA (P < 0.0001, F = 7.393, df = 3) followed by a post hoc Newman-Keuls multiple comparison test (*, P ≤ 0.05; **, P ≤ 0.01). B, Meal frequency (meals/hour) was analyzed hourly during the 7 d of the experiment and normalized to average control values (%). The dark circles represent EB-treated females (mean ± sem for the previous hour), and the gray triangles represent STX-treated females. The arrow indicates time of injection (0800 h), and the dark bars above the x-axis represent lights off. Meal frequency was analyzed using a two-way ANOVA repeated measures (P < 0.0001, F = 154.18, df = 1 for EB; P < 0.001, F = 12.42, df = 1 for STX).
STX mimics the effects of EB on cancellous bone density in the tibia
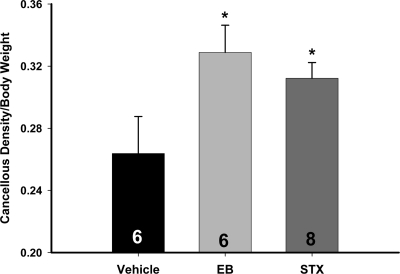
Both EB and STX increased the bone density of the metaphysis of the tibia compared with vehicle (P < 0.05) (Fig. 7). Bone density normalized to body weight at time of death for the vehicle was 0.26 ± 0.02 (mg/cm3)/g, whereas for EB and STX, the normalized bone density was 0.33 ± 0.02 and 0.31 ± 0.01 (mg/cm3)/g, respectively. The EB-induced increase in bone density was not significantly greater than that of the STX-treated females. This is another positive hormone effect of STX revealed in our guinea pig model that could be mediated by the central nervous system (21).
Figure 7.
Cancellous bone density of the proximal tibia in ovariectomized female guinea pig was increased by EB and STX. After the 52-d treatment, ovariectomized females treated with EB (20 μg/kg) or STX (12 mg/kg) had significantly higher bone density in the proximal tibia compared with the vehicle-treated females. On d 52, the animals were killed and tibiae were harvested. The trabecular bone region was scanned using quantitative computer tomography (30). A one-way ANOVA (P < 0.05, F = 3.616, df = 2) followed by a post hoc Newman-Keuls multiple comparison test (*, P < 0.05) was used to compare significance between each treatment.
E2 replacement is known to increase uterine weight via a classical receptor mediated mechanism (34). We have previously shown that STX does not have uterine hypertrophic effects (22,23,24). As expected, in the current experiment, EB significantly increased uterine weight (3.58 ± 0.29 g; P < 0.001) compared with vehicle (0.27 ± 0.03 g), whereas STX had no uterotropic effects and was not significantly different from vehicle (0.23 ± 0.02 g). ERα activation is necessary for the hypertrophic effect of E2 on the uterus (34) and is a physiological confirmation of the lack of STX binding to ERα.
Discussion
In the current study, we have demonstrated that ovariectomy will increase Tc in female guinea pigs and that both E2 and STX, which does not bind to the classical nuclear receptors (ERα and ERβ) (22), are equally efficacious in lowering Tc in this rodent model. In two separate studies, STX (6 and 12 mg/kg QOD) attenuated the postovariectomy increase in Tc. In addition, STX and E2 reduced food intake and fat accumulation. Further investigation demonstrated that STX reduced food intake by a differential effect on the microstructural parameters of meal pattern compared with E2. Finally, STX, like E2, maintained tibial bone density in ovariectomized females. The cumulative findings would indicate that the Gq-PLC-PKC-PKA signaling pathway activated by E2 and STX is involved in maintaining homeostasis in the female.
Central control of thermoregulation
Hot flushes in humans are characterized by periods of sweating and peripheral vasodilation (7) and can be defined as an exaggerated heat dissipation response. Although the mechanism is unknown, numerous observations have found that hot flushes are commonly preceded by elevation in Tc (8,9), which may serve as one trigger of hot flushes (7,10). We have developed a reliable animal model for measuring Tc in hypoestrogenic females. An implanted datalogger was used to record abdominal Tc, offering a more discrete determination of the real-time Tc compared with rectal temperature measurements. Temperature recordings using the dataloggers provided a visible picture of the daily guinea pig temperature rhythm and the effects of E2 and STX. Previously, experiments that examined the E2 effects on guinea pig Tc used rectal temperature probes at different depths in intact and ovariectomized females (4). The deeper probes, which supposedly measured Tc, did not detect a temperature change with E2 treatment, whereas the more superficially placed probes measured an increase. The difference from our findings is due most likely to differences in experimental procedures. Similarly, early data from rectal temperature measurements in rats showed that E2 decreased Tc (35), increased Tc (36), or had little effect on Tc (37). The differences between these experiments may be due to the method of E2 administration [silastic capsules (32,34) or injections (33)] that produced plasma E2 levels that correlate to either diestrous (34) or proestrous (32) E2 levels. Recent experiments using telemetry recordings of Tc during E2 and 17α-ethinyl-estradiol treatment are also ambiguous (38,39,40). However, our data suggests that E2, alone, does indeed reduce abdominal Tc significantly in ovariectomized guinea pigs compared with vehicle-treated controls and that it does so, in part, by the activation of the STX-activated Gq-mER signaling pathway.
Although the cellular mechanism for the estrogenic control of thermoregulation is not known, one potential mechanism for the central control of thermoregulation is the direct actions of E2 on thermosensitive (GABAergic) neurons in the preoptic area (POA) of the hypothalamus, a key brain area for the control of thermoregulation via the integration of central and peripheral temperature signals (15,41,42). In warm-sensitive neurons, the temperature-induced increase in neuronal activity is associated with a decrease in a potassium (A-type) conductance, leading to an increase in the depolarizing prepotential that is enhanced by a cAMP-dependent process (43). The effect of E2 on warm-sensitive POA neurons has to our knowledge not been explored in females. However, in males, E2 predominantly increases single unit firing of warm-sensitive neurons (44), indicating a pre- or postsynaptic effect to alter the excitability of these cells. Our studies of female guinea pig medial POA GABAergic neurons have revealed that E2 acts via a membrane-mediated action to attenuate GABAB autoinhibition, leading to increased firing of these neurons (45). However, we do not know if these E2-sensitive neurons are also the same warm-sensitive neurons characterized by Boulant (43).
There is compelling evidence that norepinephrine plays a central role in the temperature regulation disturbances leading to hot flushes (7). One model is that estrogen withdrawal leads to increased hypothalamic norepinephrine levels and increased activation of α2-adrenergic receptors. This eventually leads to autonomic counterregulatory measures to cool the body resulting in a hot flush. Norepinephrine acts via α2 (Gαi/o-coupled) receptors to inhibit hypothalamic neurons in an E2-dependent manner (46). In addition, E2 can also modulate norepinephrine-mediated actions via the α1 (Gαq-coupled) receptor in POA neurons (47). However, the modulation of catecholamine release and action on the temperature-sensitive POA neurons by E2 or STX has yet to be determined.
Other Gq-coupled GPCRs are involved in thermoregulation, including receptors for serotonin. Serotonin binds to Gαq-coupled receptors (5HT2A/2C) to activate signaling pathways that ultimately lower Tc and have been implicated in thermoregulation dysfunction caused by ovariectomy (48,49). Clinically, selective serotonin reuptake inhibitors, which elevate endogenous serotonin levels, are efficacious for treating hot flushes in women (19) and can significantly decrease the effects of postovariectomy thermoregulatory dysfunction in rodents (50). Both individually and in an additive manner, serotonin and E2 (STX) activate Gαq-coupled receptors in arcuate POMC neurons to attenuate inhibitory postsynaptic (GABA) signals that activate G-protein coupled inwardly rectifying potassium channel activity (51). A similar mechanism may be operating in hypothalamic thermoregulatory neurons, including POMC neurons, which are involved in the control of numerous neuroendocrine and autonomic functions.
Central control of energy homeostasis
During menopause, women tend to gain body fat that appears to be a consequence of the decline in endogenous estrogens (52). In rodent models, hypoestrogenic states (ovariectomy) are associated with a decrease in activity, an increase in food intake and body weight, and a change in body composition, which is reversed with estrogen replacement (53,54,55). The effects of E2 on energy balance (energy intake and expenditure, etc.) are thought to be mediated through its actions in the central nervous system [the hypothalamus and the nucleus tractus solitarius (NTS)] (33,56,57). Previously, E2 was thought to regulate appetite, energy expenditure, and fat accumulation via the nuclear receptor ERα (32,58). In the current study, we found that STX decreased food intake during the long-term experiments on group-housed females and during the 7-d assessment of meal patterns in individual females. Furthermore, long-term treatment of STX significantly decreased fat accumulation, which is associated with the reduction in energy intake. The clear effects of STX on food intake and fat accumulation suggest that the activation of a Gq-mER contributes to the control of energy homeostasis by E2 in addition to the actions via ERα.
We have previously reported that STX, like E2, attenuates the postovariectomy body weight gain in female guinea pigs (23,24). The reduction in body weight gain was driven, in part, by a significant reduction in food intake as seen in the present experiments. However, the effects of E2 on meal patterns in guinea pigs appear to be different from its effects on female rat meal pattern. In female rats, cyclical E2 treatment decreased meal size and increased meal frequency (54), and the ERα agonist propyl pyrazole triol decreased meal size but had no effect on meal frequency (59). The driving force behind the reduction in food intake by E2 in the guinea pig is a reduction in the number of meals, whereas in the rat, the driving force is a reduction in meal size. In the rat and the mouse, E2 via ERα potentiates the satiety signals from the gut, such as cholecystokinin, through interactions with NTS neurons to decrease meal size and duration (56,58,60). However, in the guinea pig, EB treatment increased meal duration and size, indicating that E2 activates a different central mechanism in these rodents. This E2-responsive mechanism may account, in part, for the differences between E2 and STX on guinea pig meal duration. The STX-activated Gq-mER has been functionally characterized in hypothalamic POMC neurons, and presumably, the reduction in daily food intake (meal frequency) in females by STX involves these POMC hypothalamic and/or brain stem (NTS) neurons (56,60). STX also reduces the mRNA expression of arcuate neuropeptide Y after long-term treatment in female guinea pigs, which may also influence the feeding pattern (24). However, the lack of STX’s effects on meal duration may indicate that the STX-activated Gq-mER is not expressed in the NTS, where the major satiety signals from the gut are incorporated to control the cessation of individual meals (61). We cannot eliminate the possible effects of STX on other hypothalamic nuclei or the NTS, which will need further investigation.
Thermoregulation and energy homeostasis are two interrelated physiological phenomena. And, although it is well known that food or caloric restriction will suppress Tc (62), the females in our study were allowed free access to food. We are unaware of any evidence suggesting that a decrease in food intake due to modulation of feeding behavior contributes significantly to a decrease in Tc. Furthermore, in comparing the Tc data (Figs. 2 and 3) and the meal frequency data (Fig. 6B), there was no correlation between the reduction in feeding, which occurred primarily during the 12 h after injection of STX, and the overall reduction in Tc by STX. In fact, STX had an acute thermogenic effect while simultaneously reducing meal frequency.
Central control of bone density
The effects of STX mimic the effects of E2 on tibial bone density in the ovariectomized guinea pigs. We hypothesize that STX is controlling bone density via a central mechanism involving the preautonomic paraventricular nucleus of the hypothalamus (PVH) neurons that control bone remodeling. Currently, the activity of a Gq-mER has been localized to the arcuate nucleus and the POA (22,23). However, it may also be expressed in other hypothalamic nuclei involved in E2’s actions on autonomic functions, such as the ventromedial nucleus of the hypothalamus (VMH). The VMH has been thought to play a key role in the regulation of bone density by leptin and serotonin (21). Neurons in the VMH are proposed to modulate the PVH neurons that drive sympathetic activity, although the authors do not show any discreet anatomical data (63). The sympathetic nervous system in turn controls bone remodeling via the β2 adrenergic receptor (Adrβ2) activity (63). The loss of the Adrβ2 in Adrβ2−/− mice increases bone formation and decreases bone resorption (64), and gonadectomy of Adrβ2−/− mice does not alter bone mass or bone resorption parameters, indicating that increased sympathetic activity may be responsible for the bone loss in hypoestrogenic states.
Because the STX-activated Gq-mER has been functionally characterized in arcuate POMC neurons (22,23), another possible mechanism behind the effects on bone remodeling is a direct effect on the preautonomic PVH neurons. POMC neurons project to the paraventricular nucleus (65,66). Furthermore, POMC neurons are sensitive to monosodium glutamate toxicity especially during development (67), and monosodium glutamate-sensitive arcuate neurons, which do not express neuropeptide Y, are thought to be involved in bone remodeling (68). Also, arcuate POMC neurons express cocaine- and amphetamine-regulated transcript (CART), which has recently been identified as a regulator of leptin-dependent bone resorption (64). The STX-activated Gq-mER signaling pathway may modulate the regulation of CART by leptin or the release of CART (and/or some other signal) to control bone remodeling via the sympathetic nervous system.
The effect of STX on bone density lends credence to our hypothesis that a putative hypothalamic Gq-mER is involved in E2’s central control of multiple homeostatic functions. However, we cannot rule out direct peripheral effects of STX, because E2, through a GPCR-PLC-PKC signaling pathway, regulates chondrocyte function (69) and activates MAPK in osteoblasts (70). Furthermore, E2 regulates the expression of cytokines (IL-6, etc.) and their respective activity on osteoclast and osteoblast function through nuclear-initiated and membrane-initiated mechanisms (25,71). In addition, E2 inhibits PTH stimulation of osteoclasts by attenuating the PTH receptor-mediated signaling pathways (PKA and PKC) (72). Although both osteoblasts and osteoclasts express an E2-responsive GPCR (GPR30) (73), E2 (and STX) can activate the PLC-PKC-PKA pathway in hypothalamic neurons from GPR30 KO mice (27), indicating that GPR30 is not necessary for STX-induced signaling. The presence of membrane-initiated E2 signaling in bone remodeling suggests that STX may have a potential peripheral receptor to control bone homeostasis. However, central and peripheral mechanisms are not mutually exclusive.
Summary
In the current study, we have found that E2 suppresses the ovariectomy-induced increase in Tc through, in part, a membrane-mediated mechanism initiated by the activation of a Gq-mER both via E2 and the receptor’s selective ligand, STX, in the hypothalamus. Therefore, a component of E2 thermoregulation may be due to the STX-activated Gq-mER pathway. Recent data suggests that E2 may also use the nuclear receptor, ERβ, to control the estrogenic thermoregulation of tail skin temperature (74), although E2 can suppress the postovariectomy increase in tail skin temperature in both ERα and ERβ KO mice (75). Furthermore, the Gq-mER signaling pathway may be involved in the estrogenic control of energy (food intake) and bone homeostasis (bone density), functioning in tandem with the classical nuclear receptors. Disruptions in all three of these hypothalamic functions are hallmarks of hypoestrogenic states. The multiple positive effects of the activation of a Gq-mER via STX on Tc, energy balance, and bone remodeling strongly indicate that this particular mER is an excellent target for postmenopausal hormonal replacement therapies with little to no proliferative reproductive side effects (uterine hypertrophy, etc.). Recently, STX, like E2, was also shown to have neuroprotective effects on hippocampal CA1 neurons after a global ischemia insult when administered via lateral intracerebroventricular infusion in ovariectomized, middle-aged rats (76). Future investigations will elucidate the cellular targets of STX to mediate these multiple homeostatic and neuroprotective effects.
Acknowledgments
STX receptor binding profile information was provided by Dr. Bryan L. Roth, M.D., Ph.D. (University of North Carolina at Chapel Hill, NC), Director of the National Institute of Mental Health (NIMH)’s Psychoactive Drug Screening Program (Project Officer Jamie Driscol at the NIMH, Bethesda, MD), Contract HHSN-271-2008-00025-C (NIMH Psychoactive Drug Screening Program).
Footnotes
This work was supported by Public Health Service Grants NS43330 (to O.K.R.), NS38809 (to M.J.K.), DK68098 (to M.J.K.), and DA 023414 (to E.J.W.) and by the Medical Technology Acceleration Program of the Charitable Leadership Foundation (M.J.K, O.K.R., and T.S.S.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online August 4, 2010
Abbreviations: Adrβ2, β2 Adrenergic receptor; CART, cocaine- and amphetamine-regulated transcript; CLAMS, Comprehensive Lab Animal Monitoring System; E2, 17β-estradiol; EB, E2 benzoate; ER, estrogen receptor; GABA, γ-aminobutyric acid; GPCR, G-protein coupled receptor; GPR30, G-protein coupled receptor 30; Gq-mER, Gq-coupled membrane estrogen receptor; KO, knockout; M-W-F, Monday, Wednesday, and Friday; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; POA, preoptic area; POMC, proopiomelanocortin; PPG, propylene glycol; PVH, paraventricular nucleus of the hypothalamus; QD, every day; QOD, every other day; Tc, core body temperature; VMH, ventromedial nucleus of the hypothalamus.
References
- Koehl M, Battle SE, Turek FW 2003 Sleep in female mice: a strain comparison across the estrous cycle. Sleep 26:267–272 [DOI] [PubMed] [Google Scholar]
- Rashotte ME, Ackert AM, Overton JM 2002 Ingestive behavior and body temperature during the ovarian cycle in normotensive and hypertensive rats. Am J Physiol Regul Integr Comp Physiol 282:R216–R225 [DOI] [PubMed] [Google Scholar]
- Yochim JM, Spencer F 1976 Core temperature in the female rat: effect of ovariectomy and induction of pseudopregnancy. Am J Physiol 231:361–365 [DOI] [PubMed] [Google Scholar]
- Czaja JA, Butera PC 1986 Body temperature and temperature gradients: changes during the estrous cycle and in response to ovarian steroids. Physiol Behav 36:591–596 [DOI] [PubMed] [Google Scholar]
- Coyne MD, Kesick CM, Doherty TJ, Kolka MA, Stephenson LA 2000 Circadian rhythem changes in core temperature over the menstrual cycle: method for noninvasive monitoring. Am J Physiol Regul Intergr Comp Physiol 279:R1316–R1320 [DOI] [PubMed] [Google Scholar]
- Moline ML, Broch L, Zak R, Gross V 2003 Sleep in women across the life cycle from adulthood through menopause. Sleep Med Rev 7:155–177 [DOI] [PubMed] [Google Scholar]
- Rapkin AJ 2007 Vasomotor symptoms in menopause: physiologic condition and central nervous system approaches to treatment. Am J Obstet Gynecol 196:97–106 [DOI] [PubMed] [Google Scholar]
- Freedman RR, Norton D, Woodward S, Cornelissen G 1995 Core body temperature and circadian rhythm of hot flashes in meopaursal women. J Clin Endo Metab 80:2354–2358 [DOI] [PubMed] [Google Scholar]
- Freedman RR 2005 Hot flashes: behavorial treatments, mechanisms, and relation to sleep. Am J Med 118:1245–1305 [DOI] [PubMed] [Google Scholar]
- Freedman RR, Blacker CM 2002 Estrogen raises the sweating threshold in postmenopausal women with hot flashes. Fertil Steril 77:487–490 [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Nicholas WC, Deaver DR, Mikita D, Kenney WL 1992 Estrogen replacement in middle-aged women: thermoregulatory responses to exercise in the heat. J Appl Physiol 73:1238–1245 [DOI] [PubMed] [Google Scholar]
- Brooks EM, Morgan AL, Pierzga JM, Wladkowski SL, O'Gorman JT, Derr JA, Kenney WL 1997 Chronic hormone replacement therapy alters thermoregulatory and vasomotor function in postmenopausal women. J Appl Physiol 97:477–484 [DOI] [PubMed] [Google Scholar]
- Brooks-Asplund EM, Cannon JG, Kenney WL 2000 Influence of hormone replacement therapy and aspirin on temperature regulation in postmenopausal women. Am J Physiol Regul Intergr Comp Physiol 279:839–848 [DOI] [PubMed] [Google Scholar]
- Boulant JA, Chow AR, Griffin JD 1997 Determinants of hypothalamic neuronal thermosensitivity. Ann NY Acad Sci 813:133–138 [DOI] [PubMed] [Google Scholar]
- Griffin JD, Saper CB, Boulant JA 2001 Synaptic and morphological characteristics of temperature-sensitive and -insensitive rat hypothalamic neurones. J Physiol 537. 2:521–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarean IV, Behrens MM, Bartfai T, Korn H 2004 Prostaglandin E2-increased thermosensitivity of anterior hypothalamic neurons is associated with depressed inhibition. Proc Natl Acad Sci USA 101:2590–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen HH 2000 The role of serotonin in hot flushes. Maturitas 36:155–164 [DOI] [PubMed] [Google Scholar]
- Sturdee DW 2008 The menopausal hot flush. Anything new? Maturitas 60:42–49 [DOI] [PubMed] [Google Scholar]
- Stearns V, Ullmer L, López JF, Smith Y, Isaacs C, Hayes DF 2002 Hot flushes. Lancet 360:1851–1861 [DOI] [PubMed] [Google Scholar]
- Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, Lee CE, Aschkenasi CJ, Zhang CY, Yu J, Boss O, Mountjoy KG, Clifton PG, Lowell BB, Friedman JM, Horvath T, Butler AA, Elmquist JK, Cowley MA 2006 Serontonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron 51:239–249 [DOI] [PubMed] [Google Scholar]
- Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G 2009 A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138:976–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ 2003 Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J Neurosci 23:9529–9540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ 2006 A G protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci 26:5649–5655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK 2008 Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology 149:6113–6124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed F, Khosla S 2005 Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun 328:688–696 [DOI] [PubMed] [Google Scholar]
- Tobias SC, Qiu J, Kelly MJ, Scanlan TS 2006 Synthesis and biological evaluation of SERMs with potent nongenomic estrogenic activity. ChemMedChem 1:565–571 [DOI] [PubMed] [Google Scholar]
- Qiu J, Rønnekleiv OK, Kelly MJ 2008 Modulation of hypothalamic neuronal activity through a novel G-protein coupled estrogen membrane receptor. Steroids 73:985–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Qiu J, Bosch MA, Rønnekleiv OK, Kelly MJ 2009 Cross-talk between membrane-initiated and nuclear-initiated oestrogen signalling in the hypothalamus. J Neuroendocrinol 21:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz S, Farhang B, Hoien J, Stahlman M, Adatia N, Cox JM, Wagner EJ 2009 Sex differences in the cannabinoid modulation of appetite, body temperature and neurotransmission at POMC synapses. Neuroendocrinology 89:424–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachoń D, Seidlová-Wuttke D, Vortherms T, Wuttke W 2007 Effects of dietary equol administration on ovariectomy induced bone loss in Sprague-Dawley rats. Maturitas 58:308–315 [DOI] [PubMed] [Google Scholar]
- Biddle C 2006 The neurobiology of the human febrile response. AANA Journal 74:145–150 [PubMed] [Google Scholar]
- Shi H, Seeley RJ, Clegg DJ 2009 Sexual differences in the control of energy homeostasis. Front Neuroendocrinol 30:396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Geary N 2006 Modulation of appetite by gonadal steroid hormones. Phil Trans R Soc B 361:1251–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Korach KS 2001 Contrasting phenotypes in reproductive tissues of female estrogen receptor null mice. Ann NY Acad Sci 948:1–8 [DOI] [PubMed] [Google Scholar]
- Wilkinson CW, Carlisle HJ, Reynolds RW 1980 Estrogenic effects on behavioral thermoregulation and body temperature of rats. Physiol Behav 24:337–340 [DOI] [PubMed] [Google Scholar]
- Marrone BL, Gentry RT, Wade GN 1976 Gonadal hormones and body temperature in rats: effects of estrous cycles, castration and steroid replacement. Physiol Behav 17:419–425 [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Wilkinson CW, Carlisle HJ, Hammel HT 1980 Energy balance in ovariectomized rats with and without estrogen replacement. Am J Physiol 238:R400–R405 [DOI] [PubMed] [Google Scholar]
- Hosono T, Chen XM, Zhang YH, Kanosue K 1997 Effects of estrogen on thermoregulatory responses in freely moving female rats. Ann NY Acad Sci 813:207–210 [DOI] [PubMed] [Google Scholar]
- Hosono T, Chen XM, Miyatsuji A, Yoda T, Yoshida K, Yanase-Fujiwara M, Kanosue K 2001 Effects of estrogen on thermoregulatory tail vasomotion and heat-escape behavior in freely moving female rats. Am J Physiol Regul Integr Comp Physiol 280:R1341–R1347 [DOI] [PubMed] [Google Scholar]
- Cosmi S, Pawlyk AC, Alfinito PD, Roman J, Zhou T, Deecher DC 2009 Simultaneous telemetric monitoring of tail-skin and core body temperature in a rat model of thermoregulatory dysfunction. J Neurosci Meth 178:270–275 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M 2002 The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci 22:4600–4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulant JA 2006 Neuronal basis of Hammel’s model for set-point thermoregulation. J Appl Physiol 100:1347–1354 [DOI] [PubMed] [Google Scholar]
- Boulant JA 1998 Hypothalamic neurons. Ann NY Acad Sci 856:108–115 [DOI] [PubMed] [Google Scholar]
- Silva NL, Boulant JA 1986 Effects of testrosterone, estradiol, and temperature on neurons in preoptic tissue slices. Am J Physiol 250:R625–R632 [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Rønnekleiv OK, Bosch MA, Kelly MJ 2001 Estrogen biphasically modifies hypothalamic GABAergic function concomitantly with negative and positive control of luteinizing hormone release. J Neurosci 21:2085–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etgen AM, Ansonoff MA, Quesada A 2001 Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: implications for female reproductive physiology. Horm Behav 40:169–177 [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Rønnekleiv OK, Kelly MJ 2001 The noradrenergic inhibition of an apamine-sensitive small conductance Ca2+-activated K+ channel in hypothalamic γ-aminobutyric acid neurons: pharmacology, estrogen sensitivity and relevance to the control of the reproductive axis. J Pharmacol Exp Ther 299:21–30 [PubMed] [Google Scholar]
- Berendsen HH, Weekers AH, Kloosterboer HJ 2001 Effect of tibolone and raloxifene on the tail temperature of oestrogen-deficient rats. Eur J Pharmacol 419:47–54 [DOI] [PubMed] [Google Scholar]
- Sipe K, Leventhal L, Burroughs K, Cosmi S, Johnston GH, Deecher DC 2004 Serotonin 2A receptors modulate tail-skin temperature in two rodent models of estrogen deficiency-related thermoregulatory dysfunction. Brain Res 1028:191–202 [DOI] [PubMed] [Google Scholar]
- Deecher DC, Alfinito PD, Leventhal L, Cosmi S, Johnston GH, Merchanthaler I, Winneker R 2007 Alleviation of thermoregulatory dysfunction with the new serotonin and norepinephrine reuptake inhibitor desvenlafaxine succinate in ovariectomized rodent models. Endocrinology 148:1376–1383 [DOI] [PubMed] [Google Scholar]
- Qiu J, Xue C, Bosch MA, Murphy JG, Fan W, Rønnekleiv OK, Kelly MJ 2007 Serotonin 5HT2c receptor signaling in hypothalamic POMC neurons: role in energy homeostasis in females. Mol Pharm acol 72:885–896 [DOI] [PubMed] [Google Scholar]
- Jasienska G, Ziomkiewicz A, Górkiewicz M, Pajak A 2005 Body mass, depressive symptoms and menopausal status: an examination of the “jolly fat” hypothesis. Women Health Iss 15:145–151 [DOI] [PubMed] [Google Scholar]
- Ahdieh HB, Wade GN 1982 Effects of hysterectomy on sexual receptivity, food intake, running wheel activity, and hypothalamic estrogen and progestin receptors in rats. J Comp Physiol Psychol 96:886–892 [PubMed] [Google Scholar]
- Asarian L, Geary N 2002 Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav 42:461–471 [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Shimizu H, Takahashi M, Sato N, Uehara Y, Fukatsu A, Negishi M, Kobayashi I, Kobayashi S 1990 The significance of decreased ambulatory activity during the generation by long-term observation of obesity in ovariectomized rats. Physiol Behav 47:155–159 [DOI] [PubMed] [Google Scholar]
- Thammacharoen S, Lutz TA, Geary N, Asarian L 2008 Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology 149:1609–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA 2009 Oestrogen modulates hypothalmic control of energy homeostasis through multiple mechanisms. J Neuroendocrinol 21:141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S 2001 Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-α null mice. Endocrinology 142:4751–4757 [DOI] [PubMed] [Google Scholar]
- Santollo J, Wiley MD, Eckel LA 2007 Acute activation of ER-α decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 293:R2194–R2201 [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N 2007 Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology 148:5656–5666 [DOI] [PubMed] [Google Scholar]
- Woods SC 2009 The control of food intake: behavioral versus molecular perspectives. Cell Metab 9:489–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy PH, Feuers RJ, Leakey JA, Nakamura K, Turturro A, Hart RW 1989 Effect of chronic caloric restriction on physiological variables related to energy metabolism in the male Fischer 344 rat. Mech Ageing Dev 48:117–133 [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G 2002 Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305–317 [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G 2005 Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520 [DOI] [PubMed] [Google Scholar]
- Kasai M, Tasker JG, Wuarin JP, Dudek FE 1993 Membrane properties of identified guinea-pig paraventricular neurons and their response to an opioid μ-receptor agonist: evidence for an increase in K+ conductance. J Neuroendocrinol 5:233–240 [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Joseph SA 1982 The distribution and cells of origin of ACTH(1-39)-stained varicosities in the paraventricular and supraoptic nuclei. Brain Res 232:365–374 [DOI] [PubMed] [Google Scholar]
- Alessi NE, Quinlan P, Khachaturian H 1988 MSG effects on β-endorphin and α-MSH in the hypothalamus and caudal medulla. Peptides 4:689–695 [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Takeda S, Liu X, Armstrong D, Karsenty G 2003 Monosodium glutamate-sensitive hypothalamic neurons contribute to the control of bone mass. Endocrinology 144:3842–3847 [DOI] [PubMed] [Google Scholar]
- Sylvia VL, Walton J, Lopez D, Dean DD, Boyan BD, Schwartz Z 2001 17β-Estradiol-BSA conjugates and 17β-estradiol regulate growth plate chondrocytes by common membrane associated mechanisms involving PKC dependent and independent signal transduction. J Cell Biochem 81:413–429 [DOI] [PubMed] [Google Scholar]
- Endoh H, Sasaki H, Maruyama K, Takeyama K, Waga I, Shimizu T, Kato S, Kawashima H 1997 Rapid activation of MAP Kinase by estrogen in the bone cell line. Biochem Biophys Res Commun 235:99–102 [DOI] [PubMed] [Google Scholar]
- Zallone A 2006 Direct and indirect estrogen actions on osteoblasts and osteoclasts. Ann NY Acad Sci 1068:173–179 [DOI] [PubMed] [Google Scholar]
- Liu BY, Wu PW, Bringhurst FR, Wang JT 2002 Estrogen inhibition of PTH-stimulated osteoclast foramtion and attachment in vitro: involvement of both PKA and PKC. Endocrinology 143:627–635 [DOI] [PubMed] [Google Scholar]
- Heino TJ, Chagin AS, Sävendahl L 2008 The novel estrogen receptor G-protein-coupled receptor 30 is expressed in human bone. J Endocrinol 197:1–6 [DOI] [PubMed] [Google Scholar]
- Opas EE, Scafonas A, Nantermet PV, Wilkening RR, Birzin ET, Wilkinson H, Colwell LF, Schaeffer JM, Towler DA, Rodan GA, Schmidt A 2009 Control of rat tail skin temperature regulation by estrogen receptor-β selective ligand. Maturitas 64:46–51 [DOI] [PubMed] [Google Scholar]
- Opas EE, Gentile MA, Kimmel DB, Rodan GA, Schmidt A 2006 Estrongenic control of thermoregulation in ERαKO and ERβKO mice. Maturitas 53:210–216 [DOI] [PubMed] [Google Scholar]
- Lebesgue D, Traub M, De Butte-Smith M, Chen C, Zukin RS, Kelly MJ, Etgen AM 2010 Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats. PLoS One 5:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]