Abstract
Rickets is a growth plate abnormality observed in growing animals and humans. Rachitic expansion of the hypertrophic chondrocyte layer of the growth plate, in the setting of hypophosphatemia, is due to impaired apoptosis of these cells. Rickets is observed in humans and mice with X-linked hypophosphatemia that is associated with renal phosphate wasting secondary to elevated levels of fibroblast growth factor-23. Rickets is also seen in settings of impaired vitamin D action, due to elevated PTH levels that increase renal phosphate excretion. However, mice with hypophosphatemia secondary to ablation of the renal sodium-dependent phosphate transport protein 2a (Npt2a), have not been reported to develop rickets. Because activation of the mitochondrial apoptotic pathway by phosphate is required for hypertrophic chondrocyte apoptosis in vivo, investigations were undertaken to address this paradox. Analyses of the Npt2a null growth plate demonstrate expansion of the hypertrophic chondrocyte layer at 2 wk of age, with resolution of this abnormality by 5 wk of age. This is temporally associated with an increase in circulating levels of 1,25-dihydroxyvitamin D. To address whether the receptor-dependent actions of this steroid hormone are required for normalization of the growth plate phenotype, the Npt2a null mice were mated with mice lacking the vitamin D receptor or were rendered vitamin D deficient. These studies demonstrate that the receptor-dependent actions of 1,25-dihydroxyvitamin D are required for maintenance of a normal growth plate phenotype in the Npt2a null mice.
The compensatory increase in circulating 1,25-dihydroxyvitamin D levels is responsible for the lack of rickets, despite the presence of hypophosphatemia, in Npt2a null mice.
Rickets is a feature of vitamin D deficiency and disorders associated with impaired renal phosphate reabsorption in humans and animals. Rickets is characterized by bowing of the extremities and expansion of the growth plate and is associated with osteomalacia due to hypomineralization of bone. The genetic engineering of mouse models of human disease as well as studies in naturally occurring mutant murine models has enabled characterization of the molecular basis for these skeletal changes.
Although rickets is a classic feature of vitamin D deficiency and vitamin D resistance syndromes, studies in both humans and mice have demonstrated that preservation of normal mineral ion homeostasis can compensate for the absence of 1,25-dihydroxyvitamin D or functional vitamin D receptors, resulting in a normal growth plate phenotype (1,2,3). These studies raised the question as to whether normalization of serum calcium, phosphate, or both was required to prevent the rachitic phenotype. Investigations in calcium sensor knockout mice, a murine model of familial hypocalciuric hypercalcemia, demonstrated a rachitic growth plate in the presence of hyperparathyroidism, the latter of which resulted in high circulating calcium and low circulating phosphate levels. Rendering these mice hypoparathyroid by matings with Gcm2 null mice resulted in hypocalcemia accompanied by normophosphatemia and a normal growth plate phenotype (4). Thus, these studies point to phosphate as an important regulator of growth plate maturation.
Insight into the molecular mechanism by which phosphate exerts its role on the growth plate has been provided by investigations in cellular models. Phosphate treatment of cultured avian and murine chondrocytes results in apoptosis in a dose- and maturation-dependent fashion (5,6,7,8). In primary murine chondrocytes, phosphate has been shown to activate the caspase-9-dependent mitochondrial apoptotic pathway in hypertrophic but not proliferative chondrocytes. In vivo studies demonstrate that administration of caspase-9 inhibitors to growing mice results in impaired hypertrophic chondrocyte apoptosis, leading to expansion of this layer of the growth plate, thus demonstrating a role for the mitochondrial apoptotic pathway in normal growth plate maturation (8).
Whereas rickets is a feature of hypophosphatemic disorders, including those secondary to deficiency of, or resistance to, vitamin D metabolites (9,10,11,12), elevations in serum fibroblast growth factor (FGF)-23 (13,14), and Fanconi’s syndrome (15), it has not been reported in mice lacking the renal sodium phosphate transporter, sodium-dependent phosphate transport protein 2a (Npt2a) (16). Investigations were therefore undertaken to characterize the growth plate phenotype in these mice and to elucidate compensatory factors that contribute to normalization of the growth plate in the setting of hypophosphatemia.
Materials and Methods
Animals.
Studies were approved by the institutional animal care committee. All mice studied were in the C57BL6/J background. Npt2a−/− mice were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were maintained in a virus- and parasite-free facility and subjected to a 12-h light, 12-h dark cycle. Mice were weaned at 18 d of age onto a diet containing 1% calcium and 0.44% phosphate with 2.4 IU/g vitamin D. To prevent the development of abnormal mineral ion homeostasis due to impaired 1,25-dihydroxyvitamin D action, mice were weaned onto a 2% calcium, 1.25% phosphate, and 20% lactose diet supplemented with 2 IU/g of vitamin D. To render mice vitamin D insufficient, this same diet, without supplemental vitamin D, was administered to mice housed in a UV-free environment.
Biochemical parameters
Serum calcium and phosphate were measured colorimetrically using kits from Genzyme Diagnostics (Cambridge, MA) and Stanbio (Boerne, TX), respectively, adapted to a plate reader. PTH was measured by a two-site sandwich mouse intact ELISA (Immunotopics, San Clemente, CA). FGF23 measurements were performed using an immunoradiometric assay from Kainos Laboratories International (Tokyo, Japan). Levels of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D were determined with a kit from DiaSorin (Stillwater, MN).
Tissue histology
Tibiae were fixed and decalcified in 10% formalin and 20% EDTA. Samples were processed, embedded in paraffin, and sectioned at 5 μm thickness. To permit evaluation of growth plate mineralization, nondecalcified femora were embedded in methylmethacrylate before sectioning. Evaluation of apoptosis was performed using the in situ cell death detection kit (Roche Diagnostics, Indianapolis, IN). In situ hybridization was performed using 35S-uridine 5-triphosphate-labeled antisense RNA probes as previously described (17). To permit evaluation of morphology, sections were stained with hematoxylin and eosin. Von Kossa staining was performed for detection of mineralized matrix. Quantitation of the changes in the length of the columns of cells expressing collagen X, osteopontin, and matrix metalloproteinase-13 was performed using digital images from at least three mice for each genotype, analyzed with Adobe Photoshop software (version 10.0.1; San Jose, CA).
Statistical analyses
Student’s t test was used to identify significant differences between wild-type and genetically engineered mice. P < 0.05 was considered significant.
Results
Mice lacking the renal sodium-dependent phosphate transporter, Npt2a, have been reported to exhibit a decrease in trabecular bone, accompanied by a delay in formation of the secondary ossification center, which reverses with age; however, a rachitic phenotype was not reported (16). This raised the question as to whether analogous abnormalities in the growth plate were present at early time points, which similarly resolved with age, and if so, what compensatory mechanism was responsible.
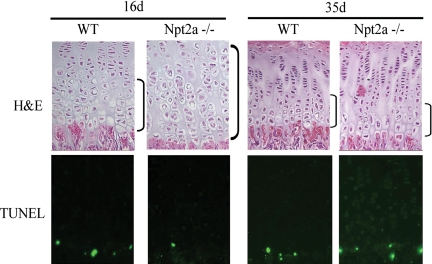
The growth plate phenotype of Npt2a−/− mice was examined at 16 and 35 d of age. As demonstrated in Fig. 1, a significant expansion of the hypertrophic chondrocyte layer of the growth plate is observed in the tibia of Npt2a knockout mice relative to wild-type littermates at 16 d of age; however, by 35 d of age, these findings are no longer apparent. Expansion of the hypertrophic chondrocyte layer at 16 d of age was accompanied by a decrease in apoptosis of late hypertrophic chondrocytes, whereas restoration of the normal growth plate phenotype at 35 d was accompanied by normalization of hypertrophic chondrocyte apoptosis in the Npt2a null mice.
Figure 1.
Growth plate phenotype of Npt2a null mice. Npt2a null mice demonstrate an expansion of the hypertrophic chondrocyte layer at 16 d of age, associated with a decrease in TUNEL-labeled nuclei. By 35 d of age, normalization of the growth plate phenotype, including apoptosis, is observed. Data are representative of that obtained with three mice of each age and genotype. TUNEL, Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling; H&E, hematoxylin and eosin.
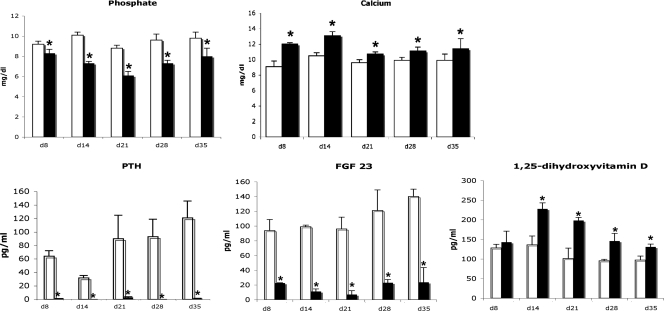
To determine whether changes in mineral ion homeostasis or hormones involved in their regulation could be implicated in this reversal of the growth plate phenotype, analyses were performed on samples obtained from 8 to 35 d of age. As demonstrated in Fig. 2, there was a significant decrease in serum phosphate levels in the Npt2a−/− mice at all time points examined. This was accompanied by hypercalcemia and suppression of PTH and FGF 23 levels at all time points. In contrast to hyp mice (model of X linked hypophosphatemia), which exhibit inappropriately normal levels of 1,25-dihydroxyvitamin D for the degree of hypophosphatemia, the 1,25-dihydroxyvitamin D levels of the Npt2a−/− mice were significantly increased from d 14 to d 35. Thus, investigations were performed to address whether the increases in 1,25-dihydroxyvitamin D levels contributed to the recovery of pathologic growth plate changes in Npt2a knockout mice.
Figure 2.
Mineral ions and hormone levels. Serum phosphate and calcium were evaluated from 8 to 35 d of age in Npt2a null (black bars) and WT mice (open bars), as were levels of PTH, FGF23, and 1,25-dihydroxyvitamin D. Data are based on results obtained in four to seven mice of each genotype at each time point. *, P < 0.05 vs. age-matched WT mice.
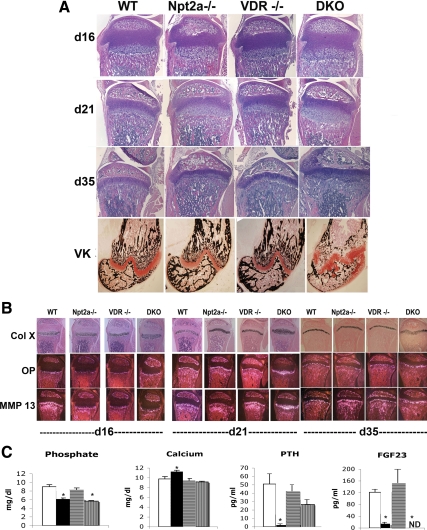
Npt2a null mice were mated with mice lacking the vitamin D receptor (VDR) to obtain mice lacking both Npt2a and the VDR. Mice were weaned onto a high-calcium, high-phosphate, lactose-supplemented diet at 18 d of age, which prevents the development of growth plate abnormalities in the VDR null mice (3,18). The growth plate phenotype of the single- and double-knockout mice was compared with that of controls before weaning (d 16) as well as at 21 and 35 d of age. Whereas the growth plate of the Npt2a−/− mice demonstrated a significant expansion of the hypertrophic chondrocyte layer at 16 and 21 d of age, by d 35, this expansion had regressed (Fig. 3A) despite persistent hypophosphatemia (Fig. 3C). Consistent with previous reports, the growth plate of the VDR knockout mice weaned onto this diet by 18 d of age was indistinguishable from that of the wild-type (WT) controls as was their serum phosphate at 35 d of age. In contrast, mice lacking both Npt2a and the VDR [double knockout (DKO)] demonstrated a significant expansion of the hypertrophic chondrocyte layer at all time points (Fig. 3A), despite the fact that, at 35 d of age, their serum phosphate level was not significantly different from that of the Npt2a null mice fed this same diet. At this age, the DKO mice exhibited significant expansion of the underlying metaphysis as well. In situ hybridization for markers of hypertrophic chondrocytes demonstrated an increase in the length of the column of hypertrophic chondrocytes expressing type X collagen in the Npt2−/− mice at d 16 and 21 (1.2- and 1.5-fold, respectively; P < 0.04 vs. WT) as well as an increase osteopontin at both these time points (1.5- and 1.8-fold, respectively; P < 0.04 vs. WT). However, by d 35, these changes had reversed, in association with normalization of the growth plate phenotype (Fig. 3B). Mice lacking both Npt2a and the VDR exhibited a marked increase in the length of the type X collagen and osteopontin expressing cell layers at 16 (1.3- and 1.5-fold, respectively; P < 0.05 vs. WT), 21 (1.8- and 2.6-fold, respectively; P < 0.04 vs. WT), and 35 (1.6- and 1.7-fold, respectively; P < 0.01 vs. WT) days of age (Fig. 3B). The domain of cells expressing matrix metalloproteinase-13 (collagenase 3) was also increased in the DKO mice at 21 and 35 d (2.1- and 1.9-fold, respectively; P < 0.03 vs. WT).
Figure 3.
Histological analyses of the growth plate of VDR and Npt2a null mice. A, Growth plate morphology was evaluated using H&E-stained sections of the tibia obtained at d 16, 21, and 35. Von Kossa staining of the distal femur was performed at 35 d of age to permit evaluation of mineralized matrix. VK, Von Kossa staining. B, In situ hybridization analyses were performed to evaluate markers of hypertrophic chondrocyte differentiation at 16, 21, and 35 d of age. Mice were weaned onto a 2% calcium, 1.25% phosphorus, and 20% lactose diet at 18 d of age. Col X, Collagen X; OP, osteopontin; MMP, matrix metalloproteinase. C, Serum biochemistries at 35 d of age. White bars, WT; black bars, Npt2a−/−; horizontally striped bars, VDR−/−; vertically striped bars, DKO. Data are representative of those obtained from three to six mice of each age and genotype. VDR−/−, VDR null; ND, not detectable.
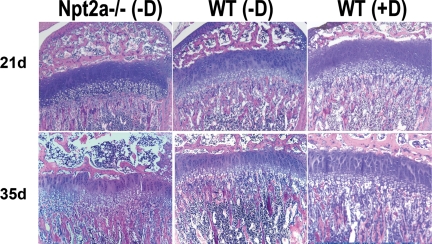
To determine whether the growth plate phenotype of the DKO was due to impaired ligand-dependent VDR action, pregnant females were placed in a UV-free environment at 17.5 d after conception and fed a high-calcium, high-phosphate, lactose-enriched diet lacking vitamin D. Pups born to these females were raised under similar vitamin D-deficient conditions and weaned onto the same diet at 18 d of age. This led to undetectable levels of 25 hydroxyvitamin D. However, the Npt2a−/− mice remained hypercalcemic (12.5 mg/dl; P < 0.01 vs. WT) and hypophosphatemic (6.0 mg/dl; P < 0.01 vs. WT) and had suppressed PTH levels (3.3 pg/ml; P < 0.01 vs. WT) relative to the WT controls raised under identical conditions. The growth plates of these pups (generation 1 vitamin D deficient) was examined at 3 and 5 wk of age. As shown in Fig. 4, the growth plate of the WT mice raised under vitamin D-deficient conditions, fed the calcium enriched diet, was indistinguishable from that of WT mice fed a similar diet that was vitamin D replete. However the growth plate of the Npt2a−/− mice demonstrated expansion of the hypertrophic chondrocyte layer both at 21 and 35 d of age. Thus, ligand-dependent actions of the VDR are required to prevent rachitic changes in the setting of hypophosphatemia associated with Npt2a ablation.
Figure 4.
Growth plate histology of vitamin D-deficient mice. Mice were born and bred under vitamin D-deficient conditions and fed a 2% calcium, 1.25% phosphorus, and 20% lactose vitamin D-deficient diet from 18 d of age. The growth plate phenotype of WT and Npt2a null mice raised under these conditions was examined and compared with that of WT mice fed the same diet supplemented with vitamin D. Data are ×10 magnifications of H&E stained sections and are representative of those obtained from three mice for each condition and time point.
Discussion
Npt2a null mice are hypophosphatemic due to a decrease in urinary phosphate reabsorption (16). Our findings demonstrate that, in these mice, hypophosphatemia is seen as early as 8 d of age and is associated with a compensatory decrease in FGF23 levels, demonstrating negative feedback regulation of this phosphaturic hormone in the setting of hypophosphatemia. Although circulating 1,25-dihydroxyvitamin D levels are not significantly elevated at 8 d of age, hypercalcemia, associated with suppressed PTH levels are observed. In contrast to the murine model of X-linked hypophosphatemia, the Npt2a null mice exhibit a normal compensatory increase in 1,25-dihydroxyvitamin D synthesis in response to hypophosphatemia, as early as 14 d postnatally. A reduction in circulating FGF23 levels likely contributes to this increase in 1,25-dihydroxyvitamin D; however, enhanced 1α-hydroxylase expression is also seen in response to hypophosphatemia.
This compensatory increase in 1,25-dihydroxyvitamin D signaling is an important distinguishing feature of the Npt2a null mice compared with other congential rachitic disorders associated with hypophosphatemia. In pseudovitamin D Deficiency Rickets and Hereditary Vitamin D Resistant Rickets, hypophosphatemia is associated with impaired 1,25-dihydroxyvitamin D synthesis and action, respectively (19). The increase in circulating FGF23 in X-linked hypophosphatemia leads to impaired vitamin D activation; thus, the levels of 1,25-dihydroxyvitamin D are inappropriate for the degree of hypophosphatemia observed. Similarly, 1,25-dihydroxyvitamin D levels are not increased appropriately for the degree of hypophosphatemia in Fanconi’s syndrome associated with rickets (15). Thus, it was intriguing that, despite persistence of hypophosphatemia, expansion of the hypertrophic chondrocyte layer of the Npt2a null growth plate observed at 16 d, resolves by 35 d. This was temporally associated with an increase in circulating levels of 1,25-dihydroxyvitamin D, which led us to address the hypothesis that the receptor-dependent actions of this steroid hormone play a role in normalization of the growth plate phenotype in the Npt2a null mice. This was confirmed by studies in mice lacking both Npt2a and the VDR, which demonstrate that the receptor-dependent actions of this steroid hormone are critical for the prevention of rickets in the presence of low circulating phosphate levels. This was further supported by investigations in first generation vitamin D-deficient Npt2a null mice. Extension of these analyses to second- and third-generation vitamin-deficient Npt2a null mice was not possible due to early mortality of the pups. However, the studies in the G1 vitamin D-deficient pups support our hypothesis that the receptor-dependent actions of 1,25-dihydroxyvitamin D can compensate for hypophosphatemia, resulting in the preservation of a normal growth plate phenotype in the Npt2a null mice.
In an analogous fashion, the maintenance of normal circulating phosphate levels prevents rachitic changes when 1,25 dihydroxyvitamin D action is impaired (3), demonstrating that phosphate and 1,25-dihydroxyvitamin D are, at least in part, functionally redundant in the maintenance of a normal growth plate phenotype. These findings have important implications for the approach to the treatment of hypophosphatemic rachitic disorders. Those associated with impaired renal phosphate reabsorption often present a therapeutic challenge. Administered phosphate is rapidly cleared; thus, it is not practicable to aim for normophosphatemia in these individuals, but rather to minimize the degree of hypophosphatemia. Although treatment with active vitamin D metabolites is currently an important part of the therapeutic regimen in X-linked hypophosphatemia, the rationale for its use is to optimize circulating phosphate levels and to prevent the development of, or attenuate hyperparathyroidism. The studies in the Npt2a null mice demonstrate that, at the time of development of the expanded hypertrophic chondrocyte layer and impaired apoptosis of these cells, serum phosphate levels are not significantly different from at 35 d when the growth plate has normalized. Furthermore, immunoreactive PTH levels are undetectable. Thus, these findings suggest that 1,25-dihydroxyvitamin D has effects on the growth plate that are not dependent on its metabolic or mineral ion regulating effects.
The role of the VDR and its ligand in the growth plate has been an area of active research. Investigations in mice with chondrocyte-specific ablation of the VDR or Cyp27b1, the enzyme required for 1,25-dihydroxyvitamin D synthesis, have demonstrated a role for the ligand-dependent actions of the VDR in growth plate chondrocytes in vivo. Absence of the VDR in chondrocytes decreases osteoclastogenesis and osteoblastic expression of FGF23, leading to an increase in serum phosphate and 1,25-dihydroxyvitamin D levels before weaning (20). This was not associated with alteration in the growth plate phenotype. Similarly, the growth plate of the mice with chondrocyte-specific ablation of Cyp27b1 remained normal postnatally (21). Interestingly, these studies demonstrate an important role for the VDR and its ligand, expressed in the growth plate, in paracrine and endocrine signaling rather than cell autonomous effects. These findings are consistent with the results of our investigations, which would have predicted that, in the absence of hypophosphatemia, a normal growth plate phenotype would be observed in these two models of impaired chondrocyte vitamin D signaling. It is of interest that, in the absence of VDR expression in proliferating chondrocytes, a decrease in FGF23 is observed, associated with elevations in 1,25-dihydroxyvitamin D and phosphate levels. Our studies in the Npt2a null mice demonstrate a suppression of FGF23 levels despite increases in circulating 1,25-dihydroxyvitamin D. These data suggest that although 1,25-dihydroxyvitamin D is an important inducer of FGF23 expression, low phosphate levels prevail, resulting in suppression of FGF23 when elevated 1,25-dihydroxyvitamin D and hypophosphatemia occur concurrently.
Footnotes
This work was supported by Grants R01 DK 46974 and P50 AR054086 from the National Institutes of Health.
Disclosure Summary: S.U.M., E.D.Z., and M.B.D. have nothing to disclose. Y.S. is currently an employee of Genzyme.
First Published Online August 4, 2010
For editorial see page 4599
Abbreviations: DKO, Double knockout; FGF, fibroblast growth factor; Npt2a, sodium-dependent phosphate transport protein 2a; VDR, vitamin D receptor; WT, wild type.
References
- Dardenne O, Prud'homme J, Glorieux FH, St-Arnaud R 2004 Rescue of the phenotype of CYP27B1 (1alpha-hydroxylase)-deficient mice. J Steroid Biochem Mol Biol 89–90:327–330 [DOI] [PubMed] [Google Scholar]
- Balsan S, Garabédian M, Larchet M, Gorski AM, Cournot G, Tau C, Bourdeau A, Silve C, Ricour C 1986 Long-term nocturnal calcium infusions can cure rickets and promote normal mineralization in hereditary resistance to 1,25-dihydroxyvitamin D. J Clin Invest 77:1661–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, Delling G, Demay MB 1998 Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology 139:4391–4396 [DOI] [PubMed] [Google Scholar]
- Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD 2003 Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. J Clin Invest 111:1029–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield K, Pucci B, Adams CS, Shapiro IM 2003 Induction of apoptosis in skeletal tissues: phosphate-mediated chick chondrocyte apoptosis is calcium dependent. Calcif Tissue Int 73:161–172 [DOI] [PubMed] [Google Scholar]
- Mansfield K, Rajpurohit R, Shapiro IM 1999 Extracellular phosphate ions cause apoptosis of terminally differentiated epiphyseal chondrocytes. J Cell Physiol 179:276–286 [DOI] [PubMed] [Google Scholar]
- Pucci B, Adams CS, Fertala J, Snyder BC, Mansfield KD, Tafani M, Freeman T, Shapiro IM 2007 Development of the terminally differentiated state sensitizes epiphyseal chondrocytes to apoptosis through caspase-3 activation. J Cell Physiol 210:609–615 [DOI] [PubMed] [Google Scholar]
- Sabbagh Y, Carpenter TO, Demay MB 2005 Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc Natl Acad Sci USA 102:9637–9642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB 1997 Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA 94:9831–9835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt A 1998 Osteomalacia and related disorders. In: Avioli LV, Krane SM, eds. Metabolic bone disease. San Diego: Academic Press; 327–386 [Google Scholar]
- Pettifor MP, Daniels ED 1997 Vitamin D deficiency and nutritional rickets in children. San Diego: Academic Press [Google Scholar]
- Rosen JF, Fleischman AR, Finberg L, Hamstra A, DeLuca HF 1979 Rickets with alopecia: an in-born error of vitamin D metabolism. J Pediatr 94:729–735 [DOI] [PubMed] [Google Scholar]
- Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H 2006 SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet 78:179–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD 2006 Pathogenic role of Fgf23 in Hyp mice. Am J Physiol 291:E38–E49 [DOI] [PubMed] [Google Scholar]
- Chesney RW, Rosen JF, Hamstra AJ, DeLuca HF 1980 Serum 1,25-dihydroxyvitamin D levels in normal children and in vitamin D disorders. Am J Dis Child 134:135–139 [DOI] [PubMed] [Google Scholar]
- Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS 1998 Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci USA 95:5372–5377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue MM, Demay MB 2002 Rickets in VDR null mice is secondary to decreased apoptosis of hypertrophic chondrocytes. Endocrinology 143:3691–3694 [DOI] [PubMed] [Google Scholar]
- Amling M, Priemel M, Holzman T, Chapin K, Rueger JM, Baron R, Demay MB 1999 Rescue of the skeletal phenotype of vitamin D receptor ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology 140:4982–4987 [DOI] [PubMed] [Google Scholar]
- Glorieux FH 1990 Calcitriol treatment in vitamin D-dependent and vitamin D-resistant rickets. Metabolism 39:10–12 [DOI] [PubMed] [Google Scholar]
- Masuyama R, Stockmans I, Torrekens S, Van Looveren R, Maes C, Carmeliet P, Bouillon R, Carmeliet G 2006 Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest 116:3150–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naja RP, Dardenne O, Arabian A, St. Arnaud R 2009 Chondrocyte-specific modulation of Cyp27b1 expression supports a role for local synthesis of 1,25-dihydroxyvitamin D3 in growth plate development. Endocrinology 150:4024–4032 [DOI] [PubMed] [Google Scholar]