Abstract
IGF-I is an anabolic factor that mediates GH and PTH actions in bone. Expression of skeletal Igf1 differs for inbred strains of mice, and Igf expression levels correlate directly with bone mass. Previously we reported that peroxisome proliferator-activated receptor-γ2 activation in bone marrow suppressed Igf1 expression and that peroxisome proliferator-activated receptor-γ2 activation-induced Nocturnin (Noc) expression, a circadian gene with peak expression at light offset, which functions as a deadenylase. In 24-h studies we found that Igf1 mRNA exhibited a circadian rhythm in femur with the lowest Igf1 transcript levels at night when Noc transcripts were highest. Immunoprecipitation/RT-PCR analysis revealed a physical interaction between Noc protein and Igf1 transcripts. To clarify which portions of the Igf1 3′ untranslated region (UTR) were necessary for regulation by Noc, we generated luciferase constructs containing various lengths of the Igf1 3′UTR. Noc did not affect the 170-bp short-form 3′UTR, but suppressed luciferase activity in constructs bearing the longer-form 3′UTR, which contains a number of potential regulatory motifs involved in mRNA degradation. C57BL/6J mice have low skeletal Igf1 mRNA compared with C3H/HeJ mice, and the Igf1 3′ UTR is polymorphic between these strains. Interestingly, the activity of luciferase constructs bearing the long-form 3′UTR from C57BL/6J mice were repressed by Noc overexpression, whereas those bearing the corresponding region from C3H/HeJ were not. In summary, Noc interacts with Igf1 in a strain- and tissue-specific manner and reduces Igf1 expression by targeting the longer form of the Igf1 3′UTR. Posttranscriptional regulation of Igf1 may be critically important during skeletal acquisition and maintenance.
Igf1 exhibits circadian expression pattern in skeleton, and nocturnin suppresses expression of Igf1 mRNA containing the long-form 3’UTR.
IGF-I is a single-chain, 70-amino acid residue polypeptide that circulates in relatively high concentrations (1). IGF-I has a critical role in bone accrual in both humans and mouse models (2,3,4,5,6,7,8,9). Circulating IGF-I predominantly arises from liver synthesis and can function in an endocrine manner in target tissues. But IGF-I is also synthesized locally, regulating cell fate and aspects of intermediary metabolism as well as skeletal growth and maintenance (1). In mouse models, overexpression of Igf1 in osteoblasts increases bone mass without affecting serum IGF-I levels, suggesting an important role of locally produced IGF-I in skeletal acquisition (8,10). Furthermore, even in inbred strains of mice, those that express different levels of skeletal Igf1 have similar differences in bone mass (11). For example, C3H/HeJ mice have high bone mass and increased skeletal Igf1 expression compared with C57BL/6J mice (11).
Although there are strong genetic determinants of IGF-I in murine bone, local regulation of IGF-I is also important, particularly during aging (12). In older mice, bone loss is associated with decreased Igf1 transcripts and enhanced Pparg expression (13,14). Furthermore, in a congenic mouse model of accelerated aging, B6.C3H.6T, we found that Igf1 in bone marrow and cortical bone was reduced and was associated with an increase in marrow adiposity (15,16). In addition, we previously showed that Igf1 expression is reduced in U-33 mesenchymal stromal cells transfected with a Pparg2 expression construct (U-33/γ2 cells) and treated with a peroxisome proliferator-activated receptor (PPAR)-γ agonist, rosiglitazone (13), a mechanism that could explain the increased marrow adiposity and decreased mineral density associated with aging. However, the underlying mechanism by which PPAR-γ suppresses Igf1 expression still remains elusive. To shed light on this issue, we performed microarray analysis on RNA extracted from rosiglitazone-treated U-33/γ2 cells and found Nocturnin (Noc; Ccrn4l) to be one of the most highly up-regulated genes (i.e. 28-fold) by PPAR-γ2 activation (14). Similarly, in the congenic B6.C3H-6T mouse strain, which has a gain-of-function polymorphism in Pparg and exhibits an accelerated aging phenotype, we observed markedly increased Noc expression, associated with impaired osteoblastogenesis and reduced Igf1 expression (15,16). Interestingly, Noc is also one of the most consistently up-regulated genes in tissues from aging rodents (17).
Noc is one of several peripheral circadian-regulated genes (18,19) whose production peaks around light offset (20) and functions as an mRNA deadenylase, and deadenylation is an important step in subsequent mRNA degradation (21,22). Because RNA-binding proteins are necessary for deadenylase recruitment and activity, it is likely that Noc binds to mRNAs by interacting with one or more RNA-binding proteins (23). The 3′ untranslated region (UTR) of Igf1 mRNA is encoded by exon 6 and the full-length (6.4 kb) 3′UTR contains at least three polyadenylation sites. Igf1 transcripts range in size from 0.8 to 7.5 kb due to variations in the length of the 3′UTR, resulting from usage of these different polyadenylation sites (24). Sequence analysis of the Igf1 3′UTR has revealed that use of the first polyadenylation site gives rise to an Igf1 transcript with a short-form 3′UTR (170 bp). The relative abundance of the short-form to the longer-form transcripts in the liver varies among studies, but several lines of evidence demonstrate that the short-form transcripts are more expressed than the longer form of transcripts in the liver (24,25,26). In contrast, the longer-form transcripts are more abundantly expressed than the short-form transcripts in extrahepatic tissues including bone (27,28). Previous data obtained in rat cells suggest that Igf1 transcripts containing the short form of the 3′ UTR are more stable than those containing the longer 6.4-kb 3′UTR (24). It is likely that the longer form of the Igf1 3′UTR contains multiple regulatory elements with which RNA-binding proteins may interact. Based on these findings, we hypothesized that Noc down-regulates Igf1 expression by destabilizing mRNA, through interaction with the 3′UTR of Igf1 transcripts, and that the longer 3′UTR containing transcripts are susceptible to recognition and deadenylation by Noc. Here we provide evidence that Igf1 expression exhibits a circadian rhythm in skeletal tissue, which is an antiphase to Noc expression, and that Noc regulates Igf1 expression in mouse bone by targeting the longer form of 3′UTR of Igf1 transcripts in a strain- and tissue-dependent manner.
Materials and Methods
Mice
Generation of Ccrn4ltmlBjc, which we refer to Nocturnin knockout mice (Noc−/− mice) has been described previously (29). Noc−/− mice lack the entire coding region of exon 3, which contains the entire catalytic domain. Noc protein is undetectable in these animals. The original mice were backcrossed onto a C57BL/6J background for at least seven generations. Colony environmental conditions included 14-h light,10-h dark cycles. All the animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of Maine Medical Center Research Institute.
Cell culture
MC3T3-E1 cells were purchased from American Type Culture Collection (Manassas, VA) and maintained in αMEM containing 10% fetal calf serum (FCS). Neonatal calvarial osteoblasts were collected from 7- to 10-d-old mice as described previously (15). Briefly, calvariae were digested five times with collagenase P and trypsin. Cells released from digests 2 through 5 were collected as primary calvarial osteoblasts and maintained in DMEM supplemented with 10% FCS and nonessential amino acids.
Western blot analysis
To prepare whole-cell lysates, bone marrow or liver was solubilized in RIPA (radioimmunoprecipitation assay) buffer (50 mm Tris, 150 mm NaCl, 1 mm EDTA, 1% Nonidet P-40, 0.25% Na-deoxycholate, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 2 μg/ml pepstatin A, 0.5 mm phenylmethylsulfonyl fluoride, and 1 mm dithiothreitol). Protein concentration was assessed by BCA protein assay kit (Thermo Scientific, Swedesboro, NJ), and an equivalent amount of protein were separated by SDS-PAGE and transferred electrophoretically to nitrocellulose membranes. Membranes were blocked in 5% BSA in Tris-buffered saline. Thereafter the membranes were immunoblotted with anti-Noc (1:2000) or anti-β-actin (1:2000) (Santa Cruz Biotechnology, Santa Cruz, CA; sc-47778) and developed with horseradish peroxidase-coupled antimouse IgG antibodies, followed by enhancement with SuperSignal West Dura extended duration substrate antibodies (Pierce Chemical Co., Rockford, IL). The rabbit polyclonal anti-Noc antibody was raised against a polypeptide corresponding to amino acids 64-429 present in mouse Noc.
Real-time PCR
Total RNA was prepared using RNeasy minikit (QIAGEN, Valencia, CA). cDNA was generated using a random hexamer and reverse transcriptase (Superscript III; Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Quantification of mRNA expression was carried out using an iQ SYBR Green supermix in a iQ5 thermal cycler and detection system (Bio-Rad Laboratories, Hercules, CA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or 18S rRNA was used as an internal standard control gene for all quantification. Specific primer sequences used in this study are listed in Supplemental Table 1, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org.
Generation of retrovirus
Full-length mouse Noc cDNA was inserted into the pBMN-IRES-GFP retroviral vector. Noc retroviruses were prepared using 293GPG packaging cells. Retroviral supernatant was collected at 48 and 72 h after transfection. MC3T3-E1 cells were infected with retrovirus in the presence of 10 μg/ml polybrene (Sigma, St. Louis, MO).
Short-hairpin RNA (shRNA)
A Noc-shRNA construct and control construct were purchased from Santa Cruz (sc-62698-SH). MC3T3-E1 cells stably expressing Noc-shRNA or control-shRNA were generated according to the manufacturer’s protocol.
Immunoprecipitation (IP)-RT-PCR
Cells were solubilized in IP buffer (150 mm NaCl; 50 mm Tris-HCl, pH 8.0; 1% Triton X-100; 2 μg/ml aprotinin; 2 μg/ml leupeptin; 2 μg/ml pepstatin A; 0.5 mm phenylmethylsulfonyl fluoride; and 1 mm dithiothreitol) and centrifuged. Two hundred units of ribonuclease inhibitor were added to supernatant. Supernatants were precleared with protein A/G-Sepharose (Santa Cruz) for 1 h. Precleared samples were centrifuged to discard the beads and incubated with antibody for 1 h, followed by immunoprecipitation with protein A/G-Sepharose (Santa Cruz) overnight. Samples were washed five times with IP buffer and then incubated with proteinase K for 30 min at 42 C. Total RNA was prepared from the immunoprecipitates, and RT-PCR for Igf1 was performed.
Constructs
Fragments of the Igf1 3′UTR from either C57BL/6J or C3H/HeJ mice were inserted into pMIR-REPORT luciferase vector (Ambion, Austin, TX). The fragments included: fragment 1 (1-170 bp 5′end of 3′UTR), fragment 2 (1-3217 bp 5′end of 3′UTR), fragment 3 (3275-5774 bp internal region of 3′UTR), fragment 4 (2698-6292 bp 3′ end of 3′UTR), fragment 5 (1-6295 bp full length 3′UTR) (see Fig. 5A). The sequence of 3′UTR fragments 1 and 2 are identical between C57BL/6J and C3H/HeJ strain, but there are polymorphic differences in fragments 3, 4, and 5 between these two strains. These polymorphic differences are shown (see Fig. 6A).
Figure 5.
Enhanced activity of luciferase fused with longer form of Igf1 3′UTR from C57BL/6J mouse in MC3T3-E1 cells stably expressing Noc-shRNA. A, Efficacy of Noc silencing was evaluated by analyzing the expression of Noc by real-time PCR (n = 3). B, Reporter assay using luciferase vector containing a variable length of 3′UTR of Igf1 mRNA from C57BL/6J (B6) mouse was performed using MC3T3-E1 cells stably expressing Noc-shRNA (MC3T3-shNoc) or Con-shRNA (MC3T3-shCon) (n = 4–6). Luciferase activity in MC3T3-shNoc cells was normalized to the luciferase activity in MC3T3-shCon cells. The fragments included: fragment 1, 1-170 bp 5′end of 3′UTR; fragment 2, 1-3217 bp 5′end of 3′UTR; fragment 3, 3275-5774 bp of 3′UTR; fragment 4, 2698-6292 bp of 3′UTR; fragment 5, 1-6295 bp full-length 3′UTR Values are expressed as the mean ± sem. *, P < 0.01; b, P < 0.01; c, P < 0.05.
Figure 6.
Noc does not change activity of luciferase-C3H/HeJ(C3H)-Igf1 3′ UTR constructs. A, Polymorphic differences in 3′UTR between C57BL/6J and C3H/HeJ mice. B and C, Reporter assay using luciferase vector containing a variable length of 3′UTR of Igf1 transcripts from C3H/HeJ (C3H) mouse was performed. MC3T3-E1 cells overexpressing either GFP (MC3T3-GFP) or Noc (MC3T3-GFP) (n = 4–6) (B) or MC3T3-E1 cells stably expressing Noc-shRNA (MC3T3-shNoc) or Con-shRNA (MC3T3-shCon) (n = 5–6) (C) were used. Luciferase activity in MC3T3-Noc cells and MC3T3-shNoc cells was normalized to the luciferase activity in MC3T3-GFP cells and MC3T3-shCon, respectively. The fragments included: fragment 3, 3275-5774 bp of 3′UTR; fragment 4, 2698-6292 bp of 3′UTR; fragment 5, 1-6295 bp full-length 3′UTR. D, Femurs were collected from 10-wk-old female C3H/HeJ mice at ZT 0, 5, 11, and 17, and RNA was isolated. Expression of Igf1 was analyzed by real-time RT-PCR (n = 3). For Igf1 detection, two sets of primers were used: exon 4 primer and exon 6 primer. Gapdh was used as an internal control for quantification. White and black bar represents light and dark cycles, respectively. E, Expression of longer form of Igf1 was analyzed using exon 6 primer in femurs collected from C57BL/6J and C3H/HeJ mice at ZT 0, 5, 11, and 17 (n = 3). Values are expressed as the mean ± sem. Values are expressed as the mean ± sem. a, P < 0.05 vs. ZT11; *, P < 0.01; **, P < 0.05.
Transient transfection and luciferase assay
Transient transfection was carried out using FuGENE 6 (Roche Applied Science, Indianapolis, IN) following the manufacturer’s protocol. The total amount of DNA added to each well was equalized using an empty vector. Transfection efficiency was normalized by cotransfection with pRL-CMV-Renilla luciferase construct (Promega, Madison, WI). Luciferase assay was performed in duplicate according to the protocol of the dual-luciferase reporter assay system (Promega). Briefly, 2 d after transfection, cells were lysed and luciferase activity was determined using specific substrates in a luminometer.
IGF-I concentration in conditioned media and bone marrow
MC3T3-E1 cells overexpressing either green fluorescent protein (GFP) or wild-type Noc were maintained until 80% confluent. The culture media were then switched to αMEM containing 0.1% BSA. Conditioned media were collected at 24 h, and IGF-I levels were measured by RIA (ALPCO, Windham, NH). Remaining cells were lysed, and total protein content was measured. IGF-I concentration in the conditioned media was normalized to the amount of the total protein from remaining cells.
Bone marrow was collected from tibia by spinning and was centrifuged at 10,000 × g for 5 min. Supernatants were collected as bone marrow fluid, and IGF-I levels were measured by an RIA (ALPCO) reported previously by our group (15,16). Remaining pellets were lysed, and total protein content was measured. IGF-I amount in the bone marrow fluid was normalized to the amount of the total protein in the remaining pellets.
Statistical analysis
All data are expressed as the mean ± sem. Results were analyzed for statistically significant differences using Student’s t test or ANOVA followed by Bonferroni multiple comparison post hoc test. Statistical significance was set at P < 0.05.
Results
Igf1 transcripts exhibit an antiphase circadian expression profile to Noc transcripts in femur
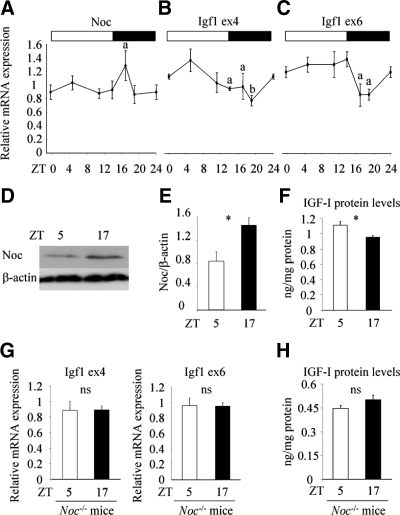
To investigate the mechanism whereby the circadian-regulated gene, Noc, affects the Igf1 expression in the skeleton, we analyzed the circadian expression profile of Igf1 in the femur of 10-wk female C57BL/6J mice. Primers to detect Igf1 expression were designed as follows: exon 4 primer sets will amplify all the transcripts of Igf1 gene; exon 6 primer sets will amplify only RNA containing the longer 3′UTR region of exon 6 and will not detect the 170-bp short-form transcripts. Noc exhibited a circadian expression pattern with peak expression around light offset, similar to the expression pattern in other tissues, including liver and kidney (Fig. 1A) (20). Consistent with this, Noc protein levels in the bone marrow were greater at nighttime [Zeitgeber time (ZT) 17] compared with daytime (ZT 5) (Fig. 1, D and E). Interestingly, Igf1 transcripts in bone analyzed by using an exon 4 primer also showed a circadian expression profile, with lowest levels at nighttime, and this expression pattern exhibited an antiphase pattern to Noc expression (Fig. 1B). A similar trend was observed when the longer form of Igf1 transcript expression was analyzed by using exon 6 primer (Fig. 1C). Because the longer-form transcripts are more abundantly expressed in bone compared with the short form (28), these data suggest that the circadian expression of Igf1 is at least partially explained by the rhythmic expression profile of the longer form of the Igf1 transcripts. Importantly, the reduction in Igf1 expression at nighttime (ZT 17) was associated with decreased IGF-I protein concentrations in bone marrow fluid compared with daytime (ZT 5) (Fig. 1F). To better understand the role of Noc in the regulation of circadian Igf1 expression profiles, we analyzed Igf1 expression in the femur of Noc−/− mice at daytime (ZT5) and nighttime (ZT17). Both total and longer form of Igf1 expression was comparable between these two time points (Fig. 1G). In addition, IGF-I protein levels in bone marrow fluid were not different between daytime (ZT5) and nighttime (ZT17) in Noc−/− mice (Fig. 1H). These data indicate that Noc plays an important role in the regulation of Igf1 circadian expression in vivo.
Figure 1.
An antiphase circadian expression pattern between Noc and Igf1 in femur. A–C, Femurs were collected from 10-wk-old female wild-type mice at ZT 0, 5, 11, 14, 17, and 19, and RNA was isolated. Expression of Noc (A) and Igf1 (B and C) was analyzed by real-time RT-PCR (n = 3). For Igf1 detection, two sets of primers were used; exon 4 primer (B) and exon 6 primer (C). Gapdh was used as an internal control for quantification. White and black bar represents light and dark cycles, respectively. D and E, Whole-cell lysates were collected from bone marrow of the tibia at daytime (ZT 5) and nighttime (ZT 17) and Noc protein expression was analyzed by Western blot analysis (D). Expression of Noc was quantified by normalizing to the expression levels of β-actin (E) (n = 3). F, Bone marrow was collected from the tibia at daytime (ZT 5) and nighttime (ZT 17) and was centrifuged. The supernatant was used as bone marrow fluid and IGF-I protein levels were analyzed by RIA. The amount of IGF-I in the bone marrow fluid was normalized to the protein amount of the remaining pellet (n = 3). G, Femurs were collected from 12-wk-old Noc−/− mice at daytime (ZT 5) and nighttime (ZT 17) and RNA was isolated. Expression of Igf1 was analyzed by real-time RT-PCR (n = 3). For Igf1 detection, two sets of primers were used; exon 4 primer and exon 6 primer. Gapdh was used as an internal control for quantification. H, Bone marrow was collected from the tibia of 12-wk-old Noc−/− mice at daytime (ZT 5) and nighttime (ZT 17) and was centrifuged. The supernatant was used as bone marrow fluid and IGF-I protein levels were analyzed by RIA. The amount of IGF-I in the bone marrow fluid was normalized to the protein amount of the remaining pellet (n = 3). Values are expressed as the mean ± sem (n = 3). The figure shown is the representative of three independent experiments. a, P < 0.05 vs. ZT 19 (A) vs. ZT 5 (B) vs. ZT 5, 11, and 14 (C); b, P < 0.01 vs. ZT 5 (B); *, P < 0.05. ns, Not significant.
Noc down-regulates Igf1 expression
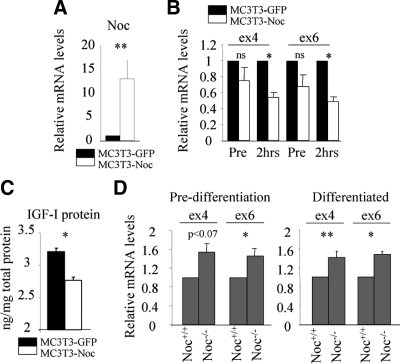
Antiphase circadian expression profiles between Noc and Igf1 led us to speculate that Noc negatively regulates Igf1 expression in bone. To answer this question, we overexpressed Noc in an osteoblastic cell line, MC3T3-E1, and analyzed Igf1 expression. Noc overexpression increased Noc mRNA levels more than 10-fold in these cells (Fig. 2A). Although Igf1 mRNA levels in MC3T3-E1 cells overexpressing Noc (MC3T3-Noc) tended to be lower compared with control MC3T3-E1 cells overexpressing GFP (MC3T3-GFP), this decrease did not reach statistical significance (Fig. 2B). However, when the cells were treated with actinomycin D for 2 h, which inhibits new RNA synthesis, the decrease in Igf1 expression in MC3T3-Noc cells became significantly different compared with MC3T3-GFP cells (Fig. 2B). Importantly, overexpression of Noc decreased IGF-I protein levels in the osteoblast conditioned media (Fig. 2C). Consistent with this, Igf1 expression was enhanced in Noc-deficient calvarial osteoblasts both before and after treatment with osteogenic media compared with wild-type cells (Fig. 2D).
Figure 2.
Noc regulates Igf1 expression in a negative direction. A, Noc was overexpressed in MC3T3-E1 cells using retrovirus vector. Efficacy of overexpression was evaluated by analyzing the expression of Noc by real-time RT-PCR (n = 3). B, MC3T3-E1 cells overexpressing GFP (MC3T3-GFP) or Noc (MC3T3-Noc) were treated with actinomycin D (10 μm) for 2 h. Expression of Igf1 was analyzed before (pre) and 2 h after treatment by real-time RT-PCR using exon 4 and exon 6 primers (n = 4). C, IGF-I protein concentration was analyzed in conditioned media from MC3T3-GFP or MC3T3-Noc cells and normalized to the amount of the protein in remaining cells (n = 4). D, Igf1 expression in primary calvarial osteoblasts before (predifferentiation) and after (differentiated) the treatment of osteogenic media (10 mm of β-glycerophosphate and 50 μg/ml of ascorbic acid in αMEM containing 10% FCS) from Noc+/+ or Noc−/− mice was determined by real-time RT-PCR using exon 4 and exon 6 primers (n = 4). 18S rRNA was used as an internal control for quantification. Values are expressed as the mean ± sem. *, P < 0.01; **, P < 0.05. ns, Not significant.
Noc protein forms a protein-RNA complex with Igf1 transcripts
Based on these findings, we speculated that Noc regulates Igf1 expression through interaction with Igf1 transcripts. To test this theory, lysates from MC3T3-Noc-Flag or control MC3T3-GFP cells were immunoprecipitated with anti-Flag antibody, and RNA was collected from immunoprecipitates. RT-PCR for Igf1 using ex4 primer sets was then performed to detect whether there is protein-RNA complex between the Noc-Flag protein and Igf1 transcripts. As shown in Fig. 3, IP/RT-PCR experiments revealed that Noc protein forms a protein-RNA complex with Igf1 transcripts. Type I collagen and GAPDH were used as negative controls for Noc recognition of RNA.
Figure 3.
Noc targets Igf1 mRNA. Lysates from MC3T3-E1 cells overexpressing either GFP or Noc-Flag were immunoprecipitated with anti-Flag antibody. Immunoprecipitants were recovered and RNA was collected. RT-PCR for Igf1 using exon 4 primer was performed (IP/RT-PCR). Whole-cell lysates (WCL) and water were used as a positive and negative control for Igf1 PCR, respectively. GAPDH and type I collagen were used as negative controls for Noc recognition of RNA. Figures shown represent at least three independent experiments. IP, Immunoprecipitation.
Noc overexpression reduces activity of luciferase-C57BL/6J-Igf1 3′UTR constructs
To investigate which region of the Igf1 3′UTR is required for regulation by Noc, luciferase reporter constructs containing variable lengths of the 3′UTR of Igf1 transcripts from C57BL/6J mouse was generated. MC3T3-Noc and MC3T3-GFP cells were transfected with these constructs and luciferase activity in MC3T3-Noc cells was normalized to the luciferase activity in MC3T3-GFP cells. Luciferase activity from constructs containing fragment 1, a 170-bp short form of 3′UTR, was not significantly affected by Noc overexpression (Fig. 4, A and B). In contrast, constructs containing fragment 2, which contains the approximately 3 kb 5′end of the 3′UTR, showed a modest but a significant decrease in luciferase activity compared with the empty vector (Fig. 4B). Luciferase constructs containing fragment 3, which includes several potential regulatory elements, resulted in a 40% decrease in luciferase activity, compared with the empty vector (Fig. 4B). Similarly, constructs containing fragments 4 and 5, which contain fragment 3, also showed a significant reduction in luciferase activity (Fig. 4B).
Figure 4.
Reduced activity of luciferase fused with longer form of Igf1 3′UTR from C57BL/6J mouse in MC3T3-E1 cells overexpressing Noc. A, Diagram of 3′UTR of Igf1 transcripts. B, Reporter assay using luciferase vector containing a variety length of 3′UTR of Igf1 transcripts from C57BL/6J (B6) mouse was performed using MC3T3-E1 cells overexpressing either GFP (MC3T3-GFP) or Noc (MC3T3-GFP) (n = 4–6). Luciferase activity in MC3T3-Noc cells was normalized to the luciferase activity in MC3T3-GFP cells. The fragments included: fragment 1, 1-170 bp 5′end of 3′UTR; fragment 2, 1-3217 bp 5′end of 3′UTR; fragment 3, 3275-5774 bp of 3′UTR; fragment 4, 2698-6292 bp of 3′UTR; fragment 5, 1-6295 bp full-length 3′UTR. Values are expressed as the mean ± sem. a, P < 0.001; c, P < 0.05.
Silencing of Noc enhances activity of luciferase-C57BL/6J-Igf1 3′UTR constructs
Next, we analyzed the effect of Noc knockdown on Igf1 3′UTR activity using MC3T3-E1 cells stably expressing Noc-shRNA (MC3T3-shNoc). shNoc construct decreased Noc mRNA levels by 70% compared with control shRNA (Fig. 5A). MC3T3-shNoc and MC3T3-E1 cells expressing control shRNA (MC3T3-shCon) were transfected with luciferase constructs containing 3′UTR from C57BL/6J mouse, and luciferase activity in MC3T3-shNoc cells was normalized by the luciferase activity in MC3T3-shCon cells. Like the empty vector, the construct containing the shortest Igf1 3′UTR (fragment 1) was not sensitive to Noc knockdown. In contrast to MC3T3-Noc cells, luciferase activity in MC3T3-shNoc cells transfected with fragment 2, 3, 4, or 5 showed an increase in luciferase activity compared with the cells transfected with empty vector (Figs. 4A and 5B). Together with our data on Noc overexpression, these lines of evidence suggest that Noc recognizes the longer form of Igf1 transcripts and mediates the down-regulation of expression.
Noc does not change the activity of luciferase-C3H/HeJ-Igf1 3′ UTR constructs
Previously we reported that the C3H/HeJ mouse, which has high bone mass, had significantly greater skeletal Igf1 expression than C57BL/6J due to both cis- and trans-acting heritable determinants (11). Because there are several polymorphic differences in the 3′UTR between C3H/HeJ and C57BL/6J, we asked whether one mechanism responsible for this difference Igf1 could involve a differential sensitivity of the C3H/HeJ and C57BL/6J 3′UTR response to Noc. The polymorphic differences between C3H/HeJ and C57BL/6J are illustrated in Fig. 6A. Hence, we generated luciferase constructs of varying 3′UTR lengths for C3H/HeJ mouse. Because fragments 1 and 2 are common between these two strains, fragments 3, 4, and 5 from C3H/HeJ mouse were used in this study. Luciferase activity of these constructs was analyzed using the Noc overexpression and knockdown systems. In contrast to the data obtained using luciferase-C57BL/6J-Igf1 3′UTR constructs, luciferase activity from luciferase-C3H/HeJ-Igf1 3′ UTR constructs containing fragment 3, 4, and 5 was not significantly affected by Noc overexpression (compare Fig. 6B with Fig. 4B). There was a trend toward enhanced activity of luciferase-C3H/HeJ fragment 3 in Noc knockdown cells, by approximately 40% compared with the activity of empty constructs (P = 0.18) (Fig. 6C). Importantly, this increase was not as great as the increase in luciferase-C57BL/6J-fragment 3 (∼90% increase) in Noc knockdown cells (compare Fig. 6C with Fig. 5B). Luciferase activity from constructs containing fragments 4 and 5 was not affected by Noc knockdown (Fig. 6C). These lines of evidence led us to ask whether the difference in Noc sensitivity between C57BL/6J and C3H/HeJ mice could cause an alteration in the Igf1 circadian expression profile. As shown in Fig. 6D, Igf1 showed a similar trend of circadian expression pattern in C3H/HeJ mice as observed in C57BL/6J mice, but the magnitude of circadian rhythm in C3H/HeJ mice was not as great as that in C57BL/6J mice (45% decrease of total Igf1 expression between ZT 5 and ZT 19 in C57BL/6J vs. 30% decrease of total Igf1 expression between ZT 11 and ZT 17 in C3H/HeJ mice). In addition, expression of the longer form of Igf1 transcripts in C3H/HeJ mice was greater than that in C57BL/6J mice at all time points analyzed (Fig. 6E). Thus, increased skeletal Igf1 expression in C3H/HeJ mice may in part be caused by the differential responsiveness of 3′UTR to Noc.
Igf1 does not exhibit circadian expression patterns in the liver
Because the liver is the main source of circulating IGF-I and liver-derived circulating IGF-I may also affect skeletal mass (4), we next investigated whether Noc has any effects on Igf1 expression patterns in the liver. As previously reported, Noc showed a circadian expression pattern in liver with peak expression around light offset (Fig. 7, A, D, and E). Unlike in the femur, however, Igf1 expression did not show any circadian expression pattern in the liver (Fig. 7, B and C), suggesting that Noc regulates the circadian expression of Igf1 in a tissue-specific manner. Consistent with this, serum IGF-I levels were not different between Noc+/+ and Noc−/− mice (Fig. 7F). The difference in circadian expression profiles for Igf1 between liver and femur may be caused by the predominance of short-form transcripts in liver (24,25), which is not regulated by Noc.
Figure 7.
Igf1 does not exhibit circadian expression pattern in liver. A–C, Liver was collected from 10-wk-old wild-type mice at ZT 0, 5, 11, 14, and 17, and RNA was isolated. Expression of Noc (A) and Igf1 (B and C) was analyzed by real-time RT-PCR (n = 3). For Igf1 detection, two sets of primers were used: exon 4 primer (B) and exon 6 primer (C). Gapdh was used as an internal control for quantification. White and black bar represents light and dark cycles, respectively. D and E, Whole-cell lysates were collected from liver at ZT 0 and ZT 14, and Noc protein expression was analyzed by Western blot analysis (D). Expression of Noc was quantified by normalizing to the expression levels of β-actin (n = 3) (E). F, Serum IGF-I levels were analyzed from Noc+/+ or Noc−/− mice at 8 wk old. Values are expressed as the mean ± sem (n = 3). The figure shown is the representative of three independent experiments. a, P < 0.01 vs. ZT 0, 5, and 17; *, P < 0.05. ns, Not significant.
Discussion
Circadian networks play critical roles in the regulation of the skeletal metabolism with evidence that two of the most common proteins, type I collagen, and osteocalcin exhibit periodicity during a 24-h cycle (30). Additionally, genetic disruption of circadian clock genes in osteoblasts such as period and cryptochrome have been shown to result in a high bone mass phenotype (31). Thus, skeletal metabolism is clearly under the control of circadian networks. Noc was originally identified in Xenopus retina as a circadian regulated gene, which has a peak expression at around light offset (20,32). Noc functions as a deadenylase, which initiates degradation of mRNA by targeting the poly-A tails in the 3′UTR of transcripts (21). A list of target transcripts deadenylated by Noc is incomplete, but several metabolic genes including Pparg appear to be regulated by Noc (29). In fact, Noc−/− mice exhibit protection from high-fat diet-induced obesity, in part by altering the genes regulating lipid metabolism (29). In this study, we provide the first evidence that Igf1 mRNA exhibits circadian rhythmicity in bone and that Igf1 mRNA is likely a target for Noc.
IGF-I is very abundant in the skeleton of rodents and humans in which it plays a critical role in bone acquisition and maintenance (8). In mice, and probably in humans, levels of IGF-I in bone vary considerably due to heritable, environmental, and nutritional determinants (1). However, the mechanisms whereby local Igf1 expression is regulated in bone are not well described. Emerging evidence emphasizes the critical role of the 3′UTR in the regulation of transcript stability and translation by microRNAs and deadenylation (23,33). With regard to the posttranscriptional regulation by deadenylation, exon 6 of Igf1 contains at least three polyadenylation sites. Use of the first polyadenylation site produces the 170-bp short-form 3′UTR, whereas use of the most downstream polyadenylation site results in production of the 6.4-kb long-form 3′UTR. Based on our previous observations that reduction of Igf1 expression by PPAR-γ2 activation is accompanied by increased expression of Noc, we speculated that Noc is one such factor regulating Igf1 mRNA. In Noc-overexpressing MC3T3-E1 cells, there was a reduction of Igf1 levels after transcription arrest with actinomycin D. These data imply that Noc may negatively regulate Igf1 transcript stability. Basal levels of Igf1 expression were not significantly different between MC3T3-E1 cells overexpressing Noc or GFP, although IGF-I protein levels in conditioned media showed a decrease from MC3T3-E1 cells overexpressing Noc. Because deadenylation can regulate translation as well as transcript stability (23), it is possible that Noc also decreases Igf1 translation, resulting in decreased IGF-I protein production in these cells.
Interestingly, the luciferase-3′ UTR assays revealed that the 170-bp short-form 3′UTR was not affected by Noc overexpression or knockdown, whereas Noc overexpression reduced the luciferase activity of constructs containing the longer form of the Igf1 3′UTR. The 3275-5774 region of the 3′UTR (fragment 3) appears to be particularly sensitive to Noc overexpression or knockdown (Figs. 4B and 5B). The full-length 3′UTR also exhibited a similar change as the 3275-5774 region of 3′UTR, but the reduction in luciferase activity by Noc overexpression was not as great as that of the 3275-5774 region of the 3′UTR. The basal level luciferase activity is lower in the full-length 3′UTR containing vector compared with that of the 3275-5774 3′UTR containing vector (data not shown). It is likely that the full-length 3′UTR contains additional regulatory regions such as binding sites for microRNAs, which could reduce the stability of transcripts. Therefore, it is possible that other trans-acting factors could affect the stability of the full-length 3′UTR and mask the effect of Noc. In addition, IP/RT-PCR analysis showed that Noc formed a protein-RNA complex with Igf1 transcripts. These data support our hypothesis that Igf1 is among the transcripts recognized by Noc, although we have to be cautious in interpreting the IP/RT-PCR data because Noc can bind to a number of mRNAs. Several RNA-binding proteins have been shown to recruit deadenylases and enhance deadenylase activity (23), and enhanced deadenylation is a frequent consequence of the interaction of microRNA/RNA-induced silencing complex complexes with 3′UTRs (34). It is likely that the longer-form 3′UTR contains sequence motifs mediating the interaction of RNA-binding proteins, necessary for Noc recruitment and effects on Igf1 transcripts.
We previously reported that C3H/HeJ mice have high bone mass together with increased IGF-I levels in bone and the circulation (11). In this study we have expanded our understanding regarding the differential regulation of Igf1 between these two strains. We found that there are polymorphic differences in the long form of 3′UTR in Igf1 transcripts between these strains, which might be responsible for the differential sensitivity to Noc. The 3′UTR of Igf1 in bone from C3H/HeJ mice was protected from Noc compared with C57BL/6J mice. Consistent with this, the magnitude of Igf1 circadian rhythmicity was lower in C3H/HeJ mice, and expression of the long-form Igf1 was enhanced in C3H/HeJ vs. C57BL/6J mice. Nevertheless, it is still unclear how genetic polymorphic differences contribute to strain difference in responsiveness to Noc. We performed a structural analysis of the 3′UTR of Igf1 transcripts and found that the 3′UTR of C3H/HeJ mice may form a loop structure at the polymorphic region of a 22T run, which was not detected in C57BL/6J mice. This alteration in three-dimensional structure may contribute to differences in Noc and/or RNA-binding protein recognition of Igf1 transcripts. However, further experiments are needed to support this hypothesis.
Although Noc exhibits a circadian expression profile in liver, surprisingly Igf1 does not. Consistent with that, serum IGF-1 levels, which are mainly derived from the liver, were not different between Noc−/− mice and wild-type controls. Because Noc decreases the activity of a luciferase vector fused with the long-form 3′UTR, not the one with the 170-bp short form, the difference in the regulation of Igf1 expression by Noc between liver and bone may be explained by the differential abundance of these transcript isoforms (i.e. predominant short form in liver vs. predominant long form in most peripheral tissues) between these two organs. Nevertheless, this does not explain the reason why the longer-form of Igf1 transcripts in liver did not show a circadian expression profile. Igf1 expression in liver is regulated by nutrient status as well as GH and other factors. Thus, multiple determinants probably affect Igf1 expression in liver, which may mask the predominant effect of Noc on the long-form transcripts.
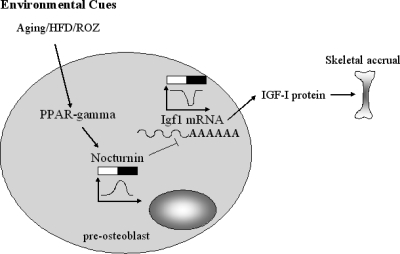
The phenotypic impacts of Noc regulation of Igf1 on the skeleton remain to be determined. Because IGF-I is anabolic to skeleton, it is possible that Noc regulates bone mass in a negative direction. Indeed, Noc-deficient osteoblasts show enhanced osteoblastogenesis and Noc−/− mice exhibit a high bone mass phenotype (35). Interestingly, the C3H/HeJ mouse with high bone mass and increased Igf1 mRNA transcripts in osteoblasts has markedly suppressed Noc expression (36). Activation of PPAR-γ regulates mesenchymal cell fate toward the adipogenic lineage and away from osteoblasts (37,38,39,40,41). In line with this, rosiglitazone treatment reduces IGF-I and bone mass but increases marrow adiposity in humans and mouse models (41,42,43). Because Noc is induced by PPAR-γ2 activation (14), the PPAR-γ-Noc-IGF-I network may partially explain the mechanism by which PPAR-γ reduces bone mass and alters lineage allocation (Fig. 8). These changes are often part of a skeletal aging phenotype that is characterized by reduced tissue IGF-I, low bone mass, and enhanced marrow adiposity. Hence, understanding the mechanisms that lead to deadenylation of IGF-I is essential for determining the role of this growth factor in the aging process.
Figure 8.
PPAR-γ-Noc-IGF-I network in preosteoblasts. IGF-I is a pivotal factor for skeletal accrual. PPAR-γ activated by a number of environmental cues including aging, high-fat diet (HFD), and PPAR-γ agonist, rosiglitazone (ROZ) enhances Noc expression. Noc suppresses Igf1 mRNA expression, probably in a circadian manner. Thus, bone loss caused by PPAR-γ activation may be in part mediated by the activation of Noc, resulting in reduced IGF-I expression. White and black bar represents light and dark cycles, respectively.
In summary, several lines of evidence demonstrate that Noc is a critical factor regulating Igf1 expression in bone. The posttranscriptional regulation of this critically important skeletal growth factor by Noc provides new insights into the regulation of Igf1 in bone and the potential downstream effects of altered time keeping on skeletal mass.
Supplementary Material
Acknowledgments
The authors thank A. Breggia, V. DeMambro, and S. Bornstein for discussion and mouse genotyping (Maine Medical Center Research Institute).
Footnotes
This work was supported by National Institutes of Health Grants AR 53853 and AR45433 (to C.J.R. and M.L.A.) and GM076626 (to C.B.G.).
Disclosure Summary: The authors have nothing to declare.
First Published Online August 4, 2010
Abbreviations: FCS, Fetal calf serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFP, green fluorescent protein; IP, immunoprecipitation; Noc, nocturnin; PPAR, peroxisome proliferator-activated receptor; shRNA, short-hairpin RNA; UTR, untranslated region; ZT, Zeitgeber time.
References
- Kawai M, Rosen CJ 2009 Insulin-like growth factor-I and bone: lessons from mice and men. Pediatr Nephrol 24:1277–1285 [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rosen CJ, Visser M, Hannan MT, Harris T, Wilson PW, Kiel DP 1998 Association between insulin-like growth factor I and bone mineral density in older women and men: the Framingham Heart Study. J Clin Endocrinol Metab 83:4257–4262 [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A 1993 Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75:59–72 [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D 2002 Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest 110:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL 2002 Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem 277:44005–44012 [DOI] [PubMed] [Google Scholar]
- He J, Rosen CJ, Adams DJ, Kream BE 2006 Postnatal growth and bone mass in mice with IGF-I haploinsufficiency. Bone 38:826–835 [DOI] [PubMed] [Google Scholar]
- Bikle D, Majumdar S, Laib A, Powell-Braxton L, Rosen C, Beamer W, Nauman E, Leary C, Halloran B 2001 The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res 16:2320–2329 [DOI] [PubMed] [Google Scholar]
- Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, Fagin JA, Clemens TL 2000 Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology 141:2674–2682 [DOI] [PubMed] [Google Scholar]
- Garnero P, Sornay-Rendu E, Delmas PD 2000 Low serum IGF-1 and occurrence of osteoporotic fractures in postmenopausal women. Lancet 355:898–899 [DOI] [PubMed] [Google Scholar]
- Elis S, Courtland HW, Wu Y, Rosen CJ, Sun H, Jepsen KJ, Majeska RJ, Yakar S 2010 Elevated serum levels of IGF-1 are sufficient to establish normal body size and skeletal properties, even in the absence of tissue IGF-1. J Bone Miner Res 25:1257–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CJ, Churchill GA, Donahue LR, Shultz KL, Burgess JK, Powell DR, Ackert C, Beamer WG 2000 Mapping quantitative trait loci for serum insulin-like growth factor-1 levels in mice. Bone 27:521–528 [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Dimai HP, Vereault D, Donahue LR, Beamer WG, Farley J, Linkhart S, Linkhart T, Mohan S, Baylink DJ 1997 Circulating and skeletal insulin-like growth factor-I (IGF-I) concentrations in two inbred strains of mice with different bone mineral densities. Bone 21:217–223 [DOI] [PubMed] [Google Scholar]
- Lecka-Czernik B, Ackert-Bicknell C, Adamo ML, Marmolejos V, Churchill GA, Shockley KR, Reid IR, Grey A, Rosen CJ 2007 Activation of peroxisome proliferator-activated receptor γ (PPARγ) by rosiglitazone suppresses components of the insulin-like growth factor regulatory system in vitro and in vivo. Endocrinology 148:903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockley KR, Lazarenko OP, Czernik PJ, Rosen CJ, Churchill GA, Lecka-Czernik B 2009 PPARγ2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem 106:232–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CJ, Ackert-Bicknell CL, Adamo ML, Shultz KL, Rubin J, Donahue LR, Horton LG, Delahunty KM, Beamer WG, Sipos J, Clemmons D, Nelson T, Bouxsein ML, Horowitz M 2004 Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program. Bone 35:1046–1058 [DOI] [PubMed] [Google Scholar]
- Ackert-Bicknell CL, Demissie S, Marin de Evsikova C, Hsu YH, DeMambro VE, Karasik D, Cupples LA, Ordovas JM, Tucker KL, Cho K, Canalis E, Paigen B, Churchill GA, Forejt J, Beamer WG, Ferrari S, Bouxsein ML, Kiel DP, Rosen CJ 2008 PPARG by dietary fat interaction influences bone mass in mice and humans. J Bone Miner Res 23:1398–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao SX, Dhahbi JM, Mote PL, Spindler SR 2001 Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc Natl Acad Sci USA 98:10630–10635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douris N, Green CB 2008 NOC out the fat: a short review of the circadian deadenylase Nocturnin. Ann Med 40:622–626 [DOI] [PubMed] [Google Scholar]
- Green CB, Besharse JC 1996 Identification of a novel vertebrate circadian clock-regulated gene encoding the protein nocturnin. Proc Natl Acad Sci USA 93:14884–14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Osterbur DL, Megaw PL, Tosini G, Fukuhara C, Green CB, Besharse JC 2001 Rhythmic expression of Nocturnin mRNA in multiple tissues of the mouse. BMC Dev Biol 1:9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggs JE, Green CB 2003 Nocturnin, a deadenylase in Xenopus laevis retina: a mechanism for posttranscriptional control of circadian-related mRNA. Curr Biol 13:189–198 [DOI] [PubMed] [Google Scholar]
- Garbarino-Pico E, Niu S, Rollag MD, Strayer CA, Besharse JC, Green CB 2007 Immediate early response of the circadian polyA ribonuclease nocturnin to two extracellular stimuli. RNA 13:745–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstrohm AC, Wickens M 2008 Multifunctional deadenylase complexes diversify mRNA control. Nat Rev Mol Cell Biol 9:337–344 [DOI] [PubMed] [Google Scholar]
- Foyt HL, LeRoith D, Roberts Jr CT 1991 Differential association of insulin-like growth factor I mRNA variants with polysomes in vivo. J Biol Chem 266:7300–7305 [PubMed] [Google Scholar]
- Bell GI, Stempien MM, Fong NM, Rall LB 1986 Sequences of liver cDNAs encoding two different mouse insulin-like growth factor I precursors. Nucleic Acids Res 14:7873–7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thissen JP, Underwood LE 1992 Translational status of the insulin-like growth factor-I mRNAs in liver of protein-restricted rats. J Endocrinol 132:141–147 [DOI] [PubMed] [Google Scholar]
- Lund PK, Moats-Staats BM, Hynes MA, Simmons JG, Jansen M, D'Ercole AJ, Van Wyk JJ 1986 Somatomedin-C/insulin-like growth factor-I and insulin-like growth factor-II mRNAs in rat fetal and adult tissues. J Biol Chem 261:14539–14544 [PubMed] [Google Scholar]
- Delany AM, Canalis E 1995 Transcriptional repression of insulin-like growth factor I by glucocorticoids in rat bone cells. Endocrinology 136:4776–4781 [DOI] [PubMed] [Google Scholar]
- Green CB, Douris N, Kojima S, Strayer CA, Fogerty J, Lourim D, Keller SR, Besharse JC 2007 Loss of Nocturnin, a circadian deadenylase, confers resistance to hepatic steatosis and diet-induced obesity. Proc Natl Acad Sci USA 104:9888–9893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundberg CM, Markowitz ME, Mizruchi M, Rosen JF 1985 Osteocalcin in human serum: a circadian rhythm. J Clin Endocrinol Metab 60:736–739 [DOI] [PubMed] [Google Scholar]
- Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G 2005 The molecular clock mediates leptin-regulated bone formation. Cell 122:803–815 [DOI] [PubMed] [Google Scholar]
- Oishi K, Miyazaki K, Kadota K, Kikuno R, Nagase T, Atsumi G, Ohkura N, Azama T, Mesaki M, Yukimasa S, Kobayashi H, Iitaka C, Umehara T, Horikoshi M, Kudo T, Shimizu Y, Yano M, Monden M, Machida K, Matsuda J, Horie S, Todo T, Ishida N 2003 Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J Biol Chem 278:41519–41527 [DOI] [PubMed] [Google Scholar]
- Bartel DP 2009 MicroRNAs: target recognition and regulatory functions. Cell 136:215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao X, Zhang X, Wu L, Belasco JG 2010 CCR4-NOT deadenylates mRNA associated with RNA-induced silencing complexes in human cells. Mol Cell Biol 30:1486–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Green CB, Lecka-Czernik B, Douris N, Gilbert MR, Kojima S, Ackert-Bicknell C, Garg N, Horowitz MC, Adamo ML, Clemmons DR, Rosen CJ 2010 A circadian-regulated gene, Nocturnin, promotes adipogenesis by stimulating PPAR-γ nuclear translocation. Proc Natl Acad Sci USA 107:10508–10513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Green CB, Horowitz M, Ackert-Bicknell C, Lecka-Czernik B, Rosen CJ 2010 Nocturnin: a circadian target of Pparg-induced adipogenesis. Ann NY Acad Sci 1192:131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H 2004 PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113:846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL 2005 Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology 146:1226–1235 [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM 2000 Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol 16:1451–1471 [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM 2008 Fat and beyond: the diverse biology of PPARγ. Annu Rev Biochem 77:289–312 [DOI] [PubMed] [Google Scholar]
- Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B 2004 Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 145:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka-Czernik B, Feingold KR, Strotmeyer ES, Resnick HE, Carbone L, Beamer BA, Park SW, Lane NE, Harris TB, Cummings SR 2006 Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab 91:3349–3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey A 2008 Skeletal consequences of thiazolidinedione therapy. Osteoporos Int 19:129–137 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.