Abstract
Alzheimer disease (AD) is multifactorial and apparently involves several different etiopathogenic mechanisms. There are at least five subgroups of AD based on cerebrospinal fluid levels of Aβ1–42, a marker of Aβ plaques, and tau and ubiquitin, two markers of neurofibrillary tangles. These different AD subgroups may respond differently to a given disease modifying drug and, hence, different therapeutic drugs for different disease subgroups might be required. Stratification of AD patients by disease subgroups in clinical trials is critical to the successful development of potent disease modifying drugs. Cerebrospinal fluid levels of disease markers are promising, both in identifying various subgroups of AD and in monitoring the response to therapeutic drugs.
Keywords: Alzheimer disease subgroups, cerebrospinal fluid, CSF biomarkers, Aβ1–42, tau, ubiquitin, Alzheimer disease therapeutics, neurofibrillary degeneration, tau, β-amyloid
1. Introduction
Alzheimer disease (AD), the single major cause of dementia in middle and old age individuals, is histopathologically characterized by brain β-amyloidosis (Aβ) and neurofibrillary degeneration. The former is seen as plaques of extracellular deposits of Aβ in the brain parenchyma and in the cerebral blood vessels, the congophilic angiopathy. The neurofibrillary degeneration is a slow and progressive retrograde neuronal degeneration that is observed as neurofibrillary tangles of paired helical filaments (PHF)/straight filaments (SF) in the cell soma, and in dystrophic neurites surrounding the plaque core β-amyloid, and in the neuropil as neuropil threads [1]. Aβ plaques identical to those in AD but lacking the dystrophic neurites with neurofibrillary pathology are also seen in the neocortex of the cognitively normal old age individuals [2]. On the other hand, neurofibrillary pathology of the AD type which is made up of PHF/SF of abnormally hyperphosphorylated tau [3, 4] is a hallmark of several related neurodegenerative diseases called tauopathies (for review see [5]. These tauopathies include frontotemporal dementia with Parkinsonism linked to chromosome 17 (FTDP-17) caused by tau mutations, corticobasal degeneration, Pick disease, dementia pugilistica, and progressive nuclear palsy. The occurrence of neurofibrillary degeneration in the neocortex in the absence of Aβ deposits in tauopathies is associated with dementia. In progressive supranuclear palsy where the hyperphosphorylated tau lesions occur in the brain stem, motor dysfunction instead of dementia is observed. Unlike aging and tauopathies, AD is characterized by the presence of both numerous β-amyloid plaques and neurofibrillary tangles of abnormally hyperphosphorylated tau filaments in the neocortex, especially the hippocampus. The abnormally hyperphosphorylated tau in neurofibrillary tangles becomes ubiquitinated [6, 7]. However, this ubiquitination occurs late, i.e. when the pathological tau is in β-pleated sheets, and is mostly unsuccessful; neurons with ubiquitinated neurofibrillary tangles survive for up to several years [8] and then on cell death are seen in the extracellular space in the brain as ghost tangles, also called tombstones.
2. Multifactorial nature and a lack of apparent temporal relationship between plaques and tangles
AD is multifactorial and heterogeneous. Less than 1% of AD cases are caused by certain mutations in three different transmembrane proteins, amyloid precursor protein (APP), presenilin 1 and presenilin 2 (for review see [9]). Furthermore, prion protein, the self-replication of the pronase resistant form of which causes prion disease, is also a transmembrane protein, and a missense mutation in this protein in Gerstman Straussler Syndrome (GSS) in a family in Indiana, referred to as Indiana kindred, has been found to be associated with numerous neurons with neurofibrillary tangles of abnormally hyperphosphorylated tau along with prion plaques [10]. Over 99% of AD cases represent the so called sporadic form of the disease which is not associated with any known mutation. The sporadic form of AD itself probably involves several different etiopathogenic mechanisms. Neuroinflammation, head trauma, and diabetes have been implicated as risk factors for AD. In the sporadic AD, the presence of one or two alleles of APOE4 as opposed to APOE2 or APOE3 increases the disease risk by several fold [11]
Although numerous plaques and neurofibrillary tangles are seen in AD brain, for reasons currently not understood these two lesions occur in disproportionate numbers in different cases of the disease, especially in the plaque-dominant and tangle-dominant AD subgroups [12, 13]. This lack of direct relationship between the numbers of plaques and tangles in AD and the presence of numerous Aβ plaques without accompanying neurofibrillary degeneration in normal aged humans are inconsistent with the Amyloid Cascade Hypothesis [14, 15], according to which Aβ, the metabolite of the β-amyloid precursor protein (βAPP), is the primary neurotoxic molecule which causes neurofibrillary degeneration and leads to dementia. In support of this hypothesis, infusion of Aβ1–42 in P301L tau transgenic mice [16] as well as crossing of P301L tau transgenic mice with APPSWE transgenic mice [17] have been shown to exacerbate the tau neurofibrillary pathology. In these studies, the infusion of Aβ1–42 or overexpression of APPSWE could have exacerbated the tau pathology by activating the stress activated protein kinases which are known to phosphorylate tau at several proline-directed sites. However, no mutations in tau have been found to date in AD and the FTDP-17/tau mutation cases do not show any Aβ deposits. Furthermore, numerous Aβ plaques in the neocortex of normal aged humans in the absence of neurofibrillary pathology [2] and a very high Aβ load in hereditary cerebral hemorrhage with amyloidosis, Dutch type (HCHWA-D) but without accompanying neurofibrillary pathology [18] occur. Thus, the Amyloid Cascade Hypothesis does not seem to hold in human brain.
Two AD Phase III clinical trials using an Aβ aggregation inhibitor, Alzhemed, and another using a γ-secretase modulating non-steroidal anti-inflammatory drug (NSAID) Flurizine (Myriad, Utah), have badly failed. A clinical trial on direct removal/clearance of β-amyloid from the brains of AD patients by Aβ vaccine (AN1792 by Elan Pharm., Ireland) had to be interrupted due to the vaccine-induced menigoencephalitis in several participants. Nevertheless, the Aβ vaccine though successful in clearing β-amyloid from the brain parenchyma of the treated AD cases studied postmortem, this treatment had failed to reduce the number of neurofibrillary tangles and significantly alter the cognitive decline [19]. Lastly, a Phase II clinical trial of AD patients treated by passive immunization with monoclonal antibody to Aβ (Elan/Wyeth Pharm) failed to show any significant clinical improvement. Thus, it appears that either Aβ deposition is not the primary cause of dementia or inhibition or clearance of Aβ without inhibition of neurofibrillary degeneration might not be sufficient to inhibit the progressive cognitive decline in AD patients. Another possibility is that Aβ initiates neurofibrillary pathology which then becomes self-propagating. In such a case, removal/clearance of Aβ from AD brain could be too late to be of any clinical benefit. However, the presence of numerous Aβ plaques without accompanying neurofibrillary pathology in the neocortex of cognitively normal aged humans and in HCHWA-D cases does not support this scenario.
Failures with Aβ-based therapies have shifted interest to developing therapeutic drugs that can inhibit Alzheimer neurofibrillary degeneration. The development of drugs that can inhibit neurofibrillary degeneration has its own challenges as well as opportunities. Neurofibrillary degeneration of AD type can result from several different etiopathogenic mechanisms and, thus, offers many therapeutic targets.
Tau protein has 80 serines/threonines and 5 tyrosines as potential sites that can be phosphorylated by protein kinases [20]. Normal brain tau has 2–3 moles phosphate/mole of the protein and this stoichiometry is apparently optimal for its biological activity which is regulated by its degree of phosphorylation. By hyperphosphorylation a neuron can reduce the microtubule network and the axonal transport and other cellular activities that depend on microtubules. However, this is reversible and plays a physiologically significant role. It is important to distinguish this type of hyperphosphorylation of tau from that which occurs in AD and related tauopathies. Two main characteristics of the AD abnormally hyperphosphorylated tau (AD P-tau) that were discovered in our lab are (i) that instead of interacting with tubulin, the AD P-tau binds to normal tau, MAP1 and MAP2, and this sequestration of normal MAPs results in depolymerization of microtubules [21–24], and (ii) that the AD P-tau can self-assemble into bundles of PHF/SF [25, 26].
Phosphorylation sites that lead to the AD type abnormal hyperphosphorylation of tau are phosphorylated by different combinations of non-proline-directed protein kinases like PKA and CaMKII with the proline directed kinases like glycogen synthase kinase 3β (GSK-3β), and cyclin dependent protein kinase 5 (cdk5), and are dephosphorylated largely by protein phosphatase-2A [26]. Furthermore, phosphorylation of tau is also regulated by its glycosylation which is altered in AD brain [27, 28]. These studies suggest that different etiopathogenic mechanisms could be involved in the abnormal hyperphosphorylation of tau.
Tau self-assembles through the β structure containing microtubule binding repeat R3 in 3R taus and through repeats R2 and R3 in 4R taus [29, 30]. Both the amino terminal and the carboxy terminal flanking regions to the microtubule binding repeats in normal tau appear to inhibit its self-assembly. Whereas on AD-type abnormal hyperphosphorylation, i.e. the phosphorylation of the amino terminal and carboxy terminal flanking regions, this inhibition is eliminated, resulting in the self-assembly of tau into tangles of PHF/SF [31, 32]. In addition to abnormal hyperphosphorylation, truncation of tau at Glu391 and Asp421 have been implicated in the pathogenesis of AD [33–35]. Truncation probably promotes self-aggregation of tau both by decreasing the inhibitory effect of the carboxy terminal domain in the case of tau391 and tau421, as well as by making the truncated proteins better substrates for phosphorylation. Transgenic rats expressing human tau truncated both N- and C-terminally, tau151–391, show marked neurofibrillary degeneration of abnormally hyperphosphorylated tau [36].
3. Different subgroups of AD
Based on the CSF levels of Aβ1–42, tau and ubiquitin studies from our lab led to the identification of five different subgroups of sporadic AD [37]. These five subgroups, called AELO, ATEO, LEBALO, HARO, and ATURO, each present a different clinical profile. Thus, it is likely that each of these sporadic AD subgroups may respond differently to any one given disease modifying drug. The subgroup AELO, which represents AD cases with low Aβ1–42 level, high incidence of APOE4, and late onset, represented almost half of the 353 AD cases examined in our study [37]. Two AD Phase II clinical trials, one with Rosiglitazone [38], also an anti-diabetic drug (Smith Kline Glaxo, USA), and another with Aβ passive immunization (Elan/Wyeth Pharm., USA), both showed a better treatment response to APOE4 non-carriers as compared with APOE4 carriers. It thus indicates that AD subgroup AELO is likely to respond poorly to Rosiglitazone and to Aβ vaccine.
The subgroup HARO, which represents AD cases with high instead of low CSF Aβ1–42 seen in the other four subgroups, is characterized by recent onset and represented <10% of the cases in our study, is likely to respond very differently from the rest of the AD cases to Aβ-based therapies. Similarly, the subgroup LEBALO, that represented AD cases with high incidence of Lewy bodies, low Aβ1–42 and late onset and constituted ~15% of the cases in our study, did not have any significantly elevated CSF tau and thus is very likely to respond very differently from the rest of the AD cases to a tau-based therapeutic drug. Thus, our current preliminary knowledge on different subgroups of AD and different response of APOE4 carriers vs. non-carriers, mentioned above, strongly suggests that the success of clinical trials of AD for disease modifying drugs can be markedly improved by stratifying test patients by subgroups. Future studies on CSF molecular markers, especially levels of different phosphotaus, may help identify additional specific subgroups of AD. Stratification of AD cases by these additional subgroups is likely to markedly increase the success of developing potent disease modifying drugs for this disease.
4. Conclusions
AD is multifactorial and heterogeneous and involves several etiopathogenic mechanisms. There are at least five subgroups of AD that can be identified by determining CSF lavels of Aβ1–42, tau, and ubiquitin. Stratification of AD patients by these subgroups and monitoring the efficacy of the drug treatment by CSF levels of the disease markers are promising approaches for the development of rational disease modifying drugs.
Acknowledgements
We are grateful to Ms. Janet Murphy for secretarial assistance. Dr. Ezzat El-Akkad helped prepare Figure 1. Studies in our laboratories were supported in part by the New York State Office of Mental Retardation and Developmental Disabilities; NIH grants AG019158 and AG028538; and Alzheimer’s Association (Chicago, IL) grant IIRG-00-2002, HRG-05-13095.
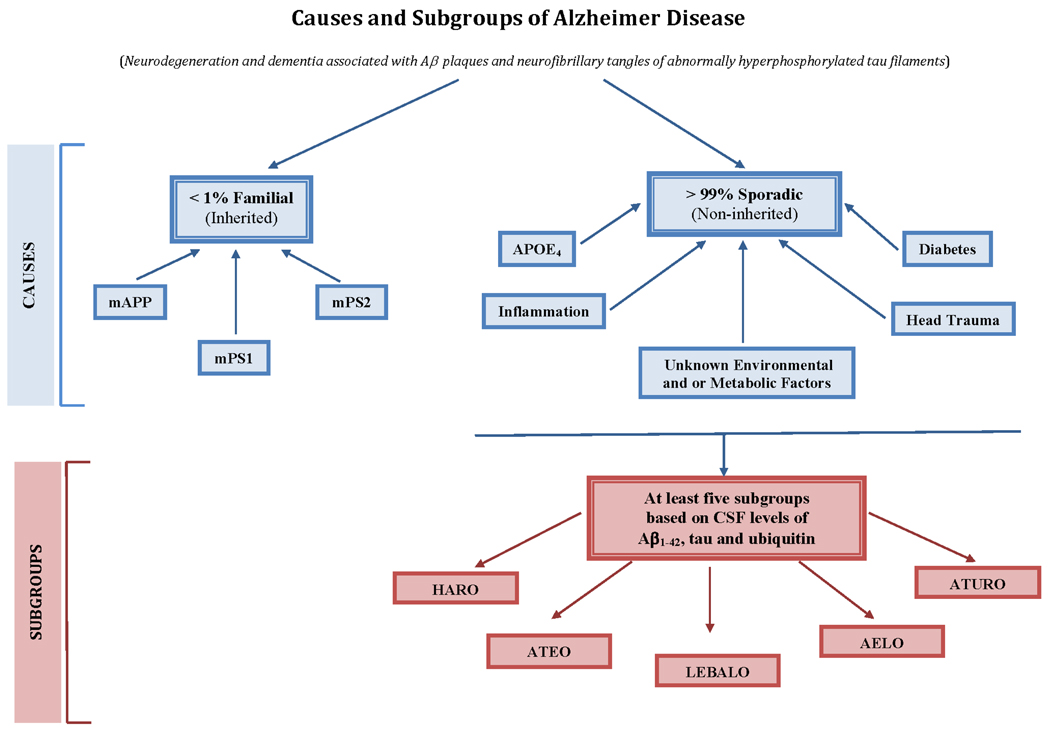
Figure 1. Causes and subgroups of AD.
Less than 1% of AD cases are familial and are caused by certain mutations in βAPP (mAPP), presenilin 1 (mPS1), or presenilin 2 (mPS2) genes which are transmitted in an autosomal dominant fashion and the resulting gene products, the mutated proteins, are dysfunctional.
Over 99% of AD cases are sporadic and have not, to date, been associated with any mutated protein, but several risk factors including the presence of one or two copies of APOE4 allele, inflammation, head trauma, diabetes/low brain glucose metabolism, and as yet unknown environmental and or metabolic factors have been implicated.
Based on CSF levels of Aβ1–42, a marker of Aβ plaques, and tau and ubiquitin, the two markers of neurofibrillary tangles, five subgroups of AD have been identified. These subgroups, AELO, ATEO, LEBALO, HARO, and ATURO, represent distinct clinical profiles. These different subgroups of AD probably involve different etiopathogenic mechanisms and may respond differently to any one given disease modifying drug.
AELO: AD cases with low Aβ1–42, high incidence of APOE4, and late onset.
ATEO: AD cases with low Aβ1–42, high tau, and early onset.
LEBALO: AD cases with high incidence of Lewy bodies, low Aβ1–42, and late onset.
HARO: AD with high Aβ1–42, and recent onset.
ATURO: AD with low Aβ1–42, high tau, high ubiquitin, and recent onset.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Braak H, Braak E, Grundke-Iqbal I, Iqbal K. Occurrence of neuropil threads in the senile human brain and in Alzheimer's disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci Lett. 1986;65:351–355. doi: 10.1016/0304-3940(86)90288-0. [DOI] [PubMed] [Google Scholar]
- 2.Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, et al. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13:179–189. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- 3.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J. Biol. Chem. 1986a;261:6084–6089. [PubMed] [Google Scholar]
- 4.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA. 1986b;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iqbal K, Liu F, Gong CX, Alonso Adel C, Grundke-Iqbal I. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 2009;118:53–69. doi: 10.1007/s00401-009-0486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mori H, Kondo J, Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science. 1987;235:1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- 7.Perry G, Friedman R, Shaw G, Chau V. Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc Natl Acad Sci U S A. 1987;84:3033–3036. doi: 10.1073/pnas.84.9.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morsch R, Simon W, Coleman PD. Neurons may live for decades with neurofibrillary tangles. J Neuropathol Exp Neurol. 1999;58:188–197. doi: 10.1097/00005072-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Bird TD. Genetic aspects of Alzheimer disease. Genet Med. 2008;10:231–239. doi: 10.1097/GIM.0b013e31816b64dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giaccone G, Tagliavini F, Verga L, Frangione B, Farlow MR, Bugiani O, et al. Neurofibrillary tangles of the Indiana kindred of Gerstmann-Straussler-Scheinker disease share antigenic determinants with those of Alzheimer disease. Brain Res. 1990;530:325–329. doi: 10.1016/0006-8993(90)91304-y. [DOI] [PubMed] [Google Scholar]
- 11.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 12.Jellinger KA, Attems J. Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol. 2007;113:107–117. doi: 10.1007/s00401-006-0156-7. [DOI] [PubMed] [Google Scholar]
- 13.Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 14.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 15.Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 16.Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 17.Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 18.Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, et al. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248:1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- 19.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 20.Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Dysregulation of protein phosphorylation/dephosphorylation in Alzheimer's disease: a therapeutic target. J Biomed Biotechnol. 2006;2006:31825. doi: 10.1155/JBB/2006/31825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc. Natl. Acad. Sci. USA. 1997;94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alonso AD, Grundke-Iqbal I, Iqbal K. Alzheimer's disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat. Med. 1996;2:783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- 23.Alonso AD, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B, Chohan MO, Grundke-Iqbal I, Iqbal K. Disruption of microtubule network by Alzheimer abnormally hyperphosphorylated tau. Acta Neuropathol (Berl) 2007;113:501–511. doi: 10.1007/s00401-007-0207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alonso AD, Zaidi T, Novak M, Barra HS, Grundke-Iqbal I, Iqbal K. Interaction of tau isoforms with Alzheimer's disease abnormally hyperphosphorylated tau and in vitro phosphorylation into the disease-like protein. J Biol Chem. 2001;276:37967–37973. doi: 10.1074/jbc.M105365200. [DOI] [PubMed] [Google Scholar]
- 26.Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci. 2007;25:59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang JZ, Grundke-Iqbal I, Iqbal K. Glycosylation of microtubule-associated protein tau: an abnormal posttranslational modification in Alzheimer's disease. Nat Med. 1996;2:871–875. doi: 10.1038/nm0896-871. [DOI] [PubMed] [Google Scholar]
- 28.Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22:1942–1950. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- 29.von Bergen M, Friedhoff P, Biernat J, Heberle J, Mandelkow EM, Mandelkow E. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif ((306)VQIVYK(311)) forming beta structure. Proc Natl Acad Sci U S A. 2000;97:5129–5134. doi: 10.1073/pnas.97.10.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arrasate M, Perez M, Armas-Portela R, Avila J. Polymerization of tau peptides into fibrillar structures. The effect of FTDP-17 mutations. FEBS Lett. 1999;446:199–202. doi: 10.1016/s0014-5793(99)00210-0. [DOI] [PubMed] [Google Scholar]
- 31.Alonso AD, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem. 2004;279:34873–34881. doi: 10.1074/jbc.M405131200. [DOI] [PubMed] [Google Scholar]
- 32.Alonso AD, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci U S A. 2001;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novak M, Jakes R, Edwards PC, Milstein C, Wischik CM. Difference between the tau protein of Alzheimer paired helical filament core and normal tau revealed by epitope analysis of monoclonal antibodies 423 and 7.51. Proc Natl Acad Sci U S A. 1991;88:5837–5841. doi: 10.1073/pnas.88.13.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, et al. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci U S A. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luna-Munoz J, Garcia-Sierra F, Falcon V, Menendez I, Chavez-Macias L, Mena R. Regional conformational change involving phosphorylation of tau protein at the Thr231, precedes the structural change detected by Alz-50 antibody in Alzheimer's disease. J Alzheimers Dis. 2005;8:29–41. doi: 10.3233/jad-2005-8104. [DOI] [PubMed] [Google Scholar]
- 36.Zilka N, Filipcik P, Koson P, Fialova L, Skrabana R, Zilkova M, et al. Truncated tau from sporadic Alzheimer's disease suffices to drive neurofibrillary degeneration in vivo. FEBS Lett. 2006;580:3582–3588. doi: 10.1016/j.febslet.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 37.Iqbal K, Flory M, Khatoon S, Soininen H, Pirttila T, Lehtovirta M, et al. Subgroups of Alzheimer's disease based on cerebrospinal fluid molecular markers. Ann Neurol. 2005;58:748–757. doi: 10.1002/ana.20639. [DOI] [PubMed] [Google Scholar]
- 38.Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]