Abstract
Epidemiological studies suggest that type 2 diabetes (T2D) significantly increases breast cancer risk and mortality. However, there is limited experimental evidence of this association. Moreover, pathophysiological pathways underlying the tumor-promoting activity of diabetes remain unidentified. In the present study, the molecular mechanisms that link T2D and breast cancer development and progression were studied using the transgenic MKR mouse model of T2D. In MKR mice, the transgene encoding a dominant-negative, kinase-dead human insulin-like growth factor I (IGF-I) receptor (IGF-IR) is expressed exclusively in the skeletal muscle where it inactivates endogenous insulin receptors (IR) and IGF-IR. MKR females are lean, yet they are hyperinsulinemic, insulin resistant and glucose intolerant. Female MKR mice demonstrate accelerated mammary gland development, enhanced phosphorylation of IR/IGF-IR and Akt in mammary tissue. The metabolic abnormalities of MKR mice accelerate development of hyperplastic precancerous lesions in transgenic PyVmT (polyoma virus middle T antigen) model and enhance tumor growth in syngeneic Met-1 and MCNeuA models of breast cancer. Normal mammary tissue and breast tumor tissue extracted from diabetic mice reveals markedly increased phosphorylation of the IR/IGF-IR and Akt, whereas ERK1/2 phosphorylation remains largely unaffected. Furthermore, pharmacological blockade of the IR/IGF-IR by the small-molecule tyrosine kinase inhibitor BMS-536924 reverses the tumor-promoting effects of diabetes. Taken together, our data provide novel and compelling experimental evidence that (i) T2D, via hyperinsulinemia, accelerates mammary gland development and mammary carcinogenesis, and (ii) the IR and/or the IGF-IR are the major mediators of this effect.
Keywords: Diabetes, breast cancer, hyperinsulinemia
Introduction
Epidemiological studies suggest that obesity and type 2 diabetes (T2D) significantly increase overall cancer risk and mortality. One of the malignancies adversely affected by obesity and T2D is breast cancer (1-5), the second most common cancer in the world, which will be diagnosed in 25 million women over the next 25 years.
Numerous factors can potentially be implicated in the tumor-promoting activity of obesity, including increased influx of nutrients, elevated circulating IGF-I levels and enhanced secretion of adipokines, proinflammatory cytokines, sex steroids and growth factors by adipose tissue (6-8). However, epidemiological data indicate that T2D exerts tumor-promoting activity independent of obesity (4, 9). This effect of T2D is thought to be mediated by the characteristic hallmarks of the disease - hyperinsulinemia, hyperglycemia and/or hyperlipidemia (10).
Animal studies investigating the relationship between obesity, diabetes and breast cancer provide scarce and inconclusive results. Most of them confirm a stimulatory effect of obesity on breast cancer development (11), but they do not differentiate between obesity- and diabetes-related effects and fail to identify pathophysiological mechanisms implicated in the tumor-promoting activity.
In the current work, we provide novel experimental evidence that diabetes-related hyperinsulinemia affects mammary gland development and breast cancer progression independent of obesity. Hyperinsulinemia mediates these effects by activating the insulin receptor (IR), the IGF-I receptor (IGF-IR) and the phosphatidylinositol-triphosphate kinase (PI3K) pathway.
Given the pandemic of obesity and T2D, this study will have a significant impact on public health and patient outcomes worldwide. It will help to develop preventative and therapeutic options, whereby breast cancer morbidity and mortality may eventually be reduced. The results of this work can be quickly translated into clinical practice that will help to optimize adjuvant and neoadjuvant therapy in breast cancer patients with T2D.
Materials and Methods
Animals
All animals were housed and maintained in an animal facility at the Mount Sinai School of Medicine. Mice were kept on a 12-hour light/dark cycle with access to a standard laboratory chow diet and fresh water ad libitum. Generation of MMTV-PyVmT and MKR transgenic mice was described previously (12, 13). MMTV-PyVmT mice were kindly donated by W.J. Muller (McGill University, Montreal, Quebec, Canada).
PyVmT/MKR transgenic tumor model
All the mice used in this study were on the FVB/N background. PyVmT+/− male mice were interbred with MKR+/+ or WT female mice to generate cohorts of WT, MKR+/+, PyVmT+/− and PyVmT+/−/MKR+/+ female mice. Only virgin female mice were analyzed in this study. Body weights were recorded weekly until termination of the study. To monitor tumor growth in mammary glands, each of the ten mammary glands were examined by finger palpation once a week starting at 6 weeks of age. Tumor volume was measured by calipers in a three-coordinate system, and calculated using the following formula: 4/3 × π × (width/2) × (length/2) × (depth/2), which represents the three-dimensional volume of an ellipse. Animals were sacrificed when one tumor dimension reached 2 cm. Before euthanasia, blood was collected from the retro-orbital sinus. After euthanasia all mammary tumors were carefully excised and weighed. Portions of the tumors were either snap-frozen in liquid nitrogen or stored in formalin for further studies.
Syngeneic orthotopic tumor models
Met-1 and MCNeuA mouse carcinoma cells derived from MMTV-PyVmT/FVB-N and MMTV-Neu/FVB-N transgenic animals were used for orthotopic inoculation into the mammary fat pads of 8 week-old MKR+/+ and WT female mice. Cells were allowed to grow until confluence, detached by non-enzymatic cell dissociation solution, and either 500,000 Met-1 cells or 1,000,000 MCNeuA cells, resuspended in 100 μl of sterile PBS were injected into the left inguinal (#4) mammary fat pad using a 30-gauge needle. Monitoring of tumor growth and processing of blood and tissues were performed as described above.
Densitometric Analysis
Densitometric analyses of immunoblots were performed using MacBAS V2.52 software.
Statistical Analysis
Results are expressed as the mean±SEM. Statistical analyses were calculated using the Student's t-test or, when appropriate, two-factor ANOVA followed by Holm-Sidak post-hoc test.
Additional experimental protocols have been described in the supplementary information.
Results
Metabolic abnormalities in MKR+/+ mice
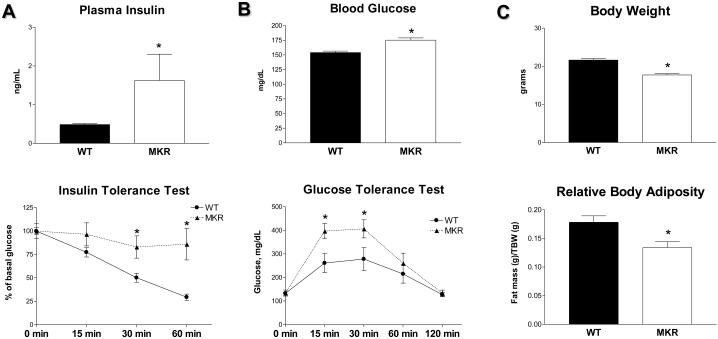
In the present work, the transgenic MKR mouse model of T2D was employed (13). In this model, the transgene encoding human insulin-like growth factor I (IGF-I) receptor has a point mutation in the ATP-binding domain and is driven by the muscle creatine kinase promoter, resulting in inactivation of insulin and IGF-I receptors (IR and IGF-IR) exclusively in skeletal muscle. Male MKR+/+ mice develop severe diabetes (13); female MKR+/+ mice were not extensively studied as they did not exhibit marked hyperglycemia. Herein, we show that female MKR+/+ mice develop a mild diabetic phenotype, which recapitulates early stages of T2D (prediabetes) in humans. These gender-specific differences may be attributed to estrogens, which exert a protective effect on pancreatic beta-cells (14). Marked hyperinsulinemia and severe insulin resistance (demonstrated by insulin tolerance test) are the major metabolic abnormalities detected in MKR+/+ female mice (Fig. 1A). In addition, MKR+/+ females display a mild dysglycemia demonstrated by slightly elevated blood glucose levels (by ~20%) and impaired glucose tolerance (Fig. 1B). Importantly, MKR+/+ females are not obese; in fact they have moderately reduced total body weight and body adiposity compared to WT controls (Fig. 1C). In addition, MKR+/+ female mice do not show exacerbated inflammatory response observed in obesity and lipoatrophic diabetes (data not shown).
Figure 1.
Metabolic abnormalities in MKR+/+ female mice. (A) Left panel: Plasma insulin levels in 8 wk old WT (close bars) and MKR+/+ (open bars) mice (n=7). Right panel: Insulin tolerance test was performed on fasted 12 wk old WT (circles) and MKR+/+ (triangles) mice (n=5) after intraperitoneal injection of insulin (0.75 U/kg). Blood samples were obtained from the tail vein, and glucose concentrations were determined at the indicated time points. (B) Left panel: Blood glucose in 12 wk old WT (close bars) and MKR+/+ (open bars) mice (n=15). Right panel: Glucose tolerance test was performed on fasted 10 wk old WT (circles) and MKR+/+ (triangles) mice (n=5) after intraperitoneal injection of glucose (2 g/kg). Blood samples were obtained from the tail vein, and glucose concentrations were determined at the indicated time points. (C) Total body weight and total body adiposity in 10-12 wk old WT (close bars) and MKR+/+ (open bars) mice (n=6). All results are expressed as a mean ± SEM. Statistically significant difference between WT and MKR groups is indicated (*), P < 0.05 (Student's t-test).
Effect of T2D on mammary gland development
Little is known about the role of insulin in mammary gland development, but it is well established that IGF-I, which is structurally related to insulin, regulates certain stages of mammary gland development (15). Since MKR+/+ females develop hyperinsulinemia at a prepubertal age (3 weeks), we assessed whether elevated insulin levels could affect mammary gland development.
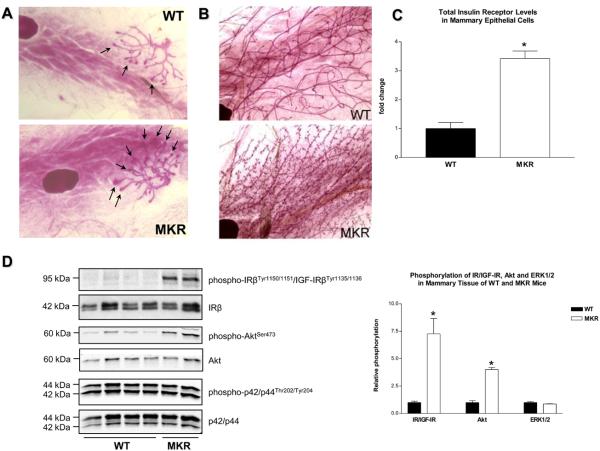
At the onset of puberty, the immature mammary gland undergoes rapid growth and differentiation mediated by terminal end buds (TEBs) that cause ductal elongation and branching (16). Prepubertal mammary glands in 3 week-old MKR+/+ mice have increased number and size of TEBs compared to WT glands (Fig. 2A). Additionally, late virgin glands obtained from 15 week old MKR+/+ females demonstrate increased number of lateral buds and enhanced side branching (Fig. 2B).
Figure 2.
Effect of type 2 diabetes on normal mammary gland development. Whole-mount analysis of (A) prepubertal (3 wk) and(B) late virgin (15 wk) mammary glands obtained from WT and MKR+/+ female mice. Arrows indicate the terminal end buds. Original magnification: x16. Representative image of 7 mice (C) Total IR levels in mammary epithelial cells (MECs) isolated from 15 wk old WT and MKR+/+ virgin female mice (n=3) were measured by ELISA. Relative levels of total IR receptor levels are expressed as fold change compared to WT. Statistically significant difference is indicated (*), P < 0.05 (Student's t-test). (D) Proteins (50 ug) extracted from mammary tissue of virgin 7 week old WT and MKR+/+ mice were size-fractionated by SDS-PAGE and immunoblotted with anti-phospho-IRβY1150/51/IGF-IRβY1135/36, anti-phospho-AktS473 and anti-phospho-p42/p44T202/Y204 antibodies. Total level of proteins was demonstrated by immunoblotting with antibodies directed against total IR(, Akt and p42/p44. At least four animals per group were analyzed, the representative blots are included. (E) The results of densitometric analyses of IR/IGF-IR, Akt and ERK1/2 phosphorylation are presented as a fold change compared to WT mammary tissue. Statistically significant difference is indicated (*), P < 0.05 (Student's t-test).
The biological effects of insulin and IGFs are mediated by the structurally related IR and IGF-IR. Both the IR and the IGF-IR bind insulin and IGFs, although each receptor binds its cognate ligand with much higher affinity. The two receptors transduce an intracellular signal primarily through the PI3K pathway and the MAPK pathway (17).
Given the different properties of each receptor, we wished to determine which receptor and signaling pathway(s) were responsible for the promoting effect of T2D on mammary gland development. For this purpose, mammary epithelial cells (MECs) were isolated from late virgin glands of 15 week-old WT and MKR+/+ female mice, and extracted cellular proteins were subjected to quantitative analysis of IR and IGF-IR levels by ELISA. Our data demonstrate that the level of IR in mammary epithelium of MKR+/+ mice is threefold higher than in WT mice (Fig. 2C). IGF-IR levels are not significantly different in WT and MKR groups (data not shown). In contrast to MECs, total IR levels in the whole breast tissue are not different in WT and MKR+/+ mice (Fig. 2 D) suggesting that the IR is upregulated in mammary epithelium, but not in the stroma of MKR+/+ mice. However, whole breast tissue obtained from MKR+/+ mice reveals markedly elevated IR/IGF-IR and Akt phosphorylation (seven- and fourfold, respectively), whereas the level of ERK1/2 phosphorylation does not differ significantly between the two groups (Fig. 2D, 2E). These results suggest that in whole mammary tissue hyperinsulinemia results in the activation of IR and/or IGF-IR and a further signal transduction primarily through the PI3K pathway.
Sex steroids and IGF-I are key regulators of mammary gland development (15). Insulin regulates the levels of total and free IGF-I and estrogens by stimulating igf1 gene expression and steroidogenesis (18, 19) and suppressing IGFBP-1, IGFBP-2 and sex hormone binding globulin expression and secretion (7). In MKR+/+ females, circulating levels of IGF-I and estradiol are not elevated. However, serum IGFBP-1 and -2 levels in MKR+/+ mice are 30% lower than in WT controls (Supplementary Fig. S1). Therefore, it is conceivable that accelerated mammary gland development in MKR+/+ mice is also mediated by local IGFs, whose bioavailability is increased due to decreased levels of IGFBP-1 and -2.
Effect of T2D on mammary carcinogenesis
To study the effect of T2D on breast cancer development, we employed several models of mouse mammary carcinogenesis including the double transgenic PyVmT/MKR model and the syngeneic Met-1 and MCNeuA orthotopic models. In the PyVmT model, the transgene encodes a powerful oncogene (PyVmT) and is controlled by the MMTV promoter, whereby the oncogene is primarily expressed in mammary epithelium (12). PyVmT-induced mammary carcinogenesis demonstrates numerous genetic, morphological and pathophysiological similarities with human breast cancer. A stage-related loss of estrogen receptors (ER) as well as ErbB2/Neu and cyclin D1 overexpression seen in human breast cancer are reproduced in this model (20).
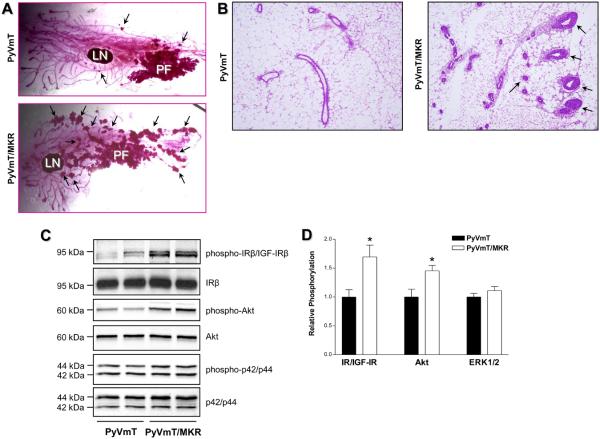
To study the effect of T2D on tumor development in the MMTV-PyVmT model, cohorts of PyVmT+/− and PyVmT+/−/MKR+/+ female mice were generated. A unique feature of tumor development in the PyVmT model is that at early stages of tumorigenesis (6 weeks), the primary tumor develops as a single focus on the ducts connected to the nipple (20). In PyVmT+/−/MKR+/+ animals, however, the tumors have a diffuse pattern, with a primary focus and multiple secondary foci (Fig. 3A). Moreover, compared to PyVmT+/− mice, PyVmT+/−/MKR+/+ mice demonstrate marked ductal hyperplasia and increased number of ducts in intact mammary tissue (Fig. 3B).
Figure 3.
Effect of type 2 diabetes on PyVmT-induced mammary hyperplasia. (A) Whole-mount and (B) histological analysis of mammary glands obtained from virgin 6 wk old PyVmT+/− and PyVmT+/−/MKR+/+ female mice. Arrows indicate secondary hyperplastic foci (A) and ductal hyperplasia (B). At least seven animals per group were analyzed, the representative images are included. LN, lymph node; PF, primary tumor focus. Original magnification: x4 (A), x100 (B). (C) Proteins (50 ug) extracted from mammary tissue of virgin 7 week old PyVmT+/− and PyVmT+/−/MKR+/+ mice were size-fractionated by SDS-PAGE and immunoblotted with anti-phospho-IRβY1150/51/IGF-IRβY1135/36, anti-phospho-AktS473 and anti-phospho-p42/p44T202/Y204 antibodies. Total level of proteins was demonstrated by immunoblotting with antibodies directed against total IRβ, Akt and p42/p44. At least seven animals per group were analyzed, the representative blots are included. The results of densitometric analysis of IR/IGF-IR, Akt and ERK1/2 phosphorylation in PyVmT+/− and PyVmT+/−/MKR+/+ mammary tissue and Met-1 orthograft tumor tissue are presented as a fold change compared to PyVmT+/− mammary tissue. Statistically significant difference is indicated (*), P < 0.05 (Student's t-test).
To explore whether hyperinsulinemia underlies the tumor-promoting effects of T2D, we studied IR and IGF-R phosphorylation as well as intracellular signal transduction in PyVmT+/− and PyVmT+/−/MKR+/+ mammary tissue. Mammary tissue from PyVmT+/−/MKR+/+ mice display markedly higher phosphorylation of the IR/IGF-IR and Akt, whereas ERK1/2 phosphorylation is unaffected (Fig. 3C, 3D). Moreover, since Western blot analysis with phospho-IR/IGF-IR antibody does not allow us to discriminate between the phosphorylated forms of the IR and IGF-IR, we employed immunoprecipitation of each receptor followed by immunodetection with the respective phosphospecific antibodies. The IR, and to lesser extent the IGF-IR, are hyperphosphorylated in mammary tumor tissue extracted from diabetic mice (1.7-fold and 1.4-fold increase, respectively) (Supplementary Fig. S2). Our Western blot data also indicate that ERα loss is not accelerated in this model (Supplementary Fig. S3).
To study the effect of T2D on breast cancer progression we also employed the syngeneic Met-1 and MCNeuA orthotopic models. These models allow us to focus on the effect of T2D on growth of fully transformed mammary epithelial cells, whereas in the double transgenic mice the entire tumorigenic process, including preneoplastic stages, is affected by the diabetic milieu. The syngeneic models enable us to study the effect of T2D at specific time points, and, in contrast to the commonly used xenograft tumor models, they allow us to observe tumorigenesis in the setting of intact immunity.
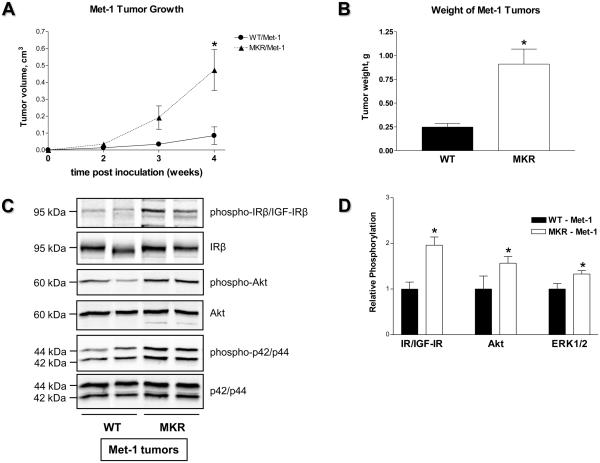
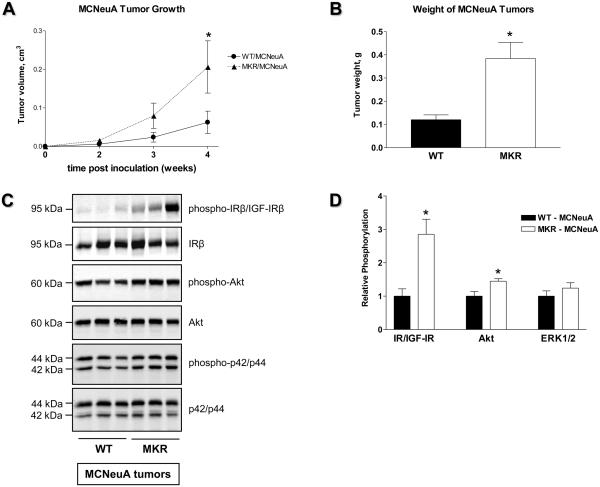
ERα-negative Met-1 and MCNeuA cell lines were originally derived from PyVmT- and Neu-induced mammary tumors, respectively (21, 22). Neu is a rodent analog of the human ERBB2 gene which is amplified and overexpressed in 30% of human breast carcinomas (23). In contrast to Met-1 cells, which demonstrate a mesenchymal phenotype and a high proliferation rate, MCNeuA cells show an epithelial phenotype and a low proliferation rate (data not shown). Both cell types are tumorigenic when implanted back into syngeneic FVB/N mice. We inoculated 500,000 Met-1 cells and 1,000,000 MCNeuA cells into the inguinal mammary fat pad (#4) of MKR+/+ and WT females and monitored tumor growth. Tumor growth is markedly increased (~ 3-5 fold) in MKR+/+ mice orthotopically inoculated with either Met-1 or MCNeuA cells (Fig. 4A, 4B, 5A, 5B). Furthermore, Met-1 and MCNeuA tumor tissues extracted from MKR+/+ mice display significantly elevated levels of IR/IGF-IR phosphorylation compared to WT controls (Fig. 4C, 4D, 5C, 5D). In addition, MKR+/+ mice also demonstrate a markedly enhanced phosphorylation of Akt and a moderate increase in ERK1/2 phosphorylation in both tumor models (Fig. 4C, 4D, 5C, 5D). Taken together, data from the transgenic and orthotopic models suggest that hyperinsulinemia, acting directly through the IR and/or indirectly through the IGF-IR, further transduces an intracellular signal primarily through the PI3K pathway and augments tumor development in MKR+/+ mice.
Figure 4.
(A) Growth and (B) terminal weight of Met-1 tumors obtained from WT and MKR+/+ female mice (7 animals per group; 4 weeks after orthotopic inoculation of 500,000 Met-1 cells). This study was replicated three times; the representative graphs are presented. Statistically significant difference is indicated (*), P < 0.05 (Student's t-test). (C) Proteins (50 ug) extracted from Met-1 tumor tissues were size-fractionated by SDS-PAGE and immunoblotted with anti-phospho-IRβY1150/51/IGF-IRβY1135/36, anti-phospho-AktS473 and anti-phospho-p42/p44T202/Y204 antibodies. Total level of proteins was demonstrated by immunoblotting with antibodies directed against total IRβ, Akt and p42/p44. Five animals per group were analyzed, the representative blots are included. (D) The results of densitometric analysis of IR/IGF-IR, Akt and ERK1/2 phosphorylation in Met-1 tumor tissue are presented as a fold change compared to WT tumor tissue. Statistically significant difference is indicated (*), P < 0.05 (Student's t-test).
Figure 5.
(A) Growth and (B) terminal weight of MCNeuA tumors obtained from WT and MKR+/+ female mice (4-7 animals per group; 4 weeks after orthotopic inoculation of 1,000,000 MCNeuA cells). This study was replicated three times; the representative graphs are presented. Statistically significant difference is indicated (*), P < 0.05 (Student's t-test). (C) Proteins (50 ug) extracted from MCNeuA tumor tissues were size-fractionated by SDS-PAGE and immunoblotted with anti-phospho-IRβY1150/51/IGF-IRβY1135/36, anti-phospho-AktS473 and anti-phospho-p42/p44T202/Y204 antibodies. Total level of proteins was demonstrated by immunoblotting with antibodies directed against total IRβ, Akt and p42/p44. 3-5 animals per group were analyzed, the representative blots are included. (D) The results of densitometric analysis of IR/IGF-IR, Akt and ERK1/2 phosphorylation in MCNeuA orthograft tumor tissue are presented as a fold change compared to WT tumor tissue. Statistically significant difference is indicated (*), P < 0.05 (Student's t-test).
Effect of pharmacological IR/IGF-IR blockade on the tumor-promoting activity of T2D
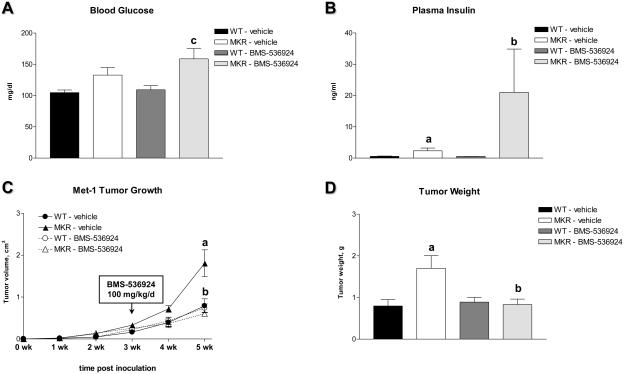
To corroborate that hyperinsulinemia, IR/IGF-IR activation and accelerated tumor growth in diabetic animals are mechanistically linked, we employed a small-molecule tyrosine kinase inhibitor, BMS-536924 (24). This compound blocks the ATP-binding cleft, which demonstrates 100% sequence identity in the IR and the IGF-IR. This mechanism of tyrosine kinase inhibition makes BMS-536924 a fully non-selective dual-receptor inhibitor. In Met-1 cells, insulin- and IGF-I-induced signaling is markedly suppressed in the presence of BMS-536924 both in vitro and in vivo (Supplementary Fig. S4, S5). To study the effect of IR and IGF-1R blockade in vivo, WT and MKR+/+ female mice were subjected to orthotopic inoculation of Met-1 cells as described above. Three weeks after cell inoculation, WT and MKR+/+ mice were treated with BMS-536924 or respective vehicle (80% polyethylene glycol, 20% water) (100 mg/kg/day, orally by gavage) for two weeks. Tumor volume and metabolic parameters were determined weekly. Our results indicate that BMS-536924 does not affect tumor growth or carbohydrate metabolism in WT mice (Fig. 6). In contrast, in MKR+/+ mice, treatment with BMS-536924 results in a moderate increase in blood glucose levels (Fig. 6A), an impairment of glucose tolerance (data not shown) and a marked elevation of plasma insulin levels (Fig. 6B). However, despite exacerbated hyperinsulinemia, tumor growth is significantly attenuated in MKR+/+ mice treated with BMS-536924 (Fig. 6C, 6D) suggesting that the IR and/or the IGF-IR are the predominant mediators of the tumor-promoting activity of T2D in general and hyperinsulinemia in particular.
Figure 6.
Effect of pharmacological IR/IGF-IR blockade on Met-1 tumor growth and carbohydrate metabolism in MKR+/+ female mice. Eight week-old WT and MKR+/+ female mice (n=5) were subjected to orthotopic inoculation of 500,000 Met-1 cells. Three weeks after tumor inoculation, small-molecule IR/IGF-IR tyrosine kinase inhibitor BMS-536924 (100 mg/kg/day orally by gavage) or the respective vehicle (80% polyethylene glycol, 20% water) was applied for the next 14 days. (A) Blood glucose and (B) plasma insulin levelsin vehicle- or BMS-536924-treated WT and MKR+/+ female mice at the end of the study. (C) Growth and (D) terminal weight of Met-1 tumors obtained from WT and MKR+/+ female mice treated with vehicle or BMS-536924. Statistically significant difference (P < 0.05, two-factor ANOVA followed by Holm-Sidak post-hoc test) is indicated: (a) WT-vehicle versus MKR-vehicle, (b) MKR-vehicle versus MKR-BMS-536924, (c) WT-BMS-536924 versus MKR-BMS-536924.
Discussion
Numerous epidemiological studies suggest that obesity and type 2 diabetes (T2D) significantly affect the development of many cancers, including breast cancer. However, a positive correlation between obesity, T2D and breast cancer is mainly observed among postmenopausal women (1, 3-5). In turn, animal studies demonstrate that diet-induced metabolic derangements promote mammary carcinogenesis irrespective of ovarian function, but they do not explain the molecular basis of this association (11). An attempt to uncouple the tumor-promoting effect of body adiposity from diabetes was done by employing the transgenic A-ZIP/F-1 mouse model of lipoatrophic form of T2D (25). Although these data clearly suggest a direct role of the diabetic milieu in tumor development, the study does not dissect a pathophysiological mechanism underlying this phenomenon.
In the current work, we employed a unique transgenic MKR model of T2D, in which severe insulin resistance and hyperinsulinemia are observed in the setting of moderately reduced body adiposity and mild dysglycemia. MKR female mice demonstrate accelerated mammary gland development and breast cancer progression independent of obesity and inflammation. In addition, we show that the signaling axis including insulin, IR/ IGF-IR and the PI3K/Akt pathway is the major pathophysiological mechanism mediating this effect.
In vivo, endogenous insulin may have a tumor-promoting activity, which has been underestimated for a long time. Reduction of insulin levels in animals with chemically-induced type 1 diabetes (T1D) resulting from the destruction of pancreatic beta cells suppresses tumor growth, whereas insulin administration abrogates this effect (26-30). Furthermore, intraportal transplantation of pancreatic islets into rats with T1D results in the development of an insulin-enriched microenvironment promoting hepatocellular transformation (31). These studies, which employed experimental models of T1D, thus link insulin and cancer mechanistically and indicate that insulin plays a role of both tumor promoter and tumor initiator. In line with these data, our results strongly support the tumor-promoting effect of elevated circulating insulin levels in T2D, in the setting of severe insulin resistance and normal ovarian function. These data are also in accordance with epidemiological studies demonstrating a positive correlation between circulating insulin levels and breast cancer risk and mortality (32).
The tumor-promoting activity of insulin can be mediated by direct and indirect mechanisms. Insulin is known to transduce the intracellular signal through its cognate receptor. Indeed, the results of our study demonstrate enhanced IR phosphorylation in mammary and tumor tissue extracted from diabetic mice. Our data, however, also show an increase in IGF-IR phosphorylation suggesting that the tumor-promoting activity of hyperinsulinemia can also be mediated, at least in part, by the IGF-IR. It is well known that at higher concentrations, insulin stimulates the IGF-I receptor (17, 33). In Met-1 cells, which were employed in the current work, IGF-IR activation is observed in response to supraphysiological concentrations of insulin (± 50 nM, physiological range 1-10 nM; unpublished data). Therefore, it is plausible that elevated circulating insulin levels may also result in IGF-IR activation in vivo. In addition, in certain cell types, insulin activates the IGF-IR at physiological concentrations (33). Moreover, some cells including human breast cancer cells express an atypical IGF-IR, which binds both IGF-I and insulin with high affinity (34). These data further support our hypothesis that the tumor-promoting activity of hyperinsulinemia can be associated with the IGF-IR. Furthermore, the tumor-promoting activity of hyperinsulinemia can also be mediated by IGFs. Mammary stroma and breast cancer cells are an abundant source of IGFs and their binding proteins (IGFBPs) (35). Insulin inhibits IGFBP-1 and IGFBP-2 expression (7) and thus increases the bioavailability of IGFs. Our results demonstrate a 30% reduction in IGFBP-1 and -2 levels in sera of diabetic mice suggesting a possible role of this mechanism in the tumor-promoting activity of T2D. Alternatively, the promoting action of hyperinsulinemia on mammary gland development and formation of PyVmT-induced hyperplastic lesions can also be mediated by ERα, which interacts with the IGF-IR/IR at multiple cellular levels (36, 37).
We also show that IR levels in mammary epithelium of diabetic mice are elevated. The role of the IR in oncogenic transformation and tumorigenesis has not been extensively studied. It is known that IR overexpression results in cellular transformation (38). A functional IR is essential for the transformation of endothelial cells infected by Kaposi sarcoma-associated herpes virus (39). IR upregulation is also observed in mouse mammary epithelium transformed by wnt-1, neu and ret (40). In addition, human mammary carcinomas show higher IR levels than normal breast tissue (38). Total IR levels in breast tumor tissue are inversely correlated with a disease-free survival (41, 42). These data strongly suggest that in breast tissue the IR has an important biological and clinical relevance.
IR- and IGF-IR transduce their intracellular signal through two principal signaling pathways, the MAPK and the PI3K pathway (17). Our data demonstrate that signaling through the PI3K pathway is enhanced in normal mammary gland and breast tumor tissue of diabetic animals suggesting that this pathway may mediate the effects of hyperinsulinemia on normal and transformed cells. Certain stages of mammary gland development are governed by the PI3K/Akt pathway (43), whereas its deregulation and hyperactivation result in tumor transformation (44-48). Targeting the PI3K/Akt pathway, therefore, may be a promising therapeutic option for breast cancer patients with T2D.
Taken together, our data provide compelling evidence that T2D accelerates mammary gland development and breast cancer progression. We hypothesize that due to hyperinsulinemia and increased insulin receptor expression, the mammary epithelium in T2D is more susceptible to mitogenic and survival signals, which accelerate mammary gland development and facilitate tumor formation and progression. Mammary carcinogenesis in T2D represents a two-hit phenomenon, with an oncogene (e.g. PyVmT or Neu) acting as a tumor initiator and hyperinsulinemia acting as a tumor promoter. The present study has an important clinical relevance and significant public health implications. Early detection and therapeutic correction of hyperinsulinemia may help to reduce breast cancer morbidity and mortality. Furthermore, our data provide the rationale for pharmacological targeting a specific signaling cascade comprising insulin, the IR/IGF-IR and the PI3K/Akt pathway in breast cancer patients with T2D.
Supplementary Material
Acknowledgements
We thank W.J. Muller (McGill University, Montreal, Quebec, Canada), S.D. Hurstings (Department of Nutritional Sciences, University of Texas, Austin, TX, and Department of Carcinogenesis, University of Texas - M.D. Anderson Cancer Center, Smithville, TX, USA) and N.P.Nunez (Department of Nutritional Sciences, University of Texas, Austin, TX, USA) and M.J. Campbell and J.F. Youngren (University of California San Francisco, San Francisco, CA, USA) for donating MMTV-PyVmT transgenic mice, Met-1 and MCNeuA cells, respectively. We also apologize to those colleagues whose publications were not cited owing to space limitations.
Financial Support
This work was funded by National Cancer Institute [1RO1CA128799-O1A1] and the Gerald J. and Dorothy R. Friedman Foundation. Danielle Lann was supported by National Institutes of Health [T32 DK007792]. Yvonne Fierz was funded by Swiss National Science Foundation [PBBSB-120851], Novartis Foundation and Roche Research Foundation grants.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Reference List
- 1.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 2.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–74. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 4.Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr. 2007;86:s823–s835. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- 5.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–64. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rose DP, Komninou D, Stephenson GD. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev. 2004;5:153–65. doi: 10.1111/j.1467-789X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 7.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 8.Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8:395–408. doi: 10.1111/j.1467-789X.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 9.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–62. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 10.Lann D, LeRoith D. The role of endocrine insulin-like growth factor-I and insulin in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:371–9. doi: 10.1007/s10911-008-9100-x. [DOI] [PubMed] [Google Scholar]
- 11.Novosyadlyy R, Vijayakumar A, Fierz Y, LeRoith D. In: Animal models of hyperinsulinemia, insulin resistance and cancer. Fantus IG, editor. Springer; New York: In press. [Google Scholar]
- 12.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–61. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez AM, Kim JK, Yakar S, et al. Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev. 2001;15:1926–34. doi: 10.1101/gad.908001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le MC, Chu K, Hu M, et al. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A. 2006;103:9232–7. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleinberg DL, Wood TL, Furth PA, Lee AV. Growth Hormone and Insulin-Like Growth Factor-I in the Transition from Normal Mammary Development to Preneoplastic Mammary Lesions. Endocr Rev. 2008 doi: 10.1210/er.2008-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev Cell. 2001;1:467–75. doi: 10.1016/s1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- 17.LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–63. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 18.Boni-Schnetzler M, Schmid C, Meier PJ, Froesch ER. Insulin regulates insulin-like growth factor I mRNA in rat hepatocytes. Am J Physiol. 1991;260:E846–E851. doi: 10.1152/ajpendo.1991.260.6.E846. [DOI] [PubMed] [Google Scholar]
- 19.Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20:535–82. doi: 10.1210/edrv.20.4.0374. [DOI] [PubMed] [Google Scholar]
- 20.Lin EY, Jones JG, Li P, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–26. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borowsky AD, Namba R, Young LJ, et al. Syngeneic mouse mammary carcinoma cell lines: two closely related cell lines with divergent metastatic behavior. Clin Exp Metastasis. 2005;22:47–59. doi: 10.1007/s10585-005-2908-5. [DOI] [PubMed] [Google Scholar]
- 22.Campbell MJ, Wollish WS, Lobo M, Esserman LJ. Epithelial and fibroblast cell lines derived from a spontaneous mammary carcinoma in a MMTV/neu transgenic mouse. In Vitro Cell Dev Biol Anim. 2002;38:326–33. doi: 10.1290/1071-2690(2002)038<0326:EAFCLD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 24.Wittman M, Carboni J, Attar R, et al. Discovery of a (1H-benzoimidazol-2-yl)-1H-pyridin-2-one (BMS-536924) inhibitor of insulin-like growth factor I receptor kinase with in vivo antitumor activity. J Med Chem. 2005;48:5639–43. doi: 10.1021/jm050392q. [DOI] [PubMed] [Google Scholar]
- 25.Nunez NP, Oh WJ, Rozenberg J, et al. Accelerated tumor formation in a fatless mouse with type 2 diabetes and inflammation. Cancer Res. 2006;66:5469–76. doi: 10.1158/0008-5472.CAN-05-4102. [DOI] [PubMed] [Google Scholar]
- 26.Heuson JC, Legros N, Heimann R. Influence of insulin administration on growth of the 7,12-dimethylbenz(a)anthracene-induced mammary carcinoma in intact, oophorectomized, and hypophysectomized rats. Cancer Res. 1972;32:233–8. [PubMed] [Google Scholar]
- 27.Heuson JC, Legros N. Influence of insulin deprivation on growth of the 7,12-dimethylbenz(a)anthracene-induced mammary carcinoma in rats subjected to alloxan diabetes and food restriction. Cancer Res. 1972;32:226–32. [PubMed] [Google Scholar]
- 28.Cohen ND, Hilf R. Influence of insulin on growth and metabolism of 7,12-dimethylbenz(alpha)anthracene-induced mammary tumors. Cancer Res. 1974;34:3245–52. [PubMed] [Google Scholar]
- 29.Shafie SM, Grantham FH. Role of hormones in the growth and regression of human breast cancer cells (MCF-7) transplanted into athymic nude mice. J Natl Cancer Inst. 1981;67:51–6. [PubMed] [Google Scholar]
- 30.Sharon R, Pillemer G, Ish-Shalom D, et al. Insulin dependence of murine T-cell lymphoma. II. Insulin-deficient diabetic mice and mice fed low-energy diet develop resistance to lymphoma growth. Int J Cancer. 1993;53:843–9. doi: 10.1002/ijc.2910530523. [DOI] [PubMed] [Google Scholar]
- 31.Dombrowski F, Bannasch P, Pfeifer U. Hepatocellular neoplasms induced by low-number pancreatic islet transplants in streptozotocin diabetic rats. Am J Pathol. 1997;150:1071–87. [PMC free article] [PubMed] [Google Scholar]
- 32.Gunter MJ, Hoover DR, Yu H, et al. Insulin, Insulin-Like Growth Factor-I, and Risk of Breast Cancer in Postmenopausal Women. J Natl Cancer Inst. 2008 doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Humpert PM, Djuric Z, Zeuge U, et al. Insulin stimulates the clonogenic potential of angiogenic endothelial progenitor cells by IGF-1 receptor-dependent signaling. Mol Med. 2008;14:301–8. doi: 10.2119/2007-00052.Humpert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milazzo G, Yip CC, Maddux BA, Vigneri R, Goldfine ID. High-affinity insulin binding to an atypical insulin-like growth factor-I receptor in human breast cancer cells. J Clin Invest. 1992;89:899–908. doi: 10.1172/JCI115670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood TL, Richert MM, Stull MA, Allar MA. The insulin-like growth factors (IGFs) and IGF binding proteins in postnatal development of murine mammary glands. J Mammary Gland Biol Neoplasia. 2000;5:31–42. doi: 10.1023/a:1009511131541. [DOI] [PubMed] [Google Scholar]
- 36.Smith CL. Cross-talk between peptide growth factor and estrogen receptor signaling pathways. Biol Reprod. 1998;58:627–32. doi: 10.1095/biolreprod58.3.627. [DOI] [PubMed] [Google Scholar]
- 37.Fagan DH, Yee D. Crosstalk between IGF1R and estrogen receptor signaling in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:423–9. doi: 10.1007/s10911-008-9098-0. [DOI] [PubMed] [Google Scholar]
- 38.Frasca F, Pandini G, Sciacca L, et al. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008;114:23–37. doi: 10.1080/13813450801969715. [DOI] [PubMed] [Google Scholar]
- 39.Rose PP, Carroll JM, Carroll PA, et al. The insulin receptor is essential for virus-induced tumorigenesis of Kaposi's sarcoma. Oncogene. 2007;26:1995–2005. doi: 10.1038/sj.onc.1210006. [DOI] [PubMed] [Google Scholar]
- 40.Frittitta L, Cerrato A, Sacco MG, et al. The insulin receptor content is increased in breast cancers initiated by three different oncogenes in transgenic mice. Breast Cancer Res Treat. 1997;45:141–7. doi: 10.1023/a:1005801713713. [DOI] [PubMed] [Google Scholar]
- 41.Mathieu MC, Clark GM, Allred DC, Goldfine ID, Vigneri R. Insulin receptor expression and clinical outcome in node-negative breast cancer. Proc Assoc Am Physicians. 1997;109:565–71. [PubMed] [Google Scholar]
- 42.Law JH, Habibi G, Hu K, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68:10238–46. doi: 10.1158/0008-5472.CAN-08-2755. [DOI] [PubMed] [Google Scholar]
- 43.Boxer RB, Stairs DB, Dugan KD, et al. Isoform-specific requirement for Akt1 in the developmental regulation of cellular metabolism during lactation. Cell Metab. 2006;4:475–90. doi: 10.1016/j.cmet.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Dupont J, Renou JP, Shani M, Hennighausen L, LeRoith D. PTEN overexpression suppresses proliferation and differentiation and enhances apoptosis of the mouse mammary epithelium. J Clin Invest. 2002;110:815–25. doi: 10.1172/JCI13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saal LH, Holm K, Maurer M, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–9. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 46.Zhao H, Cui Y, Dupont J, et al. Overexpression of the tumor suppressor gene phosphatase and tensin homologue partially inhibits wnt-1-induced mammary tumorigenesis. Cancer Res. 2005;65:6864–73. doi: 10.1158/0008-5472.CAN-05-0181. [DOI] [PubMed] [Google Scholar]
- 47.Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 2007;67:167–77. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- 48.Ju X, Katiyar S, Wang C, et al. Akt1 governs breast cancer progression in vivo. Proc Natl Acad Sci U S A. 2007;104:7438–43. doi: 10.1073/pnas.0605874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.