Abstract
Background
While there is much discussion regarding ethical payment to healthy volunteers participating in clinical research, little data exist regarding how volunteers determine fair payment.
Objective
To determine healthy volunteers’ estimates of payment for participating in hypothetical clinical trials, to explore reasons for their estimates, and to examine associations of volunteer demographics with payment estimates.
Methods
Sixty participants with previous healthy volunteer research experience were presented with four hypothetical studies and interviewed about their impressions of the studies.
Results
For each study, the range of payment estimates varied greatly. However, individuals tended to be consistent in estimate placement within this range. No demographic factor was significantly associated with the estimated study payment. Subjects frequently mentioned risk and logistical burden as factors which should determine payment levels.
Conclusions
Healthy volunteer subjects have an individualized yet consistent method of determining study payment based on perception of study burden and risk.
Keywords: Healthy volunteers, research subjects, clinical research, remuneration, payment, compensation
INTRODUCTION
Paying healthy volunteers for participating in medical research is commonplace, but there is little agreement as to what constitutes appropriate payment. Different rationales exist for providing payment. Reimbursement is provided to subjects for expenses incurred while participating in a study, such as parking fees and childcare. Compensation is payment to research subjects for injuries sustained during participation. A third form of payment, remuneration, is intended to pay volunteers for the time or inconvenience sustained by participating in the study. Finally, inducement is payment explicitly intended to provide an incentive for volunteers to participate in a study. While reimbursement and compensation generally remain uncontroversial, some authors have raised concerns about the ethical acceptability of remuneration and, particularly, inducements (1). These concerns include the possibility that such forms of payment may constitute undue inducement, potentially compromising autonomy and/or the voluntary nature of participation. This may “prompt subjects to lie, deceive, or conceal information that, if known, would disqualify them as participants in a research project” (2, 3).
Most research centers and Institutional Review Boards (IRBs) within the United States allow financial payment to volunteers and it is normative among ethicists that such payment is acceptable. Nonetheless, questions remain concerning appropriate payment for different types of studies and what factors should determine payment amounts. In satisfying ethical requirements both to avoid levels of payment that may constitute an undue inducement and to provide a reasonable level of fairness across different studies, it is useful to consider what appropriate payment means to volunteers themselves.
While the literature demonstrates widely varying views on what constitutes appropriate payment (4-6), little data exist documenting the views of research volunteers themselves. Chaput de Saintonge et al surveyed medical students as potential volunteers for clinical research (7), and found that students (who did not necessarily have research experience) expected to be paid for risks and inconvenience. Payment considerations may be different among a more diverse population of study volunteers, however, and among those with actual research experience. Volunteers’ opinions regarding payment adds valuable perspective not only to what financial value volunteers place on participation, but also, importantly, to why participants believe research studies pay the amounts that they do.
We surveyed 60 adults who served as healthy volunteers in one or more clinical trials for which they received payment. Four hypothetical studies, similar to actual studies offered at our institution and designed to be of varying levels of risk and inconvenience, were described. Volunteers were asked how much money they thought each study should pay; they also were asked about factors they believed should influence specific payment levels for different studies. Understanding healthy volunteers’ opinions regarding payment is relevant to IRBs, investigators, and institutions which establish and approve payment levels for study participation.
RESULTS
The study population consisted of 60 volunteers of whom 62% were from the community and 38% were employees of the institution. Sample characteristics are summarized in Table 1. A more complete characterization of this sample, their attitudes and experiences has been published previously (8). The majority of the participants were employed (80%), non-white (62%), unmarried (80%), and had at least some education beyond high school (60%).
Table 1. Healthy volunteers’ mean payment estimates for the four hypothetical studies stratified by demographic characteristics.
Volunteers listened to descriptions of four hypothetical studies and after each description were asked how much people should be paid to join such a study. N for each study is less than 60 because of missing data. Percentages are those of the number who provided estimates.
| Overall | Investigational Drug Study | Plant Extract Study | HIV Study | Malaria Challenge Study | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | Mean | P-value | N (%) | Mean | P-value | N (%) | Mean | P-value | N (%) | Mean | P-value | |
| Overall Experience | |||||||||||||
| 0-5 studies | 36 (60) | 35 (61) | $476 | 0.64 | 34 (59) | $1,434 | 0.80 | 36 (60) | $1,603 | 0.82 | 35 (59) | $1,299 | 0.23 |
| > 5 studies | 24 (40) | 22 (39) | $427 | 24 (41) | $1,507 | 24 (40) | $1,666 | 24 (41) | $1,699 | ||||
| Past 2 Years Experience | |||||||||||||
| 0-2 studies | 45 (75) | 43 (75) | $445 | 0.89 | 43 (74) | $1,386 | 0.34 | 45 (75) | $1,626 | 0.99 | 44 (75) | $1,397 | 0.57 |
| > 2 studies | 15 (25) | 14 (25) | $460 | 15 (26) | $1,713 | 15 (25) | $1,631 | 15 (25) | $1,613 | ||||
| Would Participate in Study | |||||||||||||
| Yes | --- | 23 (40) | $402 | 0.56a 0.20b 0.37c | 24 (41) | $1,600 | 0.38a 0.96b 0.59c | 22 (37) | $1,551 | 0.84a 0.37b 0.42c | 13 (22) | $1,321 | 0.55a 0.76b 0.42c |
| No | --- | 26 (46) | $463 | 27 (47) | $1,327 | 31 (52) | $1,607 | 39 (66) | $1,552 | ||||
| Don’t know/It depends | --- | 8 (14) | $628 | 7 (12) | $1,572 | 7 (12) | $2,001 | 7 (12) | $1,172 | ||||
| Primary motivation | |||||||||||||
| Money | 33 (57) | 30 (55) | $552 | 0.10 | 32 (57) | $1,603 | 0.44 | 33 (57) | $1,907 | 0.053 | 32 (56) | $1,784 | 0.055 |
| Other | 25 (43) | 25 (45) | $384 | 24 (43) | $1,378 | 25 (43) | $1,372 | 25 (44) | $1,164 | ||||
| Gender | |||||||||||||
| Female | 26 (43) | 24 (42) | $368 | 0.10 | 25 (43) | $1,405 | 0.72 | 26 (43) | $1,618 | 0.95 | 26 (44) | $1,247 | 0.22 |
| Male | 34 (57) | 33 (58) | $533 | 33 (57) | $1,510 | 34 (57) | $1,634 | 33 (56) | $1,631 | ||||
| Employment status* | |||||||||||||
| Employed | 44 (80) | 41 (75) | $541 | 0.12 | 42 (79) | $1,682 | 0.80 | 44 (80) | $1,682 | 0.45 | 43 (80) | $1,389 | 0.11 |
| Not employed | 11 (20) | 11 (25) | $359 | 11 (21) | $1,607 | 11 (20) | $1,946 | 11 (20) | $2,151 | ||||
| Race | |||||||||||||
| White | 23 (38) | 21 (37) | $405 | 0.41 | 22 (38) | $1,449 | 0.94 | 23 (38) | $1,533 | 0.58 | 22 (37) | $1,251 | 0.30 |
| Non-white | 37 (62) | 36 (63) | $489 | 36 (62) | $1,473 | 37 (62) | $1,689 | 37 (63) | $1,581 | ||||
| Have children | |||||||||||||
| Yes | 31 (52) | 29 (51) | $488 | 0.54 | 29 (50) | $1,628 | 0.28 | 31 (52) | $1,701 | 0.59 | 30 (51) | $1,582 | 0.41 |
| No | 29 (48) | 28 (49) | $426 | 29 (50) | $1,316 | 29 (48) | $1,553 | 29 (49) | $1,323 | ||||
| Age | |||||||||||||
| 18-44 | 36 (60) | 36 (63) | $428 | 0.44 | 35 (60) | $1,349 | 0.30 | 36 (60) | $1,476 | 0.15 | 36 (61) | $1,157 | 0.008 |
| > 44 | 24 (40) | 21 (37) | $510 | 23 (40) | $1,657 | 24 (40) | $1,884 | 23 (39) | $2,062 | ||||
| Married/with partner | |||||||||||||
| Yes | 12 (20) | 10 (18) | $720 | 0.054 | 11 (19) | $2,276 | 0.026 | 12 (20) | $2,022 | 0.19 | 11 (19) | $1,538 | 0.80 |
| No | 48 (80) | 47 (82) | $414 | 47 (81) | $1,320 | 48 (80) | $1,541 | 48 (81) | $1,429 | ||||
| Affiliation | |||||||||||||
| Institutional employee | 23 (38) | 20 (35) | $418 | 0.56 | 22 (38) | $1,503 | 0.83 | 23 (38) | $1,429 | 0.22 | 22 (37) | $1,188 | 0.16 |
| Community volunteer | 37 (62) | 37 (65) | $479 | 36 (62) | $1,440 | 37 (62) | $1,764 | 37 (63) | $1,631 | ||||
| Education | |||||||||||||
| High school or less | 24 (40) | 24 (42) | $371 | 0.11 | 24 (41) | $1,271 | 0.22 | 24 (40) | $1,494 | 0.40 | 24 (41) | $1,375 | 0.69 |
| Beyond high school | 36 (60) | 33 (58) | $530 | 34 (59) | $1,618 | 36 (60) | $1,723 | 35 (59) | $1,502 | ||||
Defined as employed at the time of participation in the clinical trials.
P-value for the Yes to No comparison.
P-value for the Yes to Don’t know/It depends comparison.
P-value for the No to Don’t know/It depends comparison.
Participants were asked if they would agree to participate in each of the four studies presented and to estimate the payment they thought volunteers who joined each study should be offered. Estimates and distribution characteristics are summarized in Table 2. The geometric mean expected payment was $456 for a single-dose Investigational Drug study (median $500, low $100, high $3,000), $1,464 for a Plant Extract study (median $1,675, low $75, high $5,500), $1,627 for an HIV study (median $1,500, low $150, high $5,500), and $1,449 for a Malaria Challenge study (median $1,500, low $250, high $10,000). Also included in Table 2 are the payments that would have been offered for each study at the time of the interviews as determined by the payment schedule of our institution’s Phase1/Phase 2 trials unit. Pairwise comparisons of the payment estimates between trials were performed, and a significant difference was found between participants’ expected payment for the Investigational Drug study as compared to participants’ expected payment for each of the other three studies (p < 0.001 for each). No statistically significant differences were found among paired estimates for the other three studies.
Table 2. Summary statistics and distribution characteristics of responses to the question, “How much do you think people should be paid to join a study like this?”.
| Investigational Drug Study |
Plant Extract Study |
HIV Study | Malaria Challenge Study |
|
|---|---|---|---|---|
| Expected Payment | ||||
| Geometric Mean | $456 | $1,464 | $1,627 | $1,449 |
| 95% CI | $367 - $568 | $1,205 - $1,778 | $1,380 - $1,920 | $1,167 - $1,799 |
| Median | $500 | $1,675 | $1,500 | $1,500 |
| Low Estimate | $100 | $75 | $150 | $250 |
| High Estimate | $3,000 | $5,500 | $5,500 | $10,000 |
| Interquartile Range | $450 | $1,500 | $1,500 | $1,700 |
| Payment per Phase I/II Unit Schedule | $270 | $1,471 | $1,000 | $1,672 |
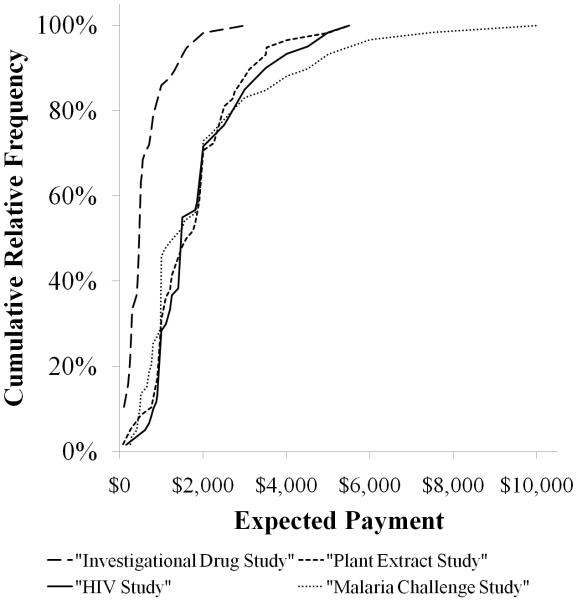
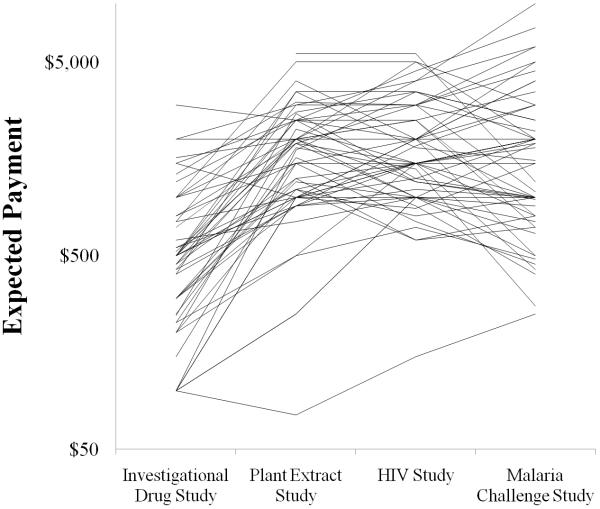
In order to characterize the range of responses for each particular study, respondents’ payment estimates were graphed against the cumulative relative frequency of respondent estimates for the four studies. As seen in Figure 1, the amounts suggested for the Investigational Drug study overall were consistently lower than the amounts suggested for the other three studies, while the amounts volunteers suggested for the three others studies were similar to each other. Furthermore, distributions were right-skewed, with approximately 70% of estimates being less than $300 for the Investigational Drug study and less than $2000 for the other three studies. Notably, the Malaria Challenge study yielded some individual estimates that were considerably higher than those provided for any of the other three studies. The relative positions of individual respondents on each of these curves were compared by the Spearman rank correlation coefficient, and individual correlations among all four studies were significant (rs = 0.34 -0.69, p ≤ 0.01 for all comparisons). Conceptually, this statistical test places the payment estimate of each respondent in rank order for each study and then compares the order among studies. The finding of statistical significance for each study compared to the others indicates that individual participants’ payment estimates were similar in rank order relative to others participants’ estimates for each of the four studies (Table 3). Figure 2 is a visual representation of this concept. First, the wide variation of estimates for a particular study is demonstrated by the spread of the individual points in each study. Second, each subject’s estimate for a particular study is connected to that subject’s estimates for the other three studies by a line. This demonstrates the relative consistency of a subject’s estimates in relation to other subjects’ estimates. In other words, subjects who gave lower estimates for one study were likely to give lower estimates for the other three studies as well.
Figure 1. Cumulative proportion of volunteers stating expected payment amounts for each study.
While payment estimates were significantly lower for the Investigational Drug Study, the other three studies were almost indistinguishable with the exception that the Malaria Challenge study yielded a few significantly higher estimates than any of the other studies.
Table 3. Spearman’s rho statistics for each study compared to the others.
| Plant Extract study |
HIV study | Malaria Challenge study |
|
|---|---|---|---|
| Investigational Drug study | 0.49** | 0.44** | 0.34* |
| Plant Extract study | 0.65** | 0.42* | |
| HIV study | 0.69** |
p ≤ 0.01,
p ≤ 0.001.
Figure 2. Estimated payment by each volunteer for each of the four hypothetical studies.
Lines connect the estimates of one participant. There is more variation in subjects’ estimates within a particular study than variation in any one subject’s estimates in relation to other participant’s estimates across the four studies.
Next, we stratified payment estimates by demographic characteristics (Table 1). While our study was underpowered to detect statistically significant differences between groups, several trends emerged. Specifically, there was a trend for males, non-white participants, those with children, those with money as their primary motivation for participation, and those with more than a high school education to provide higher estimates of payment than females, whites, those without children, those with primary motivations other than money, and those with a high school education or less. Those 45 years of age or older tended to estimate higher levels of payment than younger volunteers across all studies; this difference reached statistical significance for the Malaria Challenge study (p = 0.008). Participants who were married or had a domestic partner estimated higher levels of payment across all studies than those who were single; this difference reached statistical significance for the Plant Extract study (p= 0.026). Finally, we observed no correlation related to the extent of previous clinical trial participation, willingness to participate in the hypothetical clinical trials, employment status, or whether the volunteer was an institutional employee or a community volunteers.
In order to further explore the contributions of the demographic factors to estimates of payment, we performed multivariate linear regression analyses. The demographic factors mentioned above as suggesting a trend (gender, race, having children, education, age, and marital status) were included in the model, as were employment status and whether or not the participant was an employee of our institution. A model containing these independent variables was then generated for each of the four studies with the log-transformed payment estimate as the dependent variable. Various factors reached statistical significance for different models, but the significant factors were not consistent across models and no factors were significant across all four models. Therefore, models are not reported herein.
In order to understand the factors relevant to respondents’ payment estimates, participants were asked an open-ended question: “Why should some studies pay more and others pay less? In other words, what should payment be based on?” Ninety percent of subjects volunteered a response that related to the logistical burden of the study, including the number of procedures, visits, or medications required; the time spent in the study; or the inconvenience of participation. Sixty-five percent mentioned a topic related to the risks of the study, including the medical risks, potential side effects, or discomfort.
DISCUSSION
Determining an appropriate level of payment that upholds respect for research subjects, attracts a reasonable number of participants, and avoids creating undue influence remains a topic of much debate in bioethical and clinical research literature. An informal survey we conducted of several of the top NIH-funded institutions’ IRB policies as available on the internet revealed that there is little consistency across institutions in determining what level of payment is appropriate or how payment should be calculated (9-13). We are aware of no IRBs that formally set a policy outlining fee schedules based on different study requirements. Nearly absent from the existing literature are data documenting the level of payment healthy volunteers themselves consider acceptable and how their payment estimates are derived.
Our participants were presented with studies that we believed varied significantly in medical risk and logistical burden, and we hypothesized that payment estimates might vary in accordance with the differing levels of risk and/or burden. If volunteers thought payment should be based on medical risk, for example, and if they assessed risk comparably to the investigators, we expected estimates for the Plant Extract study to be lowest, and estimates for the Malaria Challenge study to be highest. Conversely, if logistical burden was most relevant to subjects and if they viewed the burden of these studies similarly to the investigators, then we would expect estimates to be lowest for the Investigational Drug study and highest for the Plant Extract study.
We found that subjects estimated a lower level of payment for the Investigational Drug study (which investigators viewed as intermediate medical risk but involved the least logistical burden), while suggesting higher levels of payment for the Plant Extract, HIV, and Malaria Challenge studies, all of which required more time/burden but varied from very low risk to considerably higher risk. These findings suggest that healthy volunteers may be more attuned to the tangible burdens of time spent and procedures endured than to medical risk. Interestingly, many volunteers voiced that risk is an important consideration to them in estimating appropriate payment, yet their estimates seemed to be based on time and burden, rather than risk. It could be that these participants do not believe that malaria challenge or investigational drug studies carry a greater risk of harm, or volunteers may assume that researchers would not offer participation in trials which pose significant medical risk. Further, risk may be less tangible if volunteers have never personally experienced a significant adverse event related to study participation, which indeed occurs quite rarely in studies with healthy volunteers (14, 15). Our previous work suggests that healthy volunteers very rarely mention risks associated with study participation (8). Rather, these data suggest that it may be time or logistical burden that is most relevant to volunteers in generating their payment estimates.
In considering a model for expected payment for clinical research, Chaput de Saintonge et al reported relative weights of seven variables that medical students considered when estimating payments (7). All seven variables related either to the risk or the logistical burden of the study. In order to better interpret our own findings, we adapted this system to develop a “total burden score” for our own four studies. Each variable was assigned a point value specific to each study, which was based on its burden or risk and multiplied to account for study duration, multiple drugs, or number of blood draws. That value was weighted according to its relative importance as voiced by the medical students studied by Chaput de Saintonge et al; the resulting relative burden scores for each variable were summed to get a “total burden score,” shown in Table 4.
Table 4. Relative weights of each of the seven factors reported by Chaput de Saintonge et al for each of the four hypothetical studies.
Each variable was assigned a point value specific to each study, which was based on its burden or risk and multiplied to account for study duration, multiple drugs, or number of blood draws. That value was then multiplied by a factor determined by the variable’s relative importance to the medical students studied by Chaput de Saintonge et al, and the resulting relative burden scores for each variable were added to get the “total burden score.”
| Relative Burden Scores | ||||
|---|---|---|---|---|
| Factor | Investigational Drug study |
Plant Extract Study |
HIV study | Malaria Challenge study |
| Previous testing | 71 | 24 | 24 | 71 |
| Unwanted effects | 56 | 19 | 37 | 56 |
| Study duration | 12 | 24 | 36 | 24 |
| Days in unit | 18 | 342 | 144 | 0 |
| Type of drug | 13 | 13 | 38 | 13 |
| Route of administration | 16 | 16 | 48 | 16 |
| Invasiveness of procedure | 75 | 96 | 188 | 128 |
| Total Burden Score | 261 | 534 | 515 | 308 |
Interestingly, this model predicts that the Investigational Drug study has a lower overall burden, suggesting less payment for participation. Similarly, the Plant Extract study and HIV study were comparable in burden and should therefore be associated with similar payment estimates. The model did not predict that the Malaria Challenge study was comparable in burden to the Plant Extract and HIV studies, perhaps because the model does not account for risk related to the challenge procedure.
Furthermore, our data suggest that healthy volunteers’ estimates of payment for studies relative to that of other volunteers tend to be remarkably consistent within the range of expected payments. Subjects in the top 25% of estimates for one study were likely to be in the top 25% for the other studies, while those in the bottom 25% for one study were likely to give the lowest estimates for the other three studies. This may imply that subjects have an as of yet unexplored method of determining what they think studies should pay and they apply this method consistently across different studies. Verhaggen et al have previously suggested that subjects create a “personal balance account” which integrates the positive and negative aspects of participation to facilitate the process of decision-making (16). In addition, Dunn and Gordon argue that participants consider the benefits of study enrollment (including monetary benefits) in relation to the costs, and agree to participate only if the benefits outweigh the costs (17). The consistency of subjects’ estimates in our sample suggests that participants may be evaluating the costs consistently across each of our hypothetical studies.
Although variation in any one subject’s payment estimates relative to other subjects’ estimates for the four hypothetical studies was minimal, variation among individual respondents in their estimates for a particular study was quite broad. It was surprising to us that the extent of volunteers’ experience in clinical research did not predict payment estimates. Characteristics associated with higher levels of payment estimates included being male, non-white, being 45 years of age or older, having a spouse or domestic partner, having children, having money as a primary motivation for participation, or having greater than a high school education. Although very few of these correlations reached statistical significance, future studies with larger sample sizes should investigate these demographic patterns.
Our results have important implications for IRBs, who are charged with determining the acceptability of payment levels proposed by investigators. It may be reassuring to know that the estimates provided by our respondents, which correspond generally to burden rather than risk, are consistent with IRBs’ stated policies and preferences that time and burden should dictate payment levels, rather than risk. On the other hand, that respondents so frequently stated that payment ought to be based on risk as well as burden is a reminder to IRBs that volunteers may view a low-paying study, by definition, as being of lower risk, when this may not be the case. This reinforces the importance of IRBs closely monitoring the level of risk posed by a study and requiring strict safeguards when studies impose greater than minimal risk. Finally, our data raise the question of whether it is appropriate for IRBs to consider setting standardized pay schedules for studies, at least within their own institution and/or within a given geographic region. While there may be a wide range of payment amounts that could be considered ethically acceptable for a given study, having consistency and transparency regarding payment may be viewed as helpful and fair by investigators and volunteers alike, particularly if volunteers attach meaning to different levels of payment, associated with differences in burden and risk.
Our study has several limitations. First, our sample was a small convenience sample of subjects drawn predominantly from one institution. A larger sample more representative of the entire population of healthy volunteers is needed to confirm or refute our findings. Second, we recruited subjects who had previously agreed to be re-contacted for further studies and subjects who responded to advertisements. These methods of recruitment may have underrepresented those who previously had unpleasant study-related experiences and who might now refuse to participate in further studies. However, our use of newspaper advertisements may have allowed participants with negative experiences to enroll in the study as a way to discuss their past bad experiences. Third, it is possible that some subjects had participated in trials similar to the scenarios described, and thus may have gauged their payment estimates on prior, personal experiences. However, subjects who were more study-experienced were as diverse in payment estimates as those who had only participated in one or two clinical trials.
It is important to emphasize that payment is only one of many reasons healthy volunteers choose to participate in a clinical trial (8, 18-20). It is conceivable that the wide variation in payment estimates in our hypothetical studies may be related to the influence of other factors relevant to volunteers’ motivation for participation. The data presented suggest that some demographic factors may influence subjects’ determination of payment. Future studies should examine the concordance between investigators’ and subjects’ estimates of the logistical burden, time requirement, and medical risk involved in specific clinical research. These data offer important information to investigators, funding agencies, and IRBs, that can be used to help further activities and regulations regarding the protection of human subjects.
METHODS
Subjects
Eligible subjects participated in a clinical research study as a healthy volunteer at least once in the 24 months prior to enrollment. Sources of recruitment for this study included: referrals from the Drug Development Unit (DDU) and the Center for Immunization Research (CIR) of our institution, advertisements placed in a local, free weekly newspaper that routinely publishes recruitment advertisements for clinical research studies, and participant word of mouth. The research coordinators of the DDU and CIR contacted participants who had previously consented to be notified of future studies. Those expressing interest in the study were provided the phone number of the study coordinator (RKM) and contacted the investigators directly. Individuals who self-referred through newspaper advertisement or word-of-mouth contacted the study coordinator, who determined their eligibility. We attempted to have one-third of participants in this sample be individuals who self-identified as employees of our institution and approximately two-thirds to self-identified as community-based research volunteers. The Johns Hopkins Bloomberg School of Public Health Institutional Review Board approved this study.
Interview and Data Collection
Individual, audio-recorded interviews were conducted in private exam rooms at the General Clinical Research Center of our institution. All participants provided written informed consent and verbal consent for audio recording after the tape recorder was started. Each participant was paid $25.
Each semi-structured interview took approximately 45 minutes. The interview guide included closed- and open-ended questions to explore: background and demographic information, history and experience as a research participant, motivations for joining studies, beliefs about appropriate financial payment, and beliefs about who should volunteer and participate in clinical research. The topic of undue inducement was not specifically addressed. Interviews were transcribed to facilitate qualitative coding.
Study Descriptions
Four hypothetical clinical trial scenarios varying in medical risk and logistical burden were described to participants, each based on healthy volunteer research that had been carried out at our institution (Appendix 1). The relative degree of risk and burden for each scenario was determined by consensus of four investigators (MJC, NEK, CF, EJF; Table 5). While the level of risk and burden were relevant to investigators in drafting the scenarios, respondents were not told explicitly what level of risk and burden the investigators perceived for each scenario. After each scenario was read, respondents were asked if they would join the study, why or why not they would participate, and how much they believed volunteers should be paid for participating. Finally, subjects were asked upon what they thought payment should be based.
Table 5. Investigator-assigned relative levels of medical risk and logistical burden for each of the four hypothetical studies.
| Medical Risk | Logistical Burden |
|
|---|---|---|
| Investigational Drug Study | Intermediate | Low |
| Plant Extract Study | Low | High |
| HIV Study | Intermediate | Intermediate |
| Malaria Challenge Study | High | Intermediate |
In brief, Study #1 described a one-week study in which volunteers would take a non-FDA approved medication once (Investigational Drug study). The study required two outpatient visits, one overnight hospital stay, and 10 blood draws. The investigators considered this study to be of low logistical burden but intermediate medical risk due to the investigational, non-FDA approved status of the drug. Study #2 depicted a 19-day, inpatient study of a plant extract pill (Plant Extract study). Participants would be on a restricted diet and would collect 24-hour urine samples throughout the 19 days. This study was considered to be of low medical risk but high logistical burden due to the extended inpatient stay, restricted diet, and required 24-hour urine collections. Study #3 involved taking three FDA-approved HIV medications (HIV study), lasted 42 days, and required two overnight hospital stays, five office visits, and 25 blood draws. This study was considered to be of intermediate medical risk due to the FDA-approved status of the test medications, and intermediate logistical burden due to the frequent blood draws, overnight stays, and total study length. Study #4 was a malaria challenge (Malaria Challenge study). A malaria prevention pill or placebo would be given on day one, and then volunteers would be bitten by mosquitoes carrying the malaria parasite and observed for 17 days with daily blood draws. Those contracting malaria would be treated. This study was considered to be of high medical risk due to the fact that some healthy people would likely be made severely ill, albeit with a treatable infection, and was considered to be of intermediate logistical burden.
Data Analysis
Quantitative data were summarized using medians, interquartile ranges, and geometric means. The estimates of expected remuneration were right skewed and log transformations were used to normalize the distributions before parametric analyses were performed. Two-sample t-tests were used to compare mean payment estimates between groups defined by demographics. Cumulative relative frequency distributions for payment estimates for the four studies were plotted. The Spearman rank correlation coefficient and paired t-test were used to compare payment estimates for pair-wise combinations of the four studies. Multivariate linear regression was performed for each of the four studies to model the payment estimates.
Statistical analyses were performed using SPSS version 16 (Chicago, IL) and Stata version 10.1 (College Station, TX). Significance level was set at α = 0.05, and there was no correction for multiple comparisons. Microsoft Excel 2007 (Redmond, WA) was used for graphing.
Qualitative data regarding the criteria that should be used to set volunteer payments were coded independently in their entirety by two authors (MJC, EJF). One author (MJC) generated categories of responses by reading the free responses and looking for common themes. The themes were then discussed and refined with one of the other authors (NEK). Twelve categories were generated in this way, and two authors (MJC, EJF) coded the free responses separately. The completed coding sheets were compared, and discrepancies were resolved by discussion between the two coding authors. Codes were not applied to any responses which were determined by either coder to be only marginally related to a specific code. Finally, the twelve categories were assigned to either “risk” or “burden.”
ACKNOWLEDGEMENTS
The project described was supported by Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. Funding was also provided by the Stavros Niarchos Foundation which had no role in this study.
APPENDIX 1 - Hypothetical Study Descriptions
Investigational Drug Study
Imagine a study that was testing a new medication. It was the first time this medication was ever given to humans. The medication was “experimental” or “investigational” - that means the FDA had not approved it. When it was tested in animals, it was shown to have a few side effects, but they were not too serious. The study will last one week. During that week, you would take the drug by mouth one time during an overnight stay in the hospital. You’d need to have two other office visits to the clinic during the week as well. In total, there would be 10 blood draws. So to repeat, it’s a study with an experimental drug not approved by the FDA. You’d stay in the hospital one night and come into the office 2 other times that week with 10 total blood draws.
Plant Extract Study
OK, here’s another one. Imagine a study that was testing a plant extract commonly found in some vegetables, given in a pill form twice/day. This study would last 19 days, and you’d need to stay in the hospital the whole time. During that time, you would take this plant extract pill twice a day and follow a special diet. The diet will consist of a limited list of choices of usual foods, however you will not be able to add any seasonings other than salt and pepper. You will only be allowed to eat what is provided to you by the study staff. You would also need to do 24-hour urine collections the whole time you were enrolled in the study. There would be a total of 9 blood draws during the 19-day study period. OK? So this study involved a 19 day hospital stay, a pill twice a day, eating a special diet, collecting your urine the whole time and 9 blood draws.
HIV Study
Here is the third one. Imagine a study that was looking at how three medications behave or mix, when taken together. All 3 medications are currently available by prescription, that is, the FDA has approved them. These drugs are used to treat HIV infections. In this study, you would take these medications twice a day, by mouth. The most common side effects of these drugs include nausea, diarrhea, gas, and bloating. Occasionally some subjects may experience skin rash or vivid dreams. More rarely there may be changes in blood cell counts or fat levels in the blood. These changes go away when the medications are stopped. You may experience some or none of these side effects. You will be closely monitored, and you will be under medical supervision. The study lasts 42 days (that’s about a month and a half). During this time, you would need to stay in the hospital twice, for two nights each time, a total of 4 nights. Other than that, you would need to visit the clinic for 5 office visits. You also need to keep a diary of when you take each medication every day. In total, this study requires 25 blood draws. OK, so in this study you take 3 AIDS drugs for a month and a half and you might have some side effects. You’d spend 4 nights in the hospital, have 5 office visits, 25 blood draws and keep a drug diary.
Malaria Challenge Study
OK, here is the last study I’ll ask you about. This study is being done to see if an experimental medication can prevent people from getting Malaria. Malaria is a serious disease, which can lead to severe fever and muscle aches. It can also be fatal if not treated. On the first day, the study team will give you a pill to take by mouth. A week later you will be exposed to the malaria parasite by having a mosquito infected with malaria bite you. You will have blood samples drawn once each day for 17 days, a total of 17 blood draws. If your blood shows malaria infection, you will get another drug to treat you so you will get better. So for this one, you take a drug that might or might not prevent you from getting malaria, then a mosquito with malaria bites you. Blood will be drawn for 17 days and if you actually get malaria, you will be treated.
Footnotes
CONFLICT OF INTEREST/DISCLOSURE
Dr. Charles Flexner and Edward Fuchs report receiving in the past five years research grants for studies in healthy volunteers funded by several pharmaceutical sponsors. More details can be provided in case the Editors view this as a potential conflict of interest. None of the other authors, nor our institution, has any financial conflicts or relationships of relevance to this research.
REFERENCES
- 1.McNeill P. Paying people to participate in research: why not? A response to Wilkinson and Moore. Bioethics. 1997;11:390–396. doi: 10.1111/1467-8519.00079. [DOI] [PubMed] [Google Scholar]
- 2.Latterman J, Merz JF. How much are subjects paid to participate in research? Am. J. Bioeth. 2001;1:45–46. doi: 10.1162/152651601300169040. [DOI] [PubMed] [Google Scholar]
- 3.Macklin R. On paying money to research subjects: ‘due’ and ‘undue’ inducements. IRB. 1981;3:1–6. [PubMed] [Google Scholar]
- 4.Dickert N, Grady C. What’s the price of a research subject? Approaches to payment for research participation. N. Engl. J. Med. 1999;341:198–203. doi: 10.1056/NEJM199907153410312. [DOI] [PubMed] [Google Scholar]
- 5.Ackerman TF. An ethical framework for the practice of paying research subjects. IRB. 1989;11:1–4. [PubMed] [Google Scholar]
- 6.Lemmens T, Elliott C. Guinea pigs on the payroll: the ethics of paying research subjects. Account. Res. 1999;7:3–20. doi: 10.1080/08989629908573939. [DOI] [PubMed] [Google Scholar]
- 7.Chaput de Saintonge DM, Crane GJ, Rust ND, Karadia S, Whittam LR. Modelling determinants of expected rewards in healthy volunteers. Pharmaceut. Med. 1988;3:45–54. [Google Scholar]
- 8.Kass NE, Myers R, Fuchs EJ, Carson KA, Flexner C. Balancing justice and autonomy in clinical research with healthy volunteers. Clin. Pharmacol. Ther. 2007;82:219–227. doi: 10.1038/sj.clpt.6100192. [DOI] [PubMed] [Google Scholar]
- 9.Institutional Review Board. Johns Hopkins Medicine [Accessed: 2/4/2009];Organization Policy on Payment or Remuneration to Human Subjects (Policy No. 111.2) Available: http://irb.jhmi.edu/Policies/111_2.html.
- 10.Institutional Review Board. University of Washington [Accessed: 2/4/2009];Subject Payment Guidance. Available: http://www.washington.edu/research/link.php?id=34.
- 11.Office for Protection of Research Subjects . Payment for Participation in Research. University of California; Los Angeles: [Accessed: 2/4/2009]. Available: www.oprs.ucla.edu/human/documents/pdf/31.pdf. [Google Scholar]
- 12.Office of Regulatory Affairs. University of Pennsylvania [Accessed: 2/4/2009];Guidance on Payment to Subjects Participating in Research. Available: http://www.upenn.edu/regulatoryaffairs/Pdf/paymentguidance.pdf.
- 13.The Committee of Human Research . Guidelines for Payment of Research Subjects. The University of California; San Francisco: [Accessed: 2/4/2009]. Available: http://www.research.ucsf.edu/CHR/Guide/chrPayment.asp. [Google Scholar]
- 14.Sibille M, Deigat N, Janin A, Kirkesseli S, Durand DV. Adverse events in phase-I studies: a report in 1015 healthy volunteers. Eur. J. Clin. Pharmacol. 1998;54:13–20. doi: 10.1007/s002280050413. [DOI] [PubMed] [Google Scholar]
- 15.Zarafonetis CJ, et al. Clinically significant adverse effects in a Phase 1 testing program. Clin. Pharmacol. Ther. 1978;24:127–132. doi: 10.1002/cpt1978242127. [DOI] [PubMed] [Google Scholar]
- 16.Verheggen FW, Nieman F, Jonkers R. Determinants of patient participation in clinical studies requiring informed consent: why patients enter a clinical trial. Patient Educ. Couns. 1998;35:111–125. doi: 10.1016/s0738-3991(98)00060-3. [DOI] [PubMed] [Google Scholar]
- 17.Dunn LB, Gordon NE. Improving informed consent and enhancing recruitment for research by understanding economic behavior. JAMA. 2005;293:609–612. doi: 10.1001/jama.293.5.609. [DOI] [PubMed] [Google Scholar]
- 18.Bigorra J, Banos JE. Weight of financial reward in the decision by medical students and experienced healthy volunteers to participate in clinical trials. Eur. J. Clin. Pharmacol. 1990;38:443–446. doi: 10.1007/BF02336681. [DOI] [PubMed] [Google Scholar]
- 19.Cunny KA, Miller HW. Participation in clinical drug studies: motivations and barriers. Clin. Ther. 1994;16:273–82. discussion 271-2. [PubMed] [Google Scholar]
- 20.van Gelderen CE, Savelkoul TJ, van Dokkum W, Meulenbelt J. Motives and perception of healthy volunteers who participate in experiments. Eur. J. Clin. Pharmacol. 1993;45:15–21. doi: 10.1007/BF00315344. [DOI] [PubMed] [Google Scholar]