Abstract
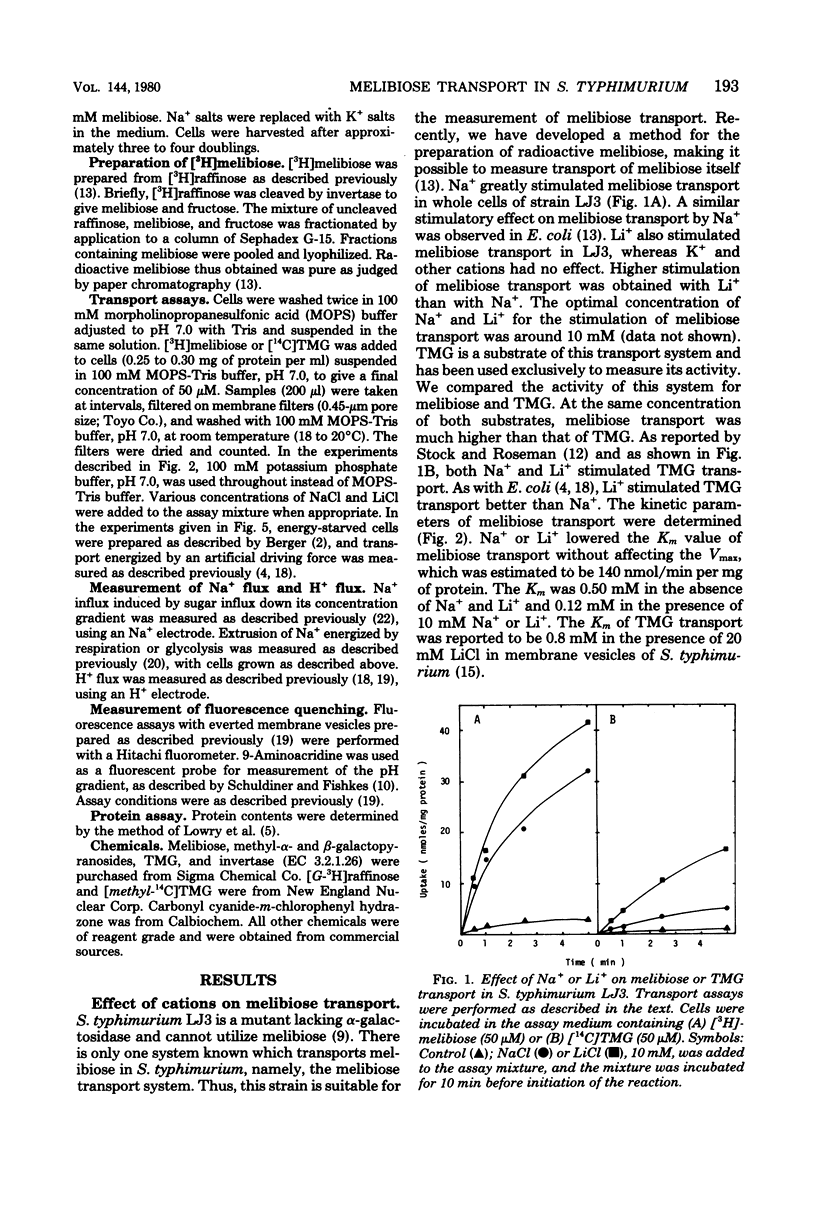
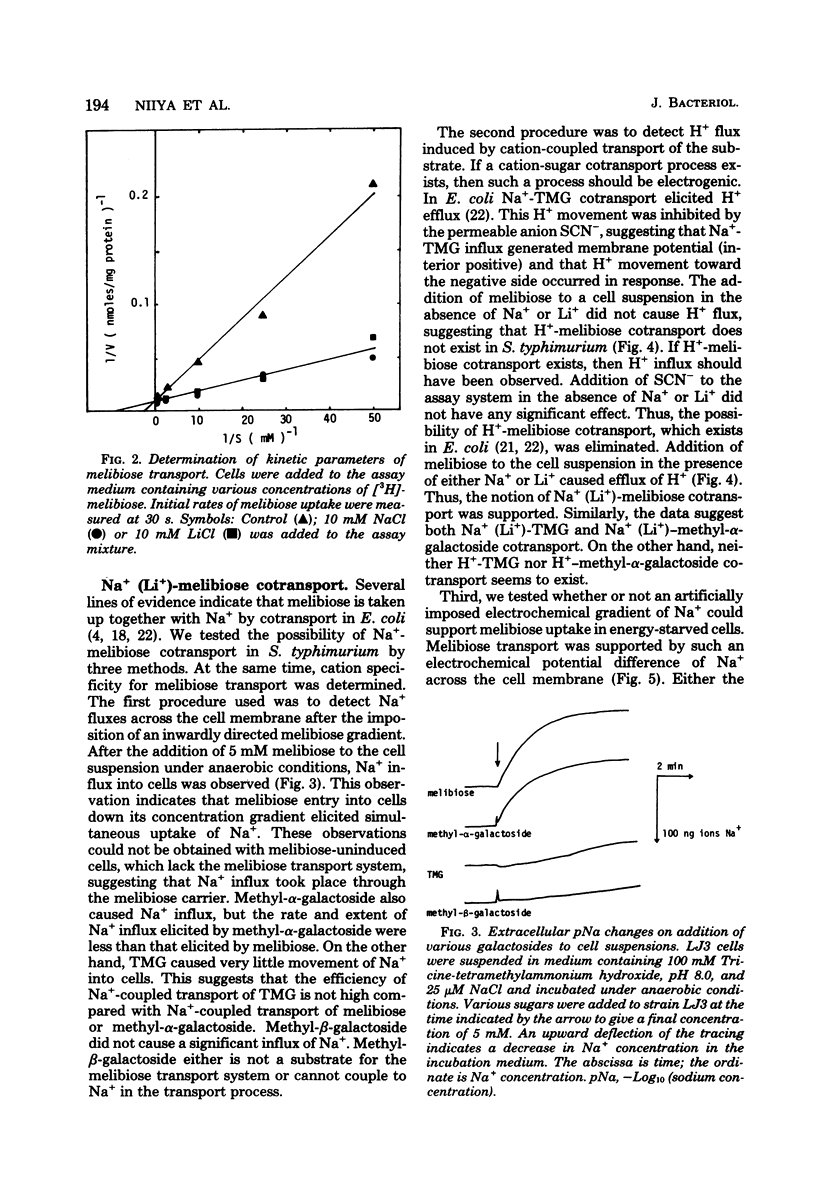
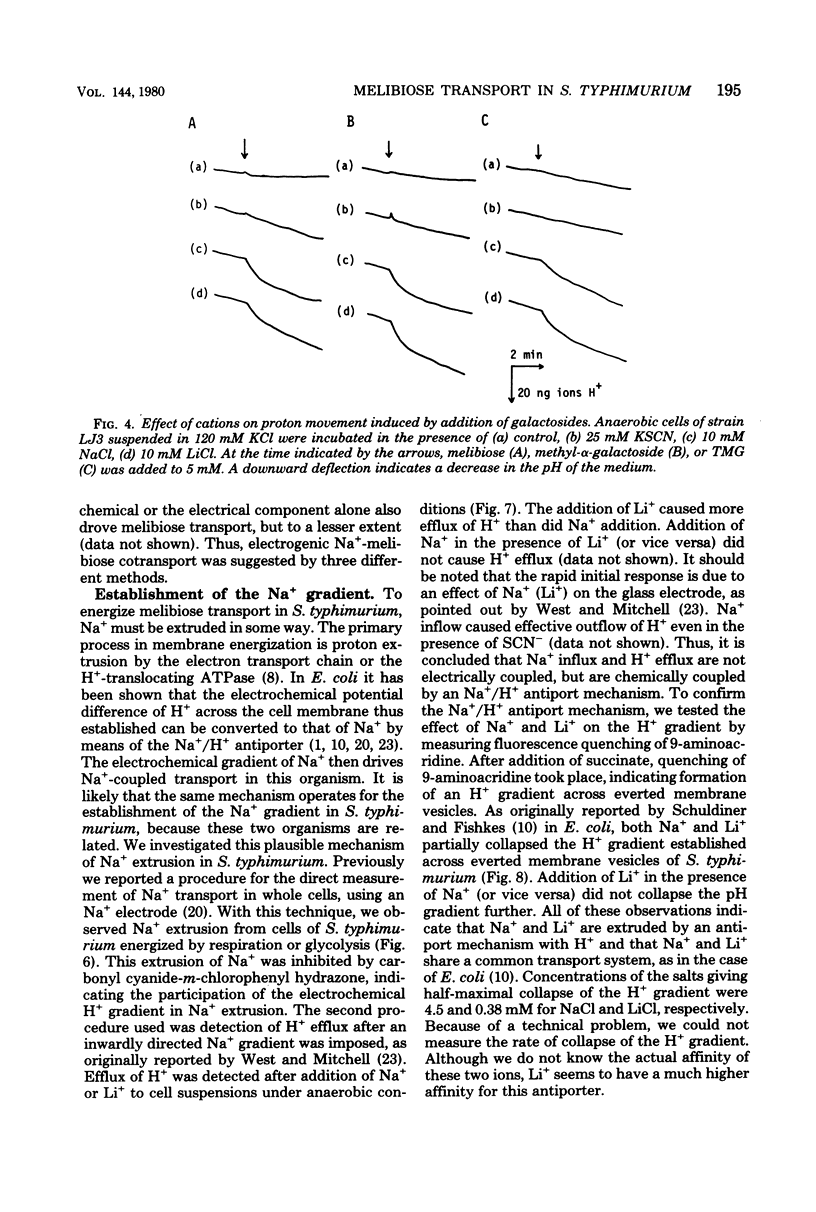
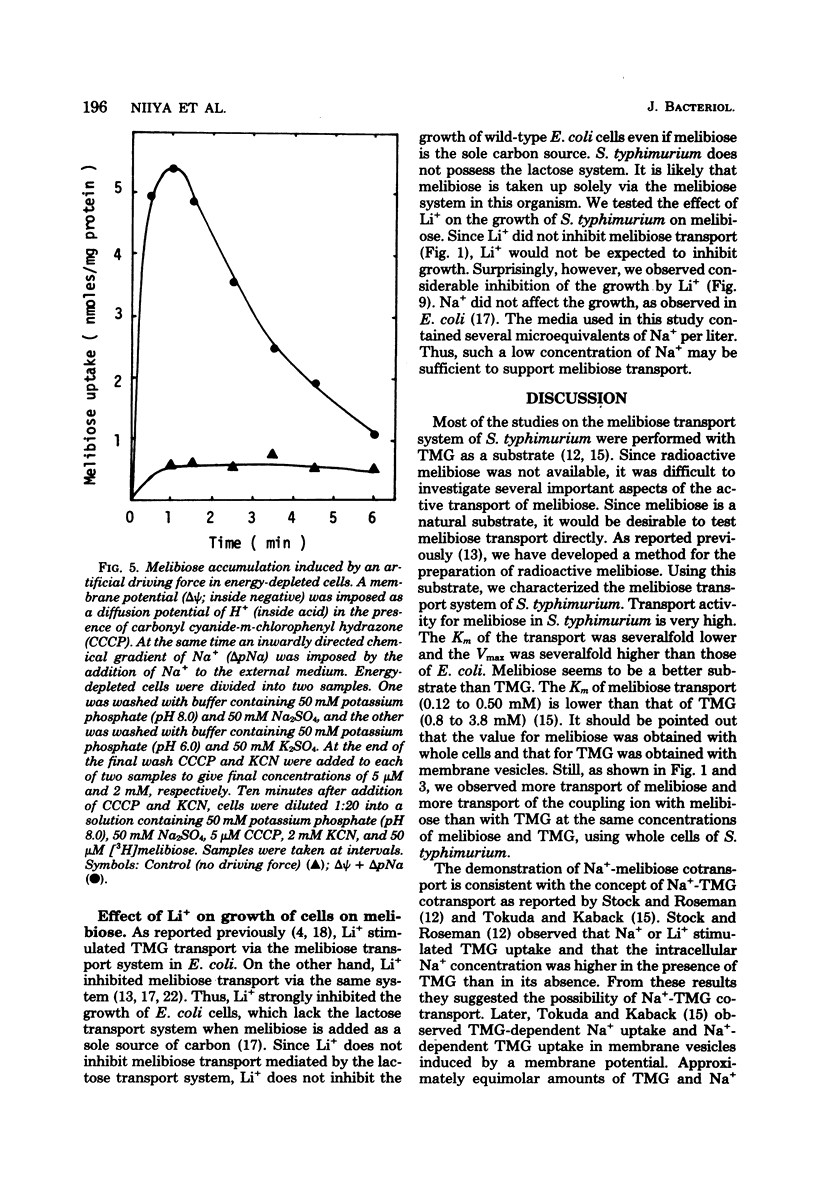
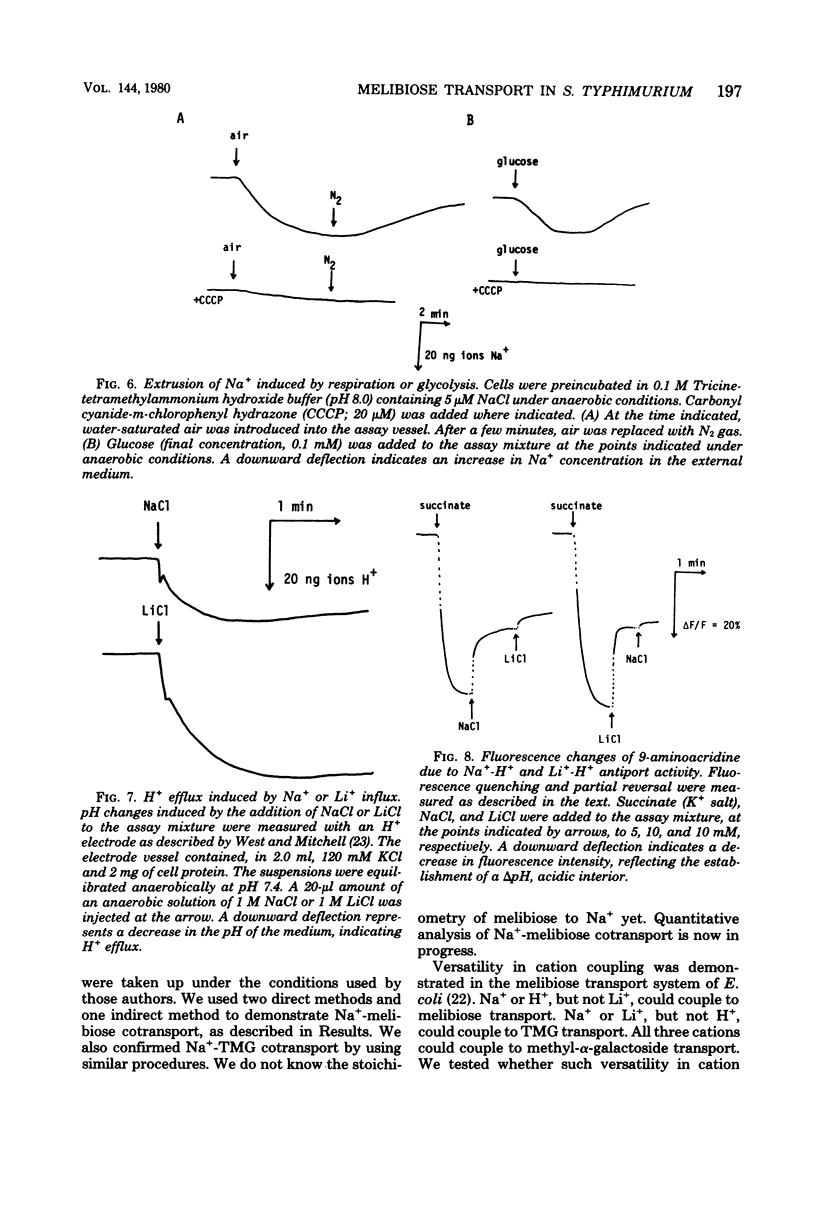
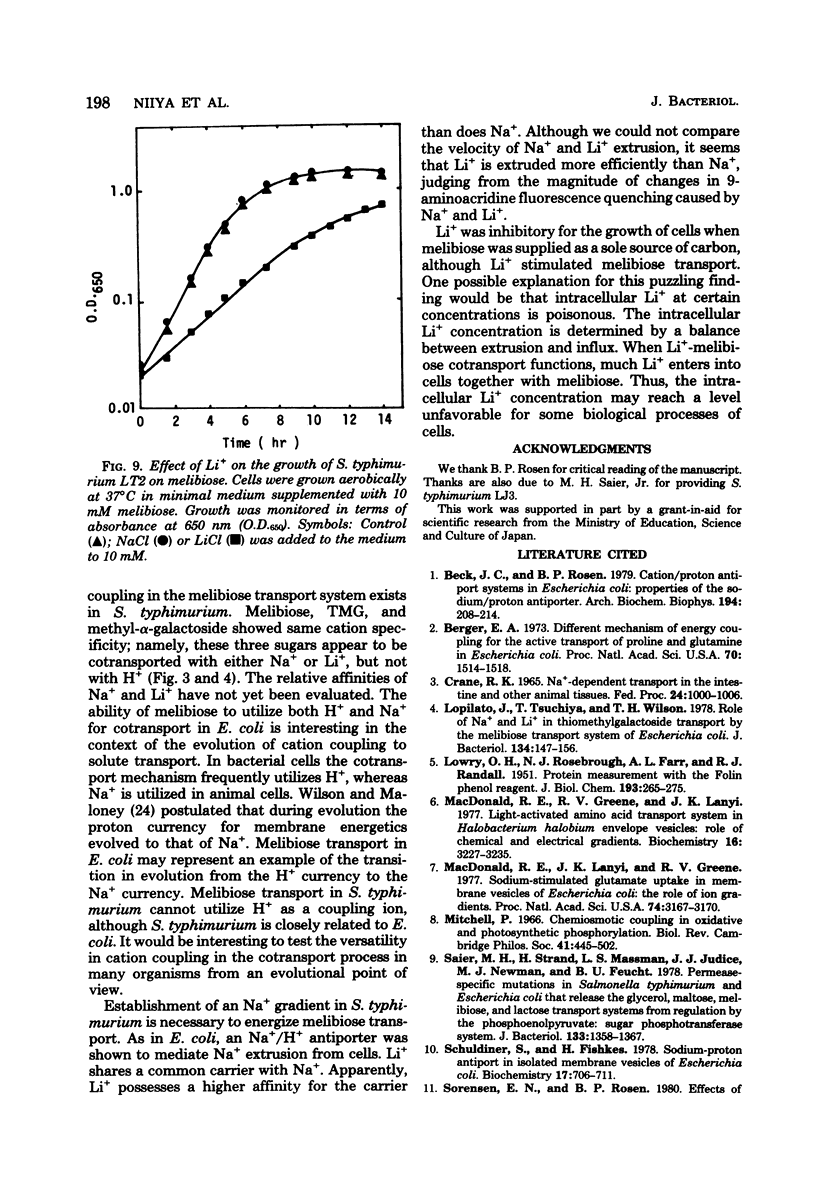
Melibiose transport in Salmonella typhimurium was investigated. Radioactive melibiose was prepared and the melibiose transport system was characterized. Na+ and Li+ stimulated transport of melibiose by lowering the Km value without affecting the Vmax value; Km values were 0.50 mM in the absence of Na+ or Li+ and 0.12 mM in the presence of 10 mM NaCl or 10 mM LiCl. The Vmax value was 140 nmol/min per mg of protein. Melibiose was a much more effective substrate than methyl-beta-thiogalactoside. An Na+-melibiose cotransport mechanism was suggested by three types of experiments. First, the influx of Na+ induced by melibiose influx was observed with melibiose-induced cells. Second, the efflux of H+ induced by melibiose influx was observed only in the presence of Na+ or Li+, demonstrating the absence of H+-melibiose cotransport. Third, either an artificially imposed Na+ gradient or membrane potential could drive melibiose uptake in cells. Formation of an Na+ gradient in S. typhimurium was shown to be coupled to H+ by three methods. First, uncoupler-sensitive extrusion of Na+ was energized by respiration or glycolysis. Second, efflux of H+ induced by Na+ influx was detected. Third, a change in the pH gradient was elicited by imposing an Na+ gradient in energized membrane vesicles. Thus, it is concluded that the mechanism for Na+ extrusion is an Na+/H+ antiport. The Na+/H+ antiporter is a transformer which converts an electrochemical H+ gradient to an Na+ gradient, which then drives melibiose transport. Li+ was inhibitory for the growth of cells when melibiose was the sole carbon source, even though Li+ stimulated melibiose transport. This suggests that high intracellular Li+ may be harmful.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck J. C., Rosen B. P. Cation/proton antiport systems in escherichia coli: properties of the sodium/proton antiporter. Arch Biochem Biophys. 1979 Apr 15;194(1):208–214. doi: 10.1016/0003-9861(79)90611-8. [DOI] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane R. K. Na+ -dependent transport in the intestine and other animal tissues. Fed Proc. 1965 Sep-Oct;24(5):1000–1006. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lopilato J., Tsuchiya T., Wilson T. H. Role of Na+ and Li+ in thiomethylgalactoside transport by the melibiose transport system of Escherichia coli. J Bacteriol. 1978 Apr;134(1):147–156. doi: 10.1128/jb.134.1.147-156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. E., Greene R. V., Lanyi J. K. Light-activated amino acid transport systems in Halobacterium halobium envelope vesicles: role of chemical and electrical gradients. Biochemistry. 1977 Jul 12;16(14):3227–3235. doi: 10.1021/bi00633a029. [DOI] [PubMed] [Google Scholar]
- MacDonald R. E., Lanyi J. K., Greene R. V. Sodium-stimulated glutamate uptake in membrane vesicles of Escherichia coli: the role of ion gradients. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3167–3170. doi: 10.1073/pnas.74.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Straud H., Massman L. S., Judice J. J., Newman M. J., Feucht B. U. Permease-specific mutations in Salmonella typhimurium and Escherichia coli that release the glycerol, maltose, melibiose, and lactose transport systems from regulation by the phosphoenolpyruvate:sugar phosphotransferase system. J Bacteriol. 1978 Mar;133(3):1358–1367. doi: 10.1128/jb.133.3.1358-1367.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Fishkes H. Sodium-proton antiport in isolated membrane vesicles of Escherichia coli. Biochemistry. 1978 Feb 21;17(4):706–711. doi: 10.1021/bi00597a023. [DOI] [PubMed] [Google Scholar]
- Sorensen E. N., Rosen B. P. Effects of sodium and lithium ions on the potassium ion transport systems of Escherichia coli. Biochemistry. 1980 Apr 1;19(7):1458–1462. doi: 10.1021/bi00548a030. [DOI] [PubMed] [Google Scholar]
- Stock J., Roseman S. A sodium-dependent sugar co-transport system in bacteria. Biochem Biophys Res Commun. 1971 Jul 2;44(1):132–138. doi: 10.1016/s0006-291x(71)80168-7. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Niiya S., Tsuchiya T. Melibiose transport of Escherichia coli. J Bacteriol. 1980 Mar;141(3):1031–1036. doi: 10.1128/jb.141.3.1031-1036.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H., Kaback H. R. Sodium-dependent methyl 1-thio-beta-D-galactopyranoside transport in membrane vesicles isolated from Salmonella typhimurium. Biochemistry. 1977 May 17;16(10):2130–2136. doi: 10.1021/bi00629a013. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Hasan S. M., Raven J. Glutamate transport driven by an electrochemical gradient of sodium ions in Escherichia coli. J Bacteriol. 1977 Sep;131(3):848–853. doi: 10.1128/jb.131.3.848-853.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Lopilato J., Wilson T. H. Effect of lithium ion on melibiose transport in Escherichia coli. J Membr Biol. 1978 Jul 21;42(1):45–59. doi: 10.1007/BF01870393. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Raven J., Wilson T. H. Co-transport of Na+ and methul-beta-D-thiogalactopyranoside mediated by the melibiose transport system of Escherichia coli. Biochem Biophys Res Commun. 1977 May 9;76(1):26–31. doi: 10.1016/0006-291x(77)91663-1. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Takeda K. Calcium/proton and sodium/proton antiport systems in Escherichia coli. J Biochem. 1979 Apr;85(4):943–951. doi: 10.1093/oxfordjournals.jbchem.a132426. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Takeda K. Extrusion of sodium ions energized by respiration and glycolysis in Escherichia coli. J Biochem. 1979 Jul;86(1):225–230. [PubMed] [Google Scholar]
- Tsuchiya T., Takeda K., Wilson T. H. H + -substrate cotransport by the melibiose membrane carrier in Escherichia coli. Membr Biochem. 1980;3(1-2):131–146. doi: 10.3109/09687688009063881. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Wilson T. H. Cation-sugar cotransport in the melibiose transport system of Escherichia coli. Membr Biochem. 1978;2(1):63–79. doi: 10.3109/09687687809063858. [DOI] [PubMed] [Google Scholar]
- West I. C., Mitchell P. Proton/sodium ion antiport in Escherichia coli. Biochem J. 1974 Oct;144(1):87–90. doi: 10.1042/bj1440087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. H., Maloney P. C. Speculations on the evolution of ion transport mechanisms. Fed Proc. 1976 Aug;35(10):2174–2179. [PubMed] [Google Scholar]


