Abstract
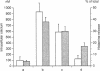
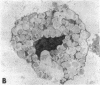
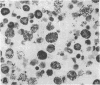
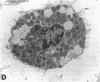
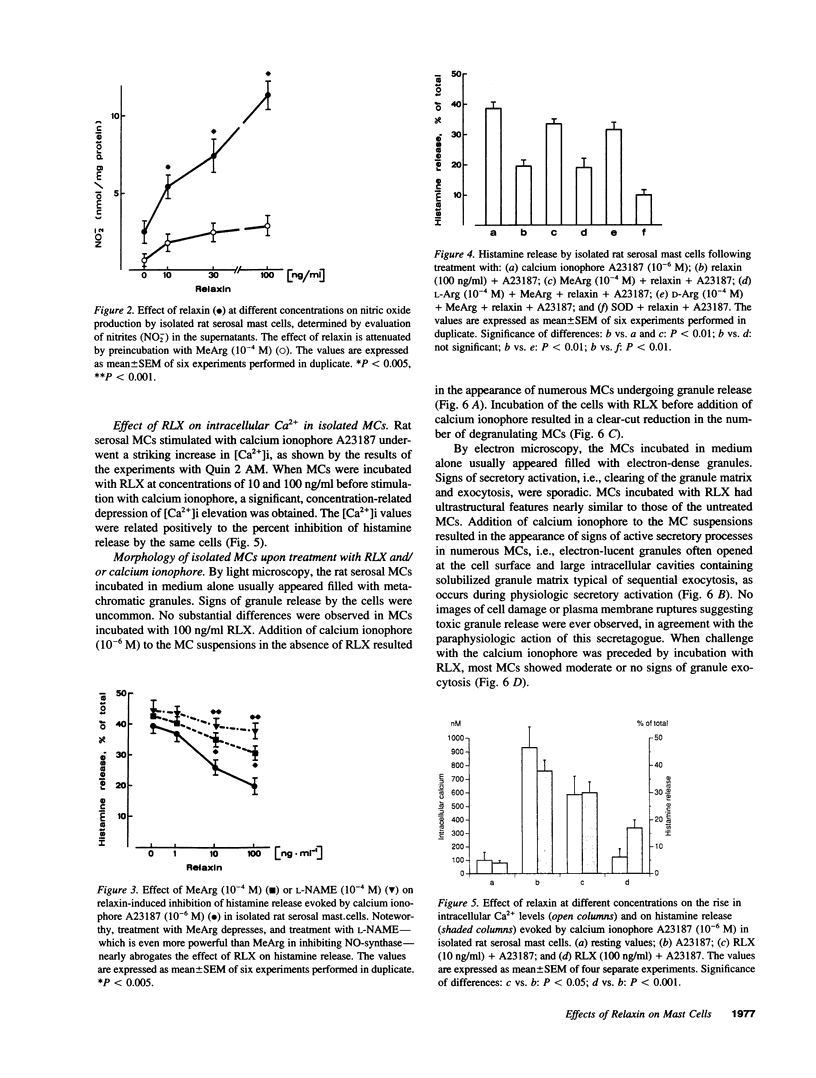
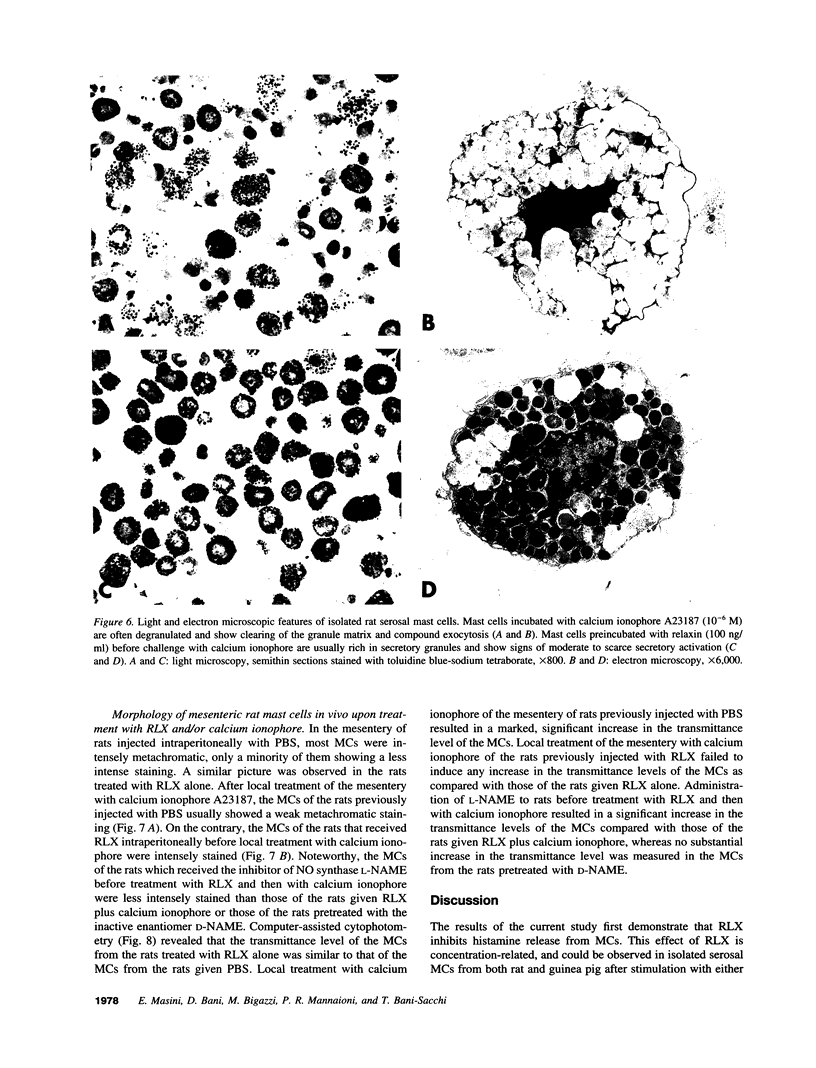
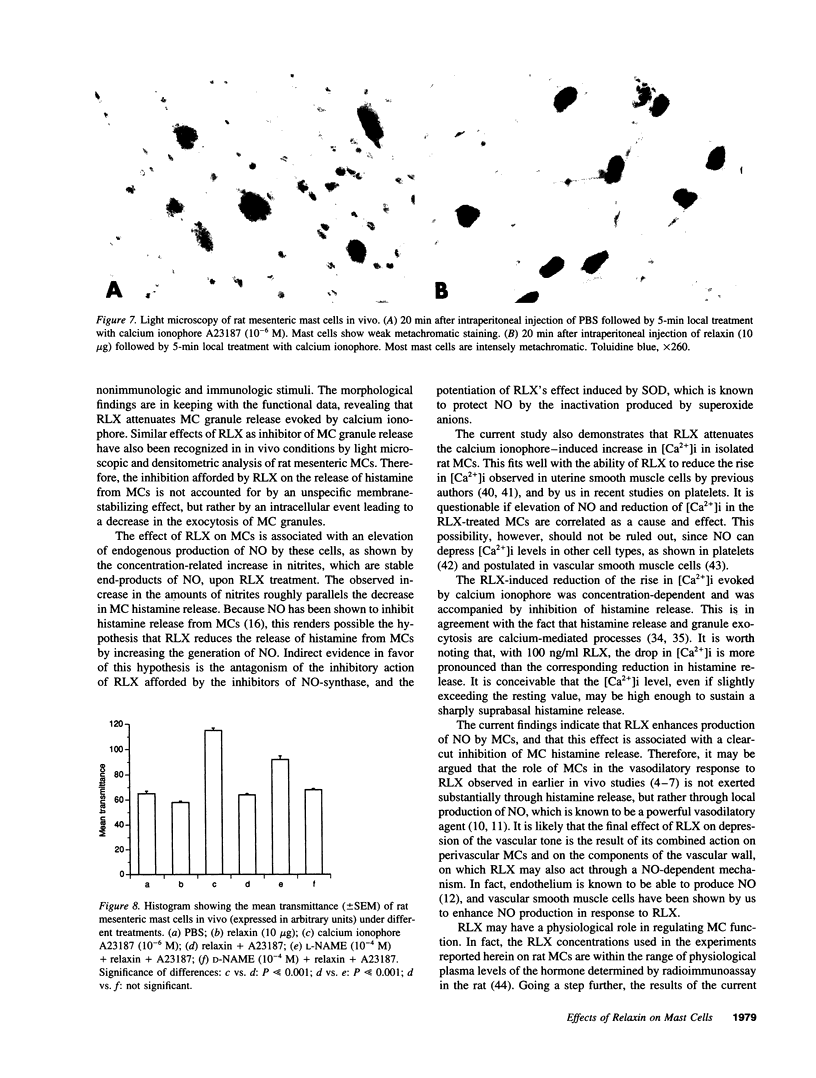
The results of the current study demonstrate that relaxin inhibits histamine release by mast cells. This effect is related to the peptide concentrations, and could be observed in both isolated rat serosal mast cells stimulated with compound 48/80 or calcium ionophore A 23187, and in serosal mast cells isolated from sensitized guinea pigs and challenged with the antigen. The morphological findings agree with the functional data, revealing that relaxin attenuates calcium ionophore-induced granule exocytosis by isolated rat serosal mast cells. Similar effects of relaxin have also been recognized in vivo by light microscopic and densitometric analysis of the mesenteric mast cells of rats which received the hormone intraperitoneally 20 min before local treatment of the mesentery with calcium ionophore. Moreover, evidence is provided that relaxin stimulates endogenous production of nitric oxide and attenuates the rise of intracellular Ca2+ concentration induced by calcium ionophore. The experiments with drugs capable of influencing nitric oxide production also provide indirect evidence that the inhibiting effect of relaxin on mast cell histamine release is related to an increased generation of nitric oxide. It is suggested that relaxin may have a physiological role in modulating mast cell function through the L-arginine-nitric oxide pathway.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bani G., Bani Sacchi T., Bigazzi M., Bianchi S. Effects of relaxin on the microvasculature of mouse mammary gland. Histol Histopathol. 1988 Oct;3(4):337–343. [PubMed] [Google Scholar]
- Bani G., Bigazzi M. Morphological changes induced in mouse mammary gland by porcine and human relaxin. Acta Anat (Basel) 1984;119(3):149–154. doi: 10.1159/000145877. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Aiken D. L., Woldemussie E., Soll A. H. Changes in histamine synthetic activity, histamine content and responsiveness to compound 48/80 with maturation of rat peritoneal mast cells. J Pharmacol Exp Ther. 1983 Mar;224(3):620–626. [PubMed] [Google Scholar]
- Bergendorff A., Uvnäs B. Storage of 5-hydroxytryptamine in rat mast cells. Evidence for an ionic binding to carboxyl groups in a granule heparin-protein complex. Acta Physiol Scand. 1972 Mar;84(3):320–331. doi: 10.1111/j.1748-1716.1972.tb05183.x. [DOI] [PubMed] [Google Scholar]
- Bigazzi M., Bani G., Sacchi T. B., Petrucci F., Bianchi S. Relaxin: a mammotropic hormone promoting growth and differentiation of the pigeon crop sac mucosa. Acta Endocrinol (Copenh) 1988 Feb;117(2):181–188. doi: 10.1530/acta.0.1170181. [DOI] [PubMed] [Google Scholar]
- Bigazzi M., Del Mese A., Petrucci F., Casali R., Novelli G. P. The local administration of relaxin induces changes in the microcirculation of the rat mesocaecum. Acta Endocrinol (Copenh) 1986 Jun;112(2):296–299. doi: 10.1530/acta.0.1120296. [DOI] [PubMed] [Google Scholar]
- Blandina P., Fantozzi R., Mannaioni P. F., Masini E. Characteristics of histamine release evoked by acetylcholine in isolated rat mast cells. J Physiol. 1980 Apr;301:281–293. doi: 10.1113/jphysiol.1980.sp013205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant-Greenwood G. D. Relaxin as a new hormone. Endocr Rev. 1982 Winter;3(1):62–90. doi: 10.1210/edrv-3-1-62. [DOI] [PubMed] [Google Scholar]
- Bydlowski S. P. Mast cell: its mediators and effects on arterial wall metabolism. Gen Pharmacol. 1986;17(6):625–631. doi: 10.1016/0306-3623(86)90290-9. [DOI] [PubMed] [Google Scholar]
- FEIGEN G. A., VAUGHAN WILLIAMS E. M., PETERSON J. K., NIELSEN C. B. Histamine release and intracellular potentials during anaphylaxis in the isolated heart. Circ Res. 1960 Jul;8:713–723. doi: 10.1161/01.res.8.4.713. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Hallett M. B., Mongar J. L. The relationship between histamine secretion and 45calcium uptake by mast cells. J Physiol. 1977 Sep;271(1):193–214. doi: 10.1113/jphysiol.1977.sp011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. Regional haemodynamic changes during oral ingestion of NG-monomethyl-L-arginine or NG-nitro-L-arginine methyl ester in conscious Brattleboro rats. Br J Pharmacol. 1990 Sep;101(1):10–12. doi: 10.1111/j.1476-5381.1990.tb12079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick D., Von Redlich D., Juhos E. T., McEwen C. R. Separation of mast cells by centrifugal elutriation. Exp Cell Res. 1971 Mar;65(1):23–26. doi: 10.1016/s0014-4827(71)80045-9. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Moncada S., Palmer R. M. Bioassay of prostacyclin and endothelium-derived relaxing factor (EDRF) from porcine aortic endothelial cells. Br J Pharmacol. 1986 Apr;87(4):685–694. doi: 10.1111/j.1476-5381.1986.tb14586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. A., Cantley T. C., Day B. N., Anthony R. V. Uterotropic actions of relaxin in prepubertal gilts. Biol Reprod. 1990 May-Jun;42(5-6):769–774. doi: 10.1095/biolreprod42.5.769. [DOI] [PubMed] [Google Scholar]
- KREMZNER L. T., WILSON I. B. A procedure for the determination of histamine. Biochim Biophys Acta. 1961 Jun 24;50:365–367. doi: 10.1016/0006-3002(61)90339-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawson D., Raff M. C., Gomperts B., Fewtrell C., Gilula N. B. Molecular events during membrane fusion. A study of exocytosis in rat peritoneal mast cells. J Cell Biol. 1977 Feb;72(2):242–259. doi: 10.1083/jcb.72.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannaioni P. F., Masini E., Pistelli A., Salvemini D., Vane J. R. Mast cells as a source of superoxide anions and nitric oxide-like factor: relevance to histamine release. Int J Tissue React. 1991;13(6):271–278. [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Masini E., Giannella E., Pistelli A., Palmerani B., Gambassi F., Occupati B., Mannaioni P. F. Histamine release by free radicals: the relationship with the signal transduction system. Agents Actions. 1989 Apr;27(1-2):72–74. doi: 10.1007/BF02222202. [DOI] [PubMed] [Google Scholar]
- Masini E., Palmerani B., Gambassi F., Pistelli A., Giannella E., Occupati B., Ciuffi M., Sacchi T. B., Mannaioni P. F. Histamine release from rat mast cells induced by metabolic activation of polyunsaturated fatty acids into free radicals. Biochem Pharmacol. 1990 Mar 1;39(5):879–889. doi: 10.1016/0006-2952(90)90203-w. [DOI] [PubMed] [Google Scholar]
- Masini E., Salvemini D., Pistelli A., Mannaioni P. F., Vane J. R. Rat mast cells synthesize a nitric oxide like-factor which modulates the release of histamine. Agents Actions. 1991 May;33(1-2):61–63. doi: 10.1007/BF01993127. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Pearce F. L., Ennis M. Isolation and some properties of mast cells from the mesentery of the rat and guinea pig. Agents Actions. 1980 Apr;10(1 Pt 2):124–131. doi: 10.1007/BF02024193. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Characterization of the L-arginine:nitric oxide pathway in human platelets. Br J Pharmacol. 1990 Oct;101(2):325–328. doi: 10.1111/j.1476-5381.1990.tb12709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Salvemini D., Masini E., Anggard E., Mannaioni P. F., Vane J. Synthesis of a nitric oxide-like factor from L-arginine by rat serosal mast cells: stimulation of guanylate cyclase and inhibition of platelet aggregation. Biochem Biophys Res Commun. 1990 Jun 15;169(2):596–601. doi: 10.1016/0006-291x(90)90372-t. [DOI] [PubMed] [Google Scholar]
- Salvemini D., Mollace V., Pistelli A., Anggard E., Vane J. Metabolism of glyceryl trinitrate to nitric oxide by endothelial cells and smooth muscle cells and its induction by Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):982–986. doi: 10.1073/pnas.89.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood C. D., O'Byrne E. M. Purification and characterization of porcine relaxin. Arch Biochem Biophys. 1974 Jan;160(1):185–196. doi: 10.1016/s0003-9861(74)80025-1. [DOI] [PubMed] [Google Scholar]
- Sherwood O. D., Crnekovic V. E., Gordon W. L., Rutherford J. E. Radioimmunoassay of relaxin throughout pregnancy and during parturition in the rat. Endocrinology. 1980 Sep;107(3):691–698. doi: 10.1210/endo-107-3-691. [DOI] [PubMed] [Google Scholar]
- Vasilenko P., Mead J. P., Weidmann J. E. Uterine growth-promoting effects of relaxin: a morphometric and histological analysis. Biol Reprod. 1986 Nov;35(4):987–995. doi: 10.1095/biolreprod35.4.987. [DOI] [PubMed] [Google Scholar]