Abstract
Background
A primary goal of recent research is the development of neurobehavioral profiles that specifically define fetal alcohol spectrum disorders (FASD), which may assist differential diagnosis or improve treatment. In the current study we define a preliminary profile using neuropsychological data from a multisite study.
Methods
Data were collected using a broad neurobehavioral protocol from two sites of a multisite study of FASD. Subjects were children with heavy prenatal alcohol exposure and unexposed controls. The alcohol-exposed group included children with and with out fetal alcohol syndrome (FAS). From 547 neuropsychological, 22 variables were selected for analysis based on their ability to distinguish children with heavy prenatal alcohol exposure from nonexposed controls. These data were analyzed using latent profile analysis (LPA).
Results
The results indicated that a 2-class model best fit the data. The resulting profile was successful at distinguishing subjects with FAS from nonexposed controls without FAS with 92% overall accuracy; 87.8% of FAS cases and 95.7% of controls were correctly classified. The same analysis was repeated with children with heavy prenatal alcohol exposure but without FAS and non-exposed controls with similar results. The overall accuracy was 84.7%; 68.4% of alcohol-exposed cases and 95% of controls were correctly classified. In both analyses, the profile based on neuropsychological variables was more successful at distinguishing the groups than was IQ alone.
Conclusions
We used data from two sites of a multisite study and a broad neuropsychological test battery to determine a profile that could be used to accurately identify children affected by prenatal alcohol exposure. Results indicated that measures of executive function and spatial processing are especially sensitive to prenatal alcohol exposure.
Keywords: Fetal alcohol syndrome, Fetal alcohol spectrum disorders, Prenatal alcohol exposure, Neurobehavioral profile, Profile analysis, International study
INTRODUCTION
The constellation of physical and neurobehavioral features, now known as fetal alcohol syndrome (FAS), was first brought to public awareness in 1973 by Jones and Smith who described 11 children born to alcohol-abusing mothers (Jones and Smith, 1973; Jones et al., 1973). This pattern of malformation, consisting of prenatal growth deficiency, developmental delay, and craniofacial abnormality remains the basis of the diagnostic criteria used today (Bertrand et al., 2005; Hoyme et al., 2005; Stratton et al., 1996). Deficits related to the central nervous system (CNS) remain part of the diagnostic criteria of FAS and can be structural, neurological, or functional; the specific nature of these deficits is not well defined and can range from severe mental retardation to subtler CNS dysfunction (e.g., attention difficulties).
Although the diagnosis of FAS can primarily be made based on physical features, the majority of children affected by prenatal alcohol exposure do not show these physical markers (Bertrand et al., 2005; Sampson et al., 1997). Several terms describe these individuals who are affected by the teratogenic nature of alcohol but who lack all or some of the physical signs required for the diagnosis of FAS. These include partial FAS (PFAS), alcohol-related neurodevelopmental disorder (ARND), alcohol-related birth defects (ARBD), fetal alcohol effects (FAE), and static encephalopathy. The Institute of Medicine guidelines (Hoyme et al., 2005; Stratton et al., 1996) provide criteria for the diagnosis of PFAS, ARND, and ARBD to facilitate identification of individuals along the spectrum of effects. Acknowledging the continuum of deleterious physical, mental and behavioral outcomes caused by prenatal alcohol exposure, the National Task Force on FAS/FAE adopted the non-diagnostic term fetal alcohol spectrum disorders (FASD) (Bertrand et al., 2004). The diagnosis of FAS is included under this umbrella term and falls at the severe end of the spectrum.
Although the first reported cases of FAS were born to known alcoholics and most early work focused on FAS specifically, more recent research indicates that similar deficits occur in children along the spectrum (e.g., Mattson et al., 1997; Mattson et al., 1998). Currently, the precise prevalence of affected children is not known (May and Gossage, 2001; May et al., 2009) perhaps because identification of children along the continuum of FASD is complicated by several factors (Bertrand et al., 2005). First, as mentioned, relying on external markers is not sufficient, as the majority of alcohol-affected children do not meet the physical criteria for FAS. In addition, the full range of effects is not known, thus children with less striking manifestations may go unnoticed or be misdiagnosed. Finally, individual neurobehavioral features, including decreases in IQ, seen in children with FASD may overlap with other clinical conditions or disorders, further decreasing the ability to accurately identify alcohol-affected individuals. Hence, research aimed at improving sensitivity and specificity of alcohol-related diagnoses is needed (Riley et al., 2003). One promising line of research is the development of a neurobehavioral profile or profiles of FAS and FASD. Such profiles will add to the armamentarium employed by clinicians and improve identification of affected children.
Developing a neurobehavioral profile that is broadly applicable requires three steps: determining the profile based on children known to be affected (i.e., children with FAS), testing and refining the profile on children with known exposure but without FAS, and finally testing the profile on independent samples of children with prenatal alcohol exposure and children with other disorders. In the current study, we define a preliminary profile using neuropsychological data from a multisite study (Mattson et al., 2010). In the first step we included only children with FAS and in the second step we tested the model on children with heavy prenatal alcohol exposure who did not meet criteria for FAS. A secondary goal of this study is to determine if the defined neurobehavioral profile improves our predictive ability over general intellectual functioning, as measured by IQ, providing evidence of specificity of the profile.
MATERIALS AND METHODS
Data analyzed in this study are the result of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), a multisite, interdisciplinary study of FASD. The general methods have been described in detail elsewhere (Mattson et al., 2010). As part of the CIFASD, a standardized neurobehavioral test battery, including neuropsychological tests and questionnaires, was administered to children at five sites in four countries. Children also were examined by a member of the CIFASD Dysmorphology Core using a standardized examination. For this study, neuropsychological data were analyzed using latent profile analysis. Subjects were between the ages of 7 and 21 years at the time of neuropsychological assessment. Analyzed data were from an extended battery that was not administered in entirety by two sites. In addition, controls were not available from a third site. Thus, data from two CIFASD sites were included in this initial set of analyses.
Subjects
Subjects included in these analyses were from the Center for Behavioral Teratology (CBT), San Diego State University in San Diego, California and the Folkhälsan Research Center in Helsinki, Finland. These two sites are characterized by middle socioeconomic status levels and generally similar postnatal environments in terms of quality of life in the childrearing years. Both retrospectively and prospectively identified subjects were included and both sites recruited exposed and control subjects. Overall, the exposed sample was heavily exposed (>4 drinks/occasion at least once/week or >13 drinks/week). In all cases, positive exposure histories were confirmed via review of records or maternal report, if available. Non-exposed, typically developing controls were recruited from ongoing studies at each site or specifically for this study. Recruitment strategies included advertisements, word of mouth, or use of national registers. Controls were screened for prenatal alcohol exposure and excluded if there was evidence, based on maternal report, of greater than minimal alcohol exposure, defined as greater than one drink per week on average and never more than 2 drinks on any one occasion during pregnancy. Only subjects with complete neuropsychological data and dysmorphology examinations were eligible. Children were excluded from the control group if direct evidence of alcohol exposure was unavailable (e.g., maternal report, laboratory testing). Children were excluded from the exposed group for the same reason, unless alcohol exposure was suspected and documented by reliable collateral reports (i.e., reporters with reasonable knowledge of the mother's alcohol use), and they met criteria for FAS, as described below. Subject demographic data is listed in Table 1.
Table 1.
Demographic data for subjects in the four study groups.
| Analysis 1 | Analysis 2 | |||||
|---|---|---|---|---|---|---|
| Variable | Alcohol-Exposed/FAS | Control/Not FAS | p value2 | Alcohol-Exposed/Deferred or Not FAS | Control/Deferred or Not FAS | p value2 |
| N | 41 | 46 | 38 | 60 | ||
| M (SD) | M (SD) | M (SD) | M (SD) | |||
|---|---|---|---|---|---|---|
| Age | 13.7 (3.47) | 13.3 (3.64) | .587 | 13.1 (3.88) | 13.0 (3.38) | .822 |
| IQ | 91.6 (14.37) | 110.0 (12.09) | <.001 | 96.0 (15.05) | 108.9 (12.52) | <.001 |
| N (%) | N (%) | N (%) | N (%) | |||
|---|---|---|---|---|---|---|
| Sex (Female) | 24 (58.5) | 29 (63.0) | .826 | 15 (39.5) | 33 (55.0) | .151 |
| Handedness (Right) | 30 (73.2) | 40 (87.0) | .110 | 31 (81.6) | 52 (86.7) | .461 |
| Country | .061 | .001 | ||||
| Finland | 33 (80.5) | 28 (60.9) | 8 (21.1) | 34 (56.7) | ||
| United States | 8 (19.5) | 18 (39.1) | 30 (78.9) | 26 (43.3) | ||
| Race (White) | 39 (95.1) | 42 (91.3) | .680 | 25 (65.8) | 53 (88.3) | .010 |
| Ethnicity (Hispanic) | 0 (0) | 2 (4.3) | .233 | 5 (13.2) | 5 (8.3) | .324 |
| Growth Deficiency | 28 (68.3) | 3 (6.5) | <.001 | 9 (23.7) | 6 (10.0) | .086 |
| Height≤ 10% | 20 (48.8) | 3 (6.5) | <.001 | 6 (15.8) | 5 (8.3) | .329 |
| Weight≤ 10% | 19 (46.3) | 0 (0) | <.001 | 9 (23.7) | 2 (3.3) | .003 |
| Microcephaly (OFC ≤ 10%) | 33 (80.5) | 0 (0) | <.001 | 5 (13.2) | 2 (3.3) | .105 |
| Structural Abnormality | 41 (100) | 0 (0) | <.001 | 9 (23.7) | 1 (1.7) | .001 |
| PFL≤10% | 35 (85.4) | 0 (0) | <.001 | 7 (18.4) | 4 (6.7) | .101 |
| Smooth Philtrum | 34 (82.9) | 0 (0) | <.001 | 14 (36.8) | 3 (5.0) | <.001 |
| Thin Vermillion Border | 40 (97.6) | 0 (0) | <.001 | 10 (26.3) | 6 (10.0) | .049 |
| Category3 | <.001 | .002 | ||||
| FAS | 41 (100.0) | -- | -- | -- | ||
| Not FAS | -- | 46 (100.0) | 17 (44.7) | 46 (76.7) | ||
| Deferred | -- | -- | 21 (55.3) | 14 (23.3) |
Two sets of analyses were conducted. The first analysis compared Alcohol-exposed subjects with FAS vs. Control subjects in the Not FAS category. The second analysis compared Alcohol-exposed subjects in the Deferred or Not FAS category vs. Control subjects in the Deferred or Not FAS category.
p values are based on ANOVA, chi square, or Fisher's exact tests.
Categories are based on physical features only and not prenatal alcohol exposure
Center for Behavioral Teratology, San Diego State University
The CBT is a university-wide research center focused on the study of brain and behavioral changes associated with prenatal exposure to drugs and alcohol (cf., Mattson et al., 2006; Mattson and Roebuck, 2002). Alcohol-exposed children are referred by Dr. Kenneth Lyons Jones (Principal Investigator of the CIFASD Dysmorphology Core), other local professionals, and self-referrals. Alcohol exposure histories were obtained from maternal report and review of medical, legal, and social service records. Controls were recruited from the community or self-referred.
Folkhälsan Research Center, Finland
Alcohol-exposed children at this site were recruited in one of two ways: from a clinical patient pool at the Hospital for Children and Adolescents, University of Helsinki, or from a prospective follow-up study (cf., Autti-Rämö, 2000; Autti-Rämö et al., 2006). Alcohol exposure histories were obtained either from the prenatal period or from medical records. Controls were recruited from a national population register using a computerized randomization method and contacted by telephone.
Dysmorphology Examinations
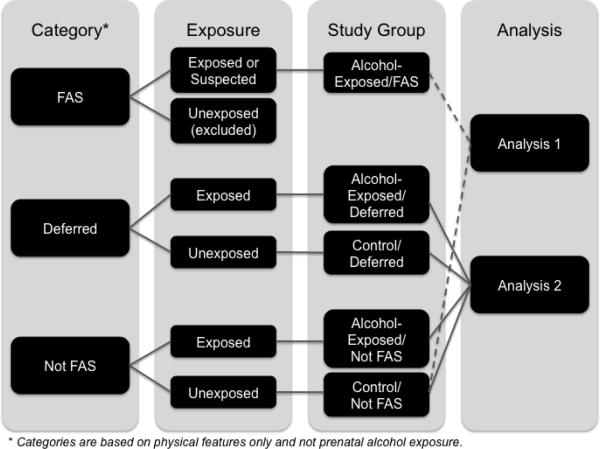
All children, including controls, were examined by a member of the CIFASD Dysmorphology core for determination of an FAS diagnosis. For the purposes of this study, only physical features are used for categorization, and not alcohol exposure or neurobehavioral outcome (Jones et al., 2006). FAS is defined by the CIFASD Dysmorphology Core as the presence of two of three key facial features typical of FAS (short palpebral fissures, smooth philtrum, thin vermillion) and either microcephaly (head circumference ≤10th percentile) or growth deficiency (weight and/or height ≤10th percentile) or both. Children in the alcohol-exposed group who do not meet these criteria are identified as either “Not FAS” or “Deferred”. Children in the Deferred category have: (1) at least one of the key features listed above or (2) microcephaly and growth deficiency or (3) microcephaly or growth deficiency and at least one additional specified feature (e.g., ptosis, camptodactly). This category was intended to be a temporary category that will be updated at the end of the study, based on results from other CIFASD studies, including neuropsychological studies. Thus, children in the Deferred category may later be considered to be affected by alcohol (i.e., fall under the spectrum of FASD) or not. Children in the Not FAS category do not qualify for the FAS or the Deferred categories, based on physical features. As indicated above, only physical features were used for categorization, and not alcohol-exposure or neurobehavioral findings. See Table 2 and Figure 1. Based on the dysmorphology examination and using the flow-chart illustrated in Figure 1, four final groups were formed: (1) children in the exposed group who met study criteria for FAS; (2) children in the control group who were in the Not FAS category; (3) children in the exposed group who were in the Not FAS or Deferred categories; and (4) children in the control group who were in the Not FAS or Deferred categories. The first set of analyses included groups 1 and 2 and the second analysis included groups 3 and 4.
Table 2.
Diagnostic criteria used by the CIFASD Dysmorphology Core.1
| Criterion | Definition |
|---|---|
| Growth Deficiency | Weight and/or Height ≤10th percentile |
| Microcephaly | Head circumference ≤10th percentile |
| Structural Abnormality | At least two of the following KEY facial features:
|
| Category2 | Required Criteria |
|---|---|
| FAS | Structural Abnormality and Growth Deficiency OR |
| Structural Abnormality and Microcephaly OR | |
| Structural Abnormality and Microcephaly and Growth Deficiency | |
| Deferred | At least one KEY facial feature (listed above) OR |
| Microcephaly and Growth Deficiency OR | |
Microcephaly or Growth Deficiency AND at least one of the following additional features:
|
|
| Not FAS | Does not meet criteria for either FAS or Deferred category |
Table originally published in Mattson et al., 2010.
Categories are based on physical features only and not prenatal alcohol exposure.
Figure 1.
Neuropsychological Measures
A standardized neuropsychological test battery was administered individually to all subjects. Age-appropriate tests were selected for this phase of the study to assess a broad range of functioning while limiting emphasis on verbal instructions and responses, given the international nature of the study. Tests were administered in the child's native language. The tests included in this battery were: Edinburgh Handedness, Leiter-R (Figure Ground, Form Completion, Sequential Order, Repeated Patterns, Attention Sustained subtests), CANTAB (Motor Screening, Big/Little Circle, Pattern Recognition Memory, Spatial Recognition Memory, Spatial Span, Spatial Working Memory subtests), Grooved Pegboard, Virtual Water Maze, NES3 - Continuous Performance Test (Animals), Visual Discrimination Reversal Learning, Progressive Planning Test, Finger Localization, Delis-Kaplan Executive Function System (Verbal Fluency, Trail Making subtests), and the Visual-Motor Integration Test. Test results were scored according to published test manuals and data were entered into a centralized data base. Scores were converted to standard scores according to age norms when available. As a result of this test battery, 547 variables were available (Arenson et al., 2010). Variables were selected based on initial determination of effect sizes; only variables with medium and greater effect sizes (Cohen's d 0.58–1.29) to detect group differences (between alcohol-exposed subjects and non-exposed controls) were retained. In addition, to further reduce the number of variables included, those that were redundant (i.e., correlated at r >.70) with other observed variables with medium to large effect sizes were excluded (variables with the larger effect sizes were retained). The resulting data set included 22 variables, which are listed in Table 3. Although Leiter Brief IQ scores were available for all subjects, IQ was not included in the initial analyses given the large amount of variance accounted for by IQ and because one of our goals is to define a neurobehavioral profile that is more specific than decreased IQ. However, as described below, supplemental analyses tested the resulting profiles after accounting for IQ.
Table 3.
Description of variables included in analyses.
| Observed Variable/Measure1 | Description | Functional Domain |
|---|---|---|
| Cambridge Neuropsychological Test Automated Battery (CANTAB) | ||
| CANTAB Spatial Recognition Memory Percent Correct (z score) | Percent correct on the spatial recognition memory test | Visual Memory, Spatial Reasoning |
| CANTAB Spatial Span Length (z score) | Longest span length with no errors on the spatial span test | Executive Function, Spatial Reasoning, Visual Memory |
| CANTAB Spatial Working Memory Strategy (z score) | Strategy score on the spatial working memory test | Executive Function, Spatial Reasoning, Visual Memory |
| CANTAB Spatial Working Memory Total Errors (z score) | Total number of errors on the spatial working memory test | Executive Function, Spatial Reasoning |
| Delis-Kaplan Executive Function System (D-KEFS) | ||
| D-KEFS Trail Making Combined Number/Letter (scaled score) | Combined score on number and letter sequencing of the trail making test | Executive Function, Sequencing |
| D-KEFS Trail Making Test – Switch vs. Number (scaled score) | Difference between switching and number sequencing on the trail making test | Executive Function, Cognitive Flexibility |
| D-KEFS Trail Making Test – Switch vs. Visual (scaled score) | Difference between switching and visual scanning on the trail making test | Executive Function |
| D-KEFS Trail Making Test – Switch Errors (scaled score) | Total number of errors on the switching subtest of the trail making test | Executive Function, Cognitive Flexibility |
| D-KEFS Verbal Fluency Total Correct Letter (scaled score) | Total number of correct words produced over 3 letters on the verbal fluency test | Executive Function, Fluency |
| D-KEFS Verbal Fluency Total Correct Category (scaled score) | Total number of correct words produced over 2 categories on the verbal fluency test | Executive Function, Fluency |
| D-KEFS Verbal Fluency Total Correct Switch (scaled score) | Total number of correct words produced during switching task, regardless of switching accuracy, on the verbal fluency test | Executive Function, Cognitive Flexibility |
| D-KEFS Verbal Fluency 2nd Interval Correct (scaled score) | Total number of correct words produced during 2nd 15 seconds summed across all trials of the verbal fluency test | Executive Function, Fluency |
| D-KEFS Verbal Fluency Set Loss Errors (scaled score) | Total number of set loss errors made during entire subtest of the verbal fluency test | Executive Function, Set Maintenance |
| Morris Virtual Water Maze (MVWM) | ||
| Morris Virtual Water Maze Time in Target Quadrant on Probe Trial (raw score) | Amount of time in seconds spent in the target quadrant during probe trial on the Morris virtual water maze test | Spatial Learning |
| Neurobehavioral Evaluation System 3 (NES3) | ||
| NES3 Animals Following subtest, Number Correct (raw score) | Number of correct responses for the CPT animal following subtest | Sustained Attention |
| NES3 Animals Repeating subtest, Number Correct (raw score) | Number of correct responses for the CPT animal repeating subtest | Sustained Attention |
| NES3 Animals Single subtest, Number Correct (raw score) | Number of correct responses for the CPT animal single subtest | Sustained Attention |
| Grooved Pegboard Test | ||
| Grooved Pegboard Test Dominant hand Completion Time (z-score) | Subject's time in seconds to complete 2 rows (≤ 8 years of age) or 5 rows (≥ 9 years of age) using dominant hand on the grooved pegboard test | Fine Motor |
| Grooved Pegboard Test Non-Dominant Hand Completion Time (z-score) | Subject's time in seconds to complete 2 rows (≤ 8 years of age) or 5 rows (≥ 9 years of age) using nondominant hand on the grooved pegboard test | Fine Motor |
| Progressive Planning Test (PPT) | ||
| Progressive Planning Test Maximally Constrained Total Score (raw score) | Total number of points on the maximally constrained condition of the progressive planning test | Executive Function, Planning |
| Visual Discrimination Reversal Learning Test (VDRL) | ||
| Visual Discrimination Reversal Learning Test Number of Reversals (raw score) | Total number of reversals within 30 trials after phase 2 on the visual discrimination reversal learning test | Executive Function, Cognitive Flexibility |
| Visual Motor Integration Test (VMI) | ||
| Visual Motor Integration Test Total (standard score) | Performance on the visual-motor integration test | Visual-Motor |
included variables were selected from 547 neuropsychologic variables collected. Only meaningful, nonredundant neuropsychologic variables that had medium to large effect sizes for detecting group differences were retained for analysis.
Statistical Analysis Plan
Latent profile analysis (LPA) was conducted to derive latent profiles that describe different categorical types of participants. LPA is a person-centered statistical approach that classifies individuals into groups based on their patterns of responses to sets of observed variables (Hagenaars and McCutcheon, 2002; Lanza et al., 2003; McCutcheon, 1987; Roesch et al., in press). The primary goal is to maximize the homogeneity within groups (i.e., individuals within a class/profile should look similar) and maximize the heterogeneity between groups (i.e., individuals between classes/profile groups should look different). These groups are represented by a categorical latent variable, as they are not directly known but are inferred from the response patterns on observed variables. LPA assumes a simple parametric model and uses the observed data to estimate parameter values for the model. This model-based approach is preferable to more subjective grouping techniques such as cluster analysis (Vermunt and Magidson, 2002). Model parameters are estimated using the maximum likelihood (ML) criterion. In the current study the 22 neuropsychological assessment variables (Table 3) were used as indicators (observed variables) to derive the latent profiles.
The determination of the optimal number of classes or profiles requires the specification and testing of multiple class solutions (1-class, 2-class, etc.). From these models, the designation of the “best-fitting” model is determined using a variety of statistical indicators. In the current study model fit was determined using the Akaike Information Critreria (Akaike, 1974) and the sample size-adjusted Bayesian Information Criterion (Sclove, 1987), with lower values for these fit indicators indicating better model fit (Tofighi and Enders, 2007; Yang, 2006). In addition, the entropy index (the percentage of individuals in the sample that were correctly classified given the specific class model) was used because it indicates how well profiles can be distinguished; this value is not meaningful in 1-class solutions. Entropy values greater than 80% are considered noteworthy (Ramaswamy et al., 1993). Once the number of profiles is determined, conditional response means (CRM) are interpreted to substantively characterize those within each profile. CRMs indicate the mean value for an observed variable within a profile. All models were estimated using MPlus (Muthén and Muthén, 2006). LPAs were conducted on two samples: (1) analysis 1: those subjects that are exposed and have FAS vs. controls that are not exposed and do not have FAS (in the Not FAS category) and (2) analysis 2: those subjects that are exposed but do not have FAS (Deferred or Not FAS) and controls that are not exposed and do not have FAS (Deferred or Not FAS). The goal of the second analysis was to determine if the same profile could be used to distinguish alcohol-exposed children without FAS from controls. The same neuropsychological variables were included in both analyses. Subsequent to the LPAs, logistic regression analyses with classification tables were evaluated. The profiles were crossed with the target comparison groups (e.g., alcohol-exposed with FAS vs. controls in the Not FAS category) to evaluate how well the classes predicted group membership. Moreover, hierarchical logistic regression analyses were conducted to determine if the profiles predicted group membership over and above IQ.
RESULTS
Demographics
Demographic data for the study groups are listed in Table 1. Groups did not differ in terms of age or distribution of sex, handedness, or ethnicity. The Alcohol-Exposed/FAS group differed from the Control/Not FAS group in terms of IQ, growth variables, microcephaly, measures of structural abnormality, diagnostic category, and were marginally different on country of origin. The Exposed/Deferred or Not FAS group differed from the Control/Deferred or Not FAS group on IQ, country of origin, race, weight, measures of structural abnormality, and diagnostic category. Specific statistical results are listed in the Table.
Latent Profile Analysis
Prior to conducting the LPA, we determined that that combining data from two sites was appropriate. We conducted univariate analyses on the 22 neuropsychological variables and the group × site interaction was significant for only one variable (NES3 Animals Single subtest, Number Correct, F (1, 135) = 5.10, p = .026). Thus, we proceeded with the LPA, combining data from the two data-collection sites. Because of the relative low sample size to observed variables ratio, 1- and 2-class solutions were evaluated.
Analysis 1: Exposed/FAS vs. Control/Not FAS
Descriptive group data for this analysis are included in Table 4. For the first analysis, comparing the exposed/FAS and Control/Not FAS groups, a 2-class solution fit better than a 1-class solution (AIC: 9281 vs. 9536; sBIC: 9235 vs. 9506; Entropy index for 2-class model = .90). For the 2-class solution, 38 participants were assigned to profile 1 (43.6% of the sample) and 49 participants were assigned to profile 2 (56.4% of the sample). As shown in Table 5, the CRMs indicate that individuals in profile 1 perform more poorly than individuals in profile 2 for each of the 22 observed variables characterizing the profiles.
Table 4.
Descriptive Data for neuropsychological tests by group for the first analysis: Alcohol-exposed subjects with FAS vs. Control subjects in the Not FAS category.
| Alcohol-Exposed/FAS | Control/Not FAS | |||
|---|---|---|---|---|
| Observed Variable/Measure | M1 | SD | M | SD |
| CANTAB Spatial Recognition Memory Percent Correct | −0.09 | 1.09 | 0.52 | 0.91 |
| CANTAB Spatial Span Length | −0.10 | 0.82 | 0.59 | 1.22 |
| CANTAB Spatial Working Memory Strategy | −0.50 | 0.64 | −0.06 | 0.95 |
| CANTAB Spatial Working Memory Total Errors | −0.84 | 0.93 | 0.05 | 1.02 |
| D-KEFS Trail Making Combined Number/Letter | 8.61 | 3.07 | 12.24 | 2.33 |
| D-KEFS Trail Making Test – Switch vs. Number | 6.98 | 3.49 | 9.57 | 2.23 |
| D-KEFS Trail Making Test – Switch vs. Visual | 8.02 | 2.97 | 9.93 | 2.84 |
| D-KEFS Trail Making Test – Switch Errors | 8.63 | 3.31 | 10.74 | 1.73 |
| D-KEFS Verbal Fluency Total Correct Letter | 7.73 | 2.82 | 11.43 | 2.99 |
| D-KEFS Verbal Fluency Total Correct Category | 9.71 | 3.19 | 12.46 | 3.44 |
| D-KEFS Verbal Fluency Total Correct Switch | 7.98 | 2.66 | 10.96 | 2.73 |
| D-KEFS Verbal Fluency 2nd Interval Correct | 8.73 | 2.89 | 11.67 | 3.34 |
| D-KEFS Verbal Fluency Set Loss Errors | 10.07 | 2.89 | 11.54 | 1.64 |
| Morris Virtual Water Maze Time in Target Quadrant on Probe Trial | 45.73 | 26.35 | 67.23 | 19.47 |
| NES3 Animals Following subtest, Number Correct | 35.46 | 7.25 | 37.89 | 2.15 |
| NES3 Animals Repeating subtest, Number Correct | 29.46 | 8.38 | 33.67 | 6.52 |
| NES3 Animals Single subtest, Number Correct | 39.02 | 2.19 | 39.61 | 0.91 |
| Grooved Pegboard Test Dominant hand Completion Time | 0.56 | 1.12 | −0.42 | 0.78 |
| Grooved Pegboard Test Non-Dominant Hand Completion Time | 0.58 | 1.38 | −0.28 | 0.74 |
| Progressive Planning Test Maximally Constrained Total Score | 30.83 | 12.85 | 55.26 | 23.82 |
| Visual Discrimination Reversal Learning Test Number of Reversals | 2.00 | 0.55 | 2.24 | 0.48 |
| Visual Motor Integration Test Total | 83.27 | 14.51 | 91.39 | 14.57 |
Data are presented as means (M) and standard deviations (SD). Based on univariate analyses, groups were significantly different (p < .05) on all variables except NES3 Animals Single subtest, Number Correct (p = .10).
Table 5.
Conditional response means and effect size differences between profiles based on the first analysis: Alcohol-exposed subjects with FAS vs. Control subjects in the Not FAS category
| Observed Variable/Measure | Profile 1 | Profile 2 | d |
|---|---|---|---|
| CANTAB Spatial Recognition Memory Percent Correct | −0.14 | 0.53 | −0.67 |
| CANTAB Spatial Span Length | −0.13 | 0.57 | −0.67 |
| CANTAB Spatial Working Memory Strategy | −0.65 | 0.03 | −0.90 |
| CANTAB Spatial Working Memory Total Errors | −0.99 | 0.12 | −1.18 |
| D-KEFS Trail Making Combined Number/Letter | 8.49 | 12.14 | −1.31 |
| D-KEFS Trail Making Test – Switch vs. Number | 6.62 | 9.70 | −1.12 |
| D-KEFS Trail Making Test – Switch vs. Visual | 7.88 | 9.94 | −0.71 |
| D-KEFS Trail Making Test – Switch Errors | 8.46 | 10.76 | −0.87 |
| D-KEFS Verbal Fluency Total Correct Letter | 7.39 | 11.49 | −1.49 |
| D-KEFS Verbal Fluency Total Correct Category | 9.20 | 12.71 | −1.10 |
| D-KEFS Verbal Fluency Total Correct Switch | 7.62 | 11.07 | −1.36 |
| D-KEFS Verbal Fluency 2nd Interval Correct | 8.38 | 11.21 | −0.93 |
| D-KEFS Verbal Fluency Set Loss Errors | 8.11 | 11.28 | −1.34 |
| Morris Virtual Water Maze Time in Target Quadrant on Probe Trial | 43.09 | 68.10 | −1.10 |
| NES3 Animals Following subtest, Number Correct | 35.17 | 37.99 | −0.52 |
| NES3 Animals Repeating subtest, Number Correct | 28.71 | 34.04 | −0.72 |
| NES3 Animals Single subtest, Number Correct | 38.99 | 39.61 | −0.36 |
| Grooved Pegboard Test Dominant hand Completion Time | 0.59 | −0.40 | 1.01 |
| Grooved Pegboard Test Non-Dominant Hand Completion Time | 0.59 | −0.24 | 0.73 |
| Progressive Planning Test Maximally Constrained Total Score | 30.34 | 54.29 | −1.25 |
| Visual Discrimination Reversal Learning Test Number of Reversals | 2.02 | 2.21 | −0.36 |
| Visual Motor Integration Test Total | 82.05 | 91.90 | −0.65 |
d = Cohen's d
Logistic regression was then used to evaluate the association between the 2 latent profiles and a binary variable representing the exposed/FAS group (coded 1) vs. the Control/Not FAS group (coded 0). The latent profile variable was significantly associated with group membership (OR = 0.006, CI = .001 to .034, p < .001), with significantly more individuals from the exposed/FAS group in profile 1 and significantly more individuals from the control group in profile 2. The profiles accounted for 92% accuracy of prediction in the two groups combined, with 87.8% accuracy in the exposed group and 95.7% accuracy in the control group. A hierarchical logistic regression was then conducted to determine if the profiles improved prediction of group membership above and beyond IQ. IQ was significantly associated with group membership, (OR = 0.90, CI = .87 to .94, p < .001), with individuals in profile 1 having significantly lower IQ scores than individuals in profile 2. IQ accounted for an overall accuracy rate of 75.9%, with 75.6% accuracy in the exposed group and 76.1% in the control group. When the profiles were entered on step 2, a significant improvement in model fit was evident, χ2 (df = 1) = 39.78, p < .001, and overall accuracy classification improved to 92%; accuracy percentages by group are identical to the values presented earlier.
Analysis 2: Exposed/Deferred or Not FAS vs. Control/Deferred or Not FAS
Descriptive group data for this analysis are included in Table 6. For the second analysis comparing subjects that are exposed (in the Deferred and Not FAS categories) and controls (in the Deferred and Not FAS categories) a 2-class solution also fit better than a 1-class solution (AIC: 10464 vs. 10833; sBIC: 10426 vs. 10808; Entropy index for 2-class model = .97). For the resulting 2-class solution, 29 participants were assigned to profile 1 (29.5% of the sample) and 69 participants were assigned to profile 2 (70.4% of the sample). As shown in Table 7, the CRMs indicate that individuals in profile 1 perform more poorly than individuals in profile 2 for each of the 22 observed variables characterizing the profiles.
Table 6.
Descriptive Data for neuropsychological tests by group for the second analysis: Alcohol-exposed subjects in the Deferred or Not FAS category vs. Control subjects in the Deferred or Not FAS category.
| Alcohol-Exposed/Deferred or Not FAS | Control/Deferred or Not FAS | |||
|---|---|---|---|---|
| Observed Variable/Measure | M1 | SD | M | SD |
| CANTAB Spatial Recognition Memory Percent Correct | −0.04 | 0.85 | 0.51 | 0.90 |
| CANTAB Spatial Span Length | 0.01 | 1.17 | 0.67 | 1.13 |
| CANTAB Spatial Working Memory Strategy | −0.54 | 0.87 | −0.05 | 0.89 |
| CANTAB Spatial Working Memory Total Errors | −0.35 | 1.14 | 0.08 | 0.99 |
| D-KEFS Trail Making Combined Number/Letter | 8.16 | 3.55 | 12.22 | 2.18 |
| D-KEFS Trail Making Test – Switch vs. Number | 7.53 | 2.74 | 9.58 | 2.41 |
| D-KEFS Trail Making Test – Switch vs. Visual | 7.29 | 3.12 | 9.90 | 2.75 |
| D-KEFS Trail Making Test – Switch Errors | 8.00 | 3.88 | 10.85 | 1.59 |
| D-KEFS Verbal Fluency Total Correct Letter | 8.32 | 3.10 | 11.10 | 3.33 |
| D-KEFS Verbal Fluency Total Correct Category | 9.03 | 2.96 | 12.52 | 3.26 |
| D-KEFS Verbal Fluency Total Correct Switch | 6.95 | 2.44 | 10.62 | 2.60 |
| D-KEFS Verbal Fluency 2nd Interval Correct | 8.29 | 2.82 | 11.60 | 3.15 |
| D-KEFS Verbal Fluency Set Loss Errors | 9.61 | 3.71 | 11.47 | 1.66 |
| Morris Virtual Water Maze Time in Target Quadrant on Probe Trial | 44.55 | 24.16 | 64.89 | 21.81 |
| NES3 Animals Following subtest, Number Correct | 32.26 | 7.98 | 37.43 | 2.70 |
| NES3 Animals Repeating subtest, Number Correct | 26.87 | 8.72 | 32.75 | 6.71 |
| NES3 Animals Single subtest, Number Correct | 37.18 | 4.35 | 39.45 | 1.40 |
| Grooved Pegboard Test Dominant hand Completion Time | 0.62 | 1.14 | −0.38 | 0.71 |
| Grooved Pegboard Test Non-Dominant Hand Completion Time | 0.93 | 1.80 | −0.22 | 0.69 |
| Progressive Planning Test Maximally Constrained Total Score | 32.61 | 20.73 | 51.80 | 23.74 |
| Visual Discrimination Reversal Learning Test Number of Reversals | 2.11 | 0.51 | 2.22 | 0.45 |
Data are presented as means (M) and standard deviations (SD). Based on univariate analyses, groups were significantly different (p < .05) on all variables except Visual Discrimination Reversal Learning Test Number of Reversals (p = .262).
Table 7.
Conditional response means and effect size differences between profiles based on the second analysis: Alcohol-exposed subjects in the Deferred or Not FAS category vs. Control subjects in the Deferred or Not FAS category.
| Observed Variable/Measure | Profile 1 | Profile 2 | d |
|---|---|---|---|
| CANTAB Spatial Recognition Memory Percent Correct | −0.27 | 0.54 | −0.97 |
| CANTAB Spatial Span Length | −0.38 | 0.74 | −1.05 |
| CANTAB Spatial Working Memory Strategy | −0.82 | 0.01 | −0.96 |
| CANTAB Spatial Working Memory Total Errors | −0.73 | 0.19 | −0.84 |
| D-KEFS Trail Making Combined Number/Letter | 7.50 | 11.96 | −1.49 |
| D-KEFS Trail Making Test – Switch vs. Number | 6.97 | 9.55 | −1.92 |
| D-KEFS Trail Making Test – Switch vs. Visual | 6.89 | 9.73 | −0.96 |
| D-KEFS Trail Making Test – Switch Errors | 7.30 | 10.77 | −1.13 |
| D-KEFS Verbal Fluency Total Correct Letter | 7.54 | 11.06 | −1.12 |
| D-KEFS Verbal Fluency Total Correct Category | 7.98 | 12.50 | −1.54 |
| D-KEFS Verbal Fluency Total Correct Switch | 7.43 | 10.89 | −1.46 |
| D-KEFS Verbal Fluency 2nd Interval Correct | 9.07 | 11.27 | −0.93 |
| D-KEFS Verbal Fluency Set Loss Errors | 8.32 | 10.29 | −0.99 |
| Morris Virtual Water Maze Time in Target Quadrant on Probe Trial | 41.23 | 63.61 | −0.96 |
| NES3 Animals Following subtest, Number Correct | 29.92 | 37.74 | −1.33 |
| NES3 Animals Repeating subtest, Number Correct | 23.48 | 33.40 | −1.41 |
| NES3 Animals Single subtest, Number Correct | 36.78 | 39.32 | −0.72 |
| Grooved Pegboard Test Dominant hand Completion Time | 0.70 | −0.29 | 0.99 |
| Grooved Pegboard Test Non-Dominant Hand Completion Time | 1.26 | −0.20 | 1.02 |
| Progressive Planning Test Maximally Constrained Total Score | 27.66 | 51.37 | −1.15 |
| Visual Discrimination Reversal Learning Test Number of Reversals | 2.00 | 2.25 | −0.53 |
| Visual Motor Integration Test Total | 77.97 | 90.06 | −1.16 |
d = Cohen's d
Logistic regression was then used to evaluate the association between the 2 latent profiles and a binary variable representing the subjects that were exposed (coded 1) vs. controls (coded 0). The latent profile variable was significantly associated with group membership (OR = 0.024, CI = .006 to .093, p < .001), with significantly more individuals that were alcohol-exposed in profile 1 and significantly more controls in profile 2. The profiles accounted for 84.7% accuracy of prediction in the two groups combined, with 68.4% accuracy in the exposed group and 95% accuracy in the controls. A hierarchical logistic regression was then conducted to determine if the profiles improved prediction of group membership above and beyond IQ. IQ was significantly associated with group membership, (OR = 0.94, CI = .91 to .97, p < .001), with individuals in profile 1 having significantly lower IQ scores than individuals in profile 2. IQ accounted for an overall accuracy rate of 73.5%. However, only 55.3% of those in the exposed group were accurately predicted, whereas 85% of controls were accurately predicted. When the profiles were entered on step 2, a significant improvement in model fit was evident, χ2 (df=1) = 30.31, p < .001. Accuracy classification improved to 84.7% overall, with the prediction accuracy increasing to 68.4% of exposed and 95% of control subjects.
Misclassified Subjects
In the first analysis, classification accuracy was 92%. Although sample sizes were small, data were examined for any sign of systematic differences between the children with FAS who were incorrectly classified as controls and children with FAS who were correctly classified. No systematic differences were noted in physical characteristics, race, ethnicity, handedness, site of testing, or age. Children identified as FAS using CIFASD criteria who were misclassified as controls (n = 5) tended to have higher IQ scores (M = 103, SD = 16.3) than children correctly classified as FAS (M = 90, SD = 13.6). However, as indicated by the logistic regression analysis, IQ was not as accurate as the combination of the neuropsychological tests at distinguishing the groups and many of the correctly (20/36; 56%) and incorrectly (4/5; 80%) classified subjects had IQ scores >90. In the second analysis, accuracy was 84.7% and similar results were noted. Alcohol-exposed children who were misclassified as controls (n = 12) tended to have higher IQ scores (M = 104, SD = 16.5) than those that were correctly classified (M = 92, SD = 13.1). In this case, 8 of the 12 (75%) misclassified subjects and 13 of 26 (50%) correctly classified subjects had IQ scores >90.
In the first analysis, there were two controls misclassified as FAS. One had growth deficiency (height ≤ 10th percentile) and an IQ score of 79 and the other had no physical features and an IQ score of 97. In the second analysis, there was only one control misclassified as exposed; this child was growth deficient and had short palpebral fissures and an IQ score of 85. In all of the misclassified controls, any alcohol use during pregnancy was denied by the biological parent.
DISCUSSION
In this study, neuropsychological data from two sites of a multisite collaborative project were analyzed using LPA. Results indicated that a specific set of neuropsychological tests could be used to distinguish children with the physical features of FAS from non-exposed controls without FAS (92% overall classification accuracy). Further, and perhaps more importantly, this same profile can be used to accurately distinguish children with prenatal alcohol exposure who do not meet the physical criteria for FAS from non-exposed controls (85% overall classification accuracy). In both cases, the neuropsychological profile was more accurate at group classification than IQ scores alone. Improvement in the ability to distinguish these groups will aid in accurate identification of affected children. While the identification of FAS is predominantly based on physical features, these markers are not useful for children who are structurally unaffected. It is this latter group who are in need of better identification. Although we did not directly compare alcohol-exposed children without FAS to children with FAS, the results of this study indicate that these groups are similar in terms of their neurobehavioral profile and that in both cases, they can be reliably distinguished from controls.
The resulting profile consisted of tests of executive function (DKEFS Verbal Fluency Test, DKEFS Trail Making Test, Progressive Planning Test, Visual Discrimination Reversal Learning), attention (NES-3), spatial learning and memory (Morris Virtual Water Maze, CANTAB Spatial Recognition Memory, CANTAB Spatial Working Memory, and CANTAB Spatial Span), fine motor speed (Grooved Pegboard), and visual motor integration (VMI). Measures that did not discriminate groups well, and were not included in the LPA, were tests of basic motor, rule learning, object memory (CANTAB Motor Screening, CANTAB Big/Little Circle, CANTAB Pattern Recognition Memory), and interhemispheric transfer (finger localization).
There are two commonalities in the measures identified by this profile: many of the measures involve executive function or spatial reasoning. Executive function has been well studied in FASD; deficits have been noted in planning (Kodituwakku et al., 1995), utilization of feedback, cognitive flexibility, response inhibition, concept formation (Mattson et al., 1999), verbal fluency (Kodituwakku et al., 2006; Kodituwakku et al., 1995; Mattson et al., 1999; Vaurio et al., 2008), and nonverbal fluency (Schonfeld et al., 2001). In the current study, several measures incorporated different aspects of executive function, including working memory, verbal fluency, planning, sequencing, cognitive flexibility, and emotional executive function. Secondly, several of the measures tapped spatial processing in some capacity. These included spatial recognition memory, spatial span, spatial working memory, spatial learning, and visual-motor integration. While performance on spatial tasks has not been well studied in FASD, there have been reports of deficits in this domain. Previous human (Hamilton et al., 2003) and animal (e.g., Johnson and Goodlett, 2002) studies show convergent and specific place learning deficits using water maze tasks. Studies of spatial recall have been less consistent. For example, impaired spatial location recall has been documented (Uecker and Nadel, 1996; Uecker and Nadel, 1998), but may be better accounted for by basic perceptual and verbal memory skills (Kaemingk and Halverson, 2000). Spatial reasoning has also been associated with low levels of prenatal alcohol exposure in the Seattle longitudinal study (Streissguth, 2007). Thus, our findings add to the existing literature by documenting deficits in this domain.
The importance of the results presented herein is that we were able to distinguish alcohol-exposed children from controls using neuropsychological measures and the same profile applied to both dysmorphic and non-dysmorphic alcohol-exposed children. While not all children exposed prenatally to alcohol are affected, improved identification of those that are affected will enhance clinical service to this population and accuracy of incidence estimates. Accurate estimates of the incidence of FASD are critical in order to gauge public health impact and societal cost (Lupton et al., 2004). Increased incidence obligates even greater efforts to enhance public awareness of the deleterious effects of alcohol.
Estimates of the rates of affected children born to alcoholic or heavy drinking women are difficult to come by, but in the general U.S. population the rate of FAS is estimated at 0.5–2.0/1000 live births. In Finland, the estimated rate is 3/1000 (Autti-Rämö et al., 2008) and in other parts of the world much higher estimates have been reported (May et al., 2006; May et al., 2007). Other disorders along the spectrum are estimated to occur three times as often (US Department of Health and Human Services, 2005). Given that the estimated U.S. birth rate for 2009 is over 4.2 million (https://www.cia.gov/library/publications/the-world-factbook/), using a relatively conservative estimate for FAS of 1/1000 births, the estimated number of new FAS cases is over 4,200 per year. Using this same estimate, the number of all affected cases (FASD, including FAS) is nearly 17,000 births per year, with three quarters of them not identifiable based on physical features. It should be noted that this is a relatively conservative estimate, and other estimates have been higher (Sampson et al., 1997).
In spite of the high classification accuracy documented in this study, not all children were correctly classified. However, there does not seem to be any systematic reason for misclassification, other than performance on the neuropsychological measures upon which the profile was determined. Although average IQ scores were higher in the misclassified exposed subjects, it was not a reliable predictor of prenatal alcohol exposure and was less successful at accurately distinguishing the groups than the combination of the neuropsychological measures, as supported by the logistic regression. In addition, although 2 of the 3 misclassified controls had 1 or more features of FAS (i.e., growth deficiency, short palpebral fissures), alcohol use during pregnancy was denied by the biological parent.
This study was limited by the measures chosen for inclusion in the test battery and the resulting profile likely excludes other measures that would be useful for distinguishing alcohol-exposed subjects from controls. Phase I of the CIFASD testing, upon which this report is based, was predominantly nonverbal in nature. This was intentional, as the test battery was set by the CIFASD Neurobehavioral Core to limit the reliance on verbal instructions and verbal responses given the multi-site and multi-lingual nature of the study. In Phase II (data currently being collected), a greater number of verbal measures are included as the data collection sites have changed and more have English as the primary language. Thus, future analyses will examine the utility of both verbal and nonverbal measures. In addition, future analyses might also examine if a more limited battery of tests is equally useful in distinguishing the groups or if more than one profile exists. The current analyses tested only 1- or 2-class solutions based on the sample size available and it is possible that larger samples might reveal that more than one profile of function exists in alcohol-exposed children.
On a related note, selection of 22 variables for analysis from 547 available variables might be seen as a limitation of this study. Clearly, our sample size did not support inclusion of all the variables in the LPA, nor would all of them provide meaningful information. We examined effect sizes and correlations to select 22 non-redundant variables. In addition, the variables used to define the neurobehavioral profile were based on group differences in the same sample. The findings would be stronger if the current profiles could be validated on a new sample, and we recognize this as a limitation to the current study. However, a multivariate technique such as LPA requires a large sample for the initial calibration sample, which precluded this possibility. We also did not complete the third step in defining a neurobehavioral profile: testing its specificity using a clinical contrast group. Future research goals for the CIFASD include testing this model on subject groups that share clinical features with FASD, like lower IQ scores or ADHD. Only then will the true value of this profile be maximized.
Additional limitations of this study also exist. Some of the alcohol-exposed subjects were recruited prospectively, while none of the controls were recruited this way. This may have impacted the accuracy of recall about alcohol exposure in both groups. Similarly, reports of alcohol-exposure were done by maternal report in some cases and thus may be subject to recall bias. However, our emphasis on heavily exposed subjects lessens the impact of this limitation. In addition, our exclusionary level of alcohol exposure (not greater than 1 drink per week on average and never more than 2 drinks on any one occasion during pregnancy) is well below the threshold of effect identified in previous neurobehavioral studies (7–28 eight drinks per week, see Jacobson and Jacobson, 1994). However, if exposure levels in controls were underreported to the extent that this threshold was exceeded, the significant group differences described in this study are conservative estimates of actual group differences.
In summary, we used data from two sites of a multisite study and a broad neuropsychological test battery to determine a neuropsychological profile that could be used to accurately identify children affected by prenatal alcohol exposure. Results indicated that measures of executive function and spatial processing are especially sensitive to prenatal alcohol exposure. Importantly, this study did not address the third step in determining a neurobehavioral profile and these results should be validated by future studies replicating the profile in both independent samples of alcohol-exposed children as well as in other clinical groups.
Acknowledgements
Research described in this paper is supported by NIAAA grant numbers U01 AA014834 (Mattson), U24 AA014830 (Riley), U24 AA014811 (Riley), U24 AA014818 (Stewart/Barnett), and U24 AA014815 (Jones).
All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol and Alcohol Abuse (NIAAA). Additional information about CIFASD can be found at www.cifasd.org.
REFERENCES
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- Arenson AD, Bakhireva L, Chambers T, Deximo C, Foroud T, Jacobson J, Jacobson S, Jones KL, Mattson S, May P, Moore E, Ogle K, Riley E, Robinson L, Rogers J, Streissguth A, Tavares M, Urbanski J, Yezerets H, Surya R, Stewart CA, Barnett WK. Implementation of a shared data repository and common data dictionary for fetal alcohol spectrum disorders research. Alcohol. 2010 doi: 10.1016/j.alcohol.2009.08.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autti-Rämö I. Twelve-year follow-up of children exposed to alcohol in utero. Developmental Medicine and Child Neurology. 2000;42:406–411. doi: 10.1017/s0012162200000748. [DOI] [PubMed] [Google Scholar]
- Autti-Rämö I, Fagerlund A, Ervalahti N, Loimu L, Korkman M, Hoyme HE. Fetal alcohol spectrum disorders in Finland: Clinical delineation of 77 older children and adolescents. American Journal of Medical Genetics. 2006;140A:137–143. doi: 10.1002/ajmg.a.31037. [DOI] [PubMed] [Google Scholar]
- Autti-Rämö I, Fagerlund A, Korkman K. Identification of foetal alcohol spectrum disorders [Miten tunnistat sikiön alkoholivauriot?] (in Finnish) Suomen Lääkärilehti [Finnish Medical Journals] 2008;63:501–506. [Google Scholar]
- Bertrand J, Floyd RL, Weber MK. Guidelines for identifying and referring persons with fetal alcohol syndrome. Morbidity and Mortality Weekly Report Recommendations and Reports. 2005;54:1–14. [PubMed] [Google Scholar]
- Bertrand J, Floyd RL, Weber MK, O'Connor M, Riley EP, Johnson KA, Cohen DE. National task force on FAS/FAE: Guidelines for referral and diagnosis. Centers for Disease Control and Prevention; Atlanta, GA: 2004. [Google Scholar]
- Hagenaars JA, McCutcheon AL. Applied latent class analysis. Cambridge University Press; Cambridge, MA: 2002. [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with fetal alcohol syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behavioural Brain Research. 2003;143:85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Prenatal alcohol exposure and neurobehavioral development: Where is the threshold? Alcohol Health and Research World. 1994;18:30–36. [PMC free article] [PubMed] [Google Scholar]
- Johnson TB, Goodlett CR. Selective and enduring deficits in spatial learning after limited neonatal binge alcohol exposure in male rats. Alcohol Clin Exp Res. 2002;26:83–93. [PubMed] [Google Scholar]
- Jones KL, Robinson LK, Bakhireva LN, Marintcheva G, Storojev V, Strahova A, Sergeevskaya S, Budantseva S, Mattson SN, Riley EP, Chambers CD. Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics. 2006;118:e1734–e1738. doi: 10.1542/peds.2006-1037. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth AP. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Kaemingk KL, Halverson PT. Spatial memory following prenatal alcohol exposure: More than a material specific memory deficit. Child Neuropsychology. 2000;6:115–128. doi: 10.1076/chin.6.2.115.7058. [DOI] [PubMed] [Google Scholar]
- Karlsson T. Terveyden ja hyvinvoinnin laitos [The alcohol situation in Finland in the early 2000s] (in Finnish) Report 15/2009. Gummerus Printing Ltd.; 2009. [Google Scholar]
- Kodituwakku PW, Adnams CM, Hay A, Kitching AE, Burger E, Kalberg WO, Viljoen DL, May PA. Letter and category fluency in children with fetal alcohol syndrome from a community in South Africa. Journal of Studies on Alcohol. 2006;67:502–509. doi: 10.15288/jsa.2006.67.502. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Handmaker NS, Cutler SK, Weathersby EK, Handmaker SD. Specific impairments in self-regulation in children exposed to alcohol prenatally. Alcohol Clin Exp Res. 1995;19:1558–1564. doi: 10.1111/j.1530-0277.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Lanza ST, Flaherty BP, Collins LM. Latent class and latent transition analysis. In: Schinka JA, Velicer WE, Weiner IB, editors. Handbook of psychology: Research methods in psychology. vol 2. Wiley; New York: 2003. pp. 663–685. [Google Scholar]
- Lupton C, Burd L, Harwood R. Cost of fetal alcohol spectrum disorders. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2004;127:42–50. doi: 10.1002/ajmg.c.30015. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Calarco KE, Lang AR. Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychology. 2006;20:361–369. doi: 10.1037/0894-4105.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund Å , Autti-Rämö I, May PA, Adnams CM, Konovalova V, Wetherill L, Arenson AD, Barnett WK, Riley EP, the CIFASD Collaborative initiative on fetal alcohol spectrum disorders: Methodology of clinical projects. Alcohol. 2010 doi: 10.1016/j.alcohol.2009.08.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 1999;23:1808–1815. [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling LJ, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. Journal of Pediatrics. 1997;131:718–721. doi: 10.1016/s0022-3476(97)70099-4. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling LJ, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12:146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2002;26:875–882. [PubMed] [Google Scholar]
- May PA, Fiorentino D, Gossage JP, Kalberg WO, Hoyme HE, Robinson LK, Coriale G, Jones KL, del Campo M, Tarani L, Romeo M, Kodituwakku PW, Deiana L, Buckley D, Ceccanti M. Epidemiology of FASD in a province in Italy: Prevalence and characteristics of children in a random sample of schools. Alcohol Clin Exp Res. 2006;30:1562–1575. doi: 10.1111/j.1530-0277.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome: A summary. Alcohol Research and Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley DG, Manning M, Hoyme EH. The prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on in-school studies. Developmental Disabilities Research Reviews. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NC, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellavato C, Viljoen DL. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug and Alcohol Dependence. 2007;88:259–271. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon AL. Latent class analysis. Sage; Newbury Park, CA: 1987. [Google Scholar]
- Muthén L, Muthén B. Mplus User's Guide. 4th ed Los Angeles, CA: 2006. [Google Scholar]
- Ramaswamy V, DeSarbo WS, Reibstein DJ, Robinson WT. An empirical pooling approach for estimating marketing mix elasticities with PIMS data. Marketing Science. 1993;12:103–124. [Google Scholar]
- Riley EP, Guerri C, Calhoun F, Charness ME, Foroud TM, Li T-K, Mattson SN, May PA, Warren KR. Prenatal alcohol exposure: Advancing knowledge through international collaborations. Alcohol Clin Exp Res. 2003;27:118–135. doi: 10.1097/01.ALC.0000047351.03586.A3. [DOI] [PubMed] [Google Scholar]
- Roesch SC, Villodas M, Villodas F. Latent class/profile analysis in maltreatment research: A commentary on Nooner et al., Pears et al., and looking beyond. Child Abuse and Neglect. doi: 10.1016/j.chiabu.2010.01.003. in press. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Schonfeld AM, Mattson SN, Lang AR, Delis DC, Riley EP. Verbal and nonverbal fluency in children with heavy prenatal alcohol exposure. Journal of Studies on Alcohol. 2001;62:239–246. doi: 10.15288/jsa.2001.62.239. [DOI] [PubMed] [Google Scholar]
- Sclove SL. Application of model-selection criteria to some problems in multivariate analysis. Psychometrika. 1987;52:333–343. [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Streissguth A. Offspring effects of prenatal alcohol exposure from birth to 25 years: The Seattle prospective longitudinal study. Journal of Clinical Psychology in Medical Settings. 2007;14:81–101. [Google Scholar]
- Tofighi D, Enders CK. Identifying the correct number of classes in growth mixture models. In: Hancock GR, Sameulsen KM, editors. Advances in latent variable mixture models. Information Age; Greenwich, CT: 2007. pp. 317–341. [Google Scholar]
- Uecker A, Nadel L. Spatial locations gone awry: Object and spatial memory deficits in children with fetal alcohol syndrome. Neuropsychologia. 1996;34:209–223. doi: 10.1016/0028-3932(95)00096-8. [DOI] [PubMed] [Google Scholar]
- Uecker A, Nadel L. Spatial but not object memory impairments in children with fetal alcohol syndrome. American Journal on Mental Retardation. 1998;103:12–18. doi: 10.1352/0895-8017(1998)103<0012:SBNOMI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services . US Surgeon General releases advisory on alcohol use in pregnancy. Washington, DC: 2005. http://www.surgeongeneral.gov/pressreleases/sg02222005.html. [Google Scholar]
- Vaurio L, Riley EP, Mattson SN. Differences in executive functioning in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society. 2008;14:119–129. doi: 10.1017/S1355617708080144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermunt JK, Magidson J. Latent class cluster analysis. In: Hagenaars JA, McCutcheon AL, editors. Applied latent class analysis. Cambridge University Press; Cambridge, United Kingdom: 2002. pp. 89–106. [Google Scholar]
- Yang C. Evaluating latent class analyses in qualitative phenotype identification. Computational Statistics and Data Analysis. 2006;50:1090–1104. [Google Scholar]