Abstract
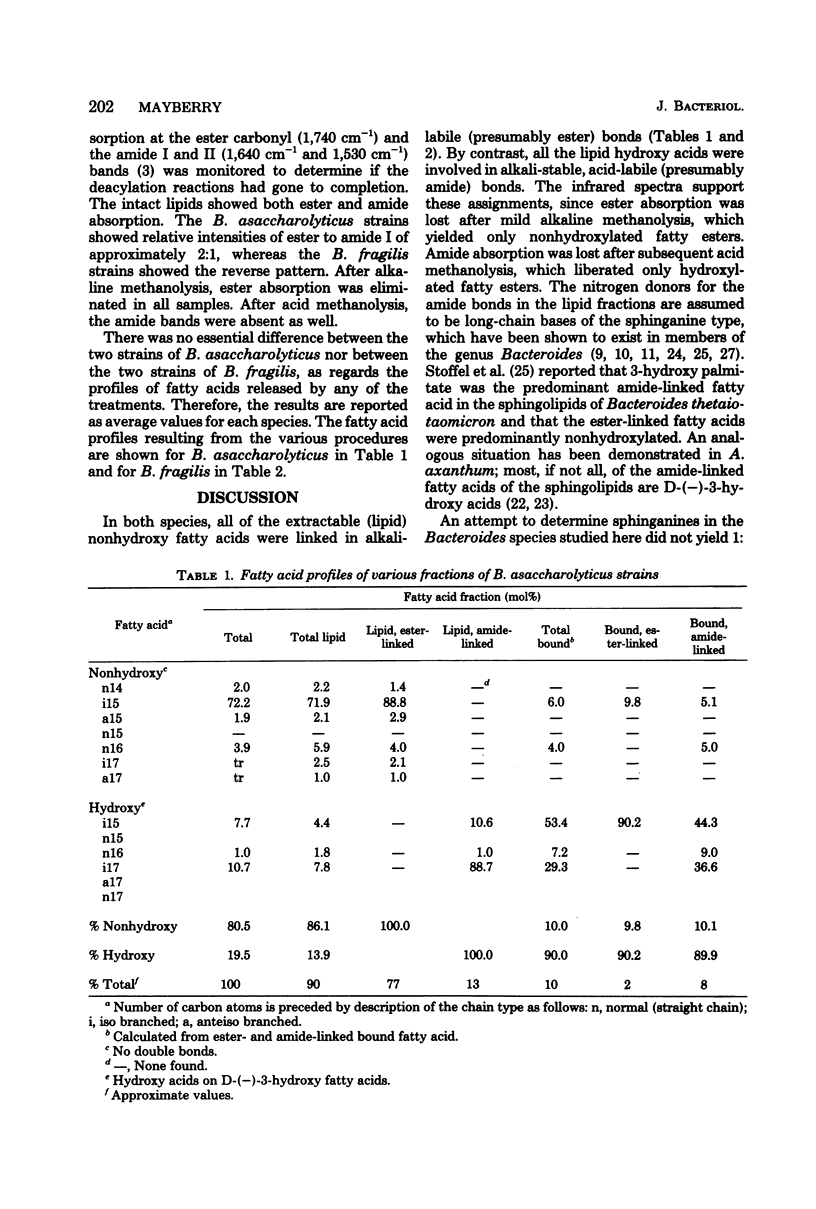
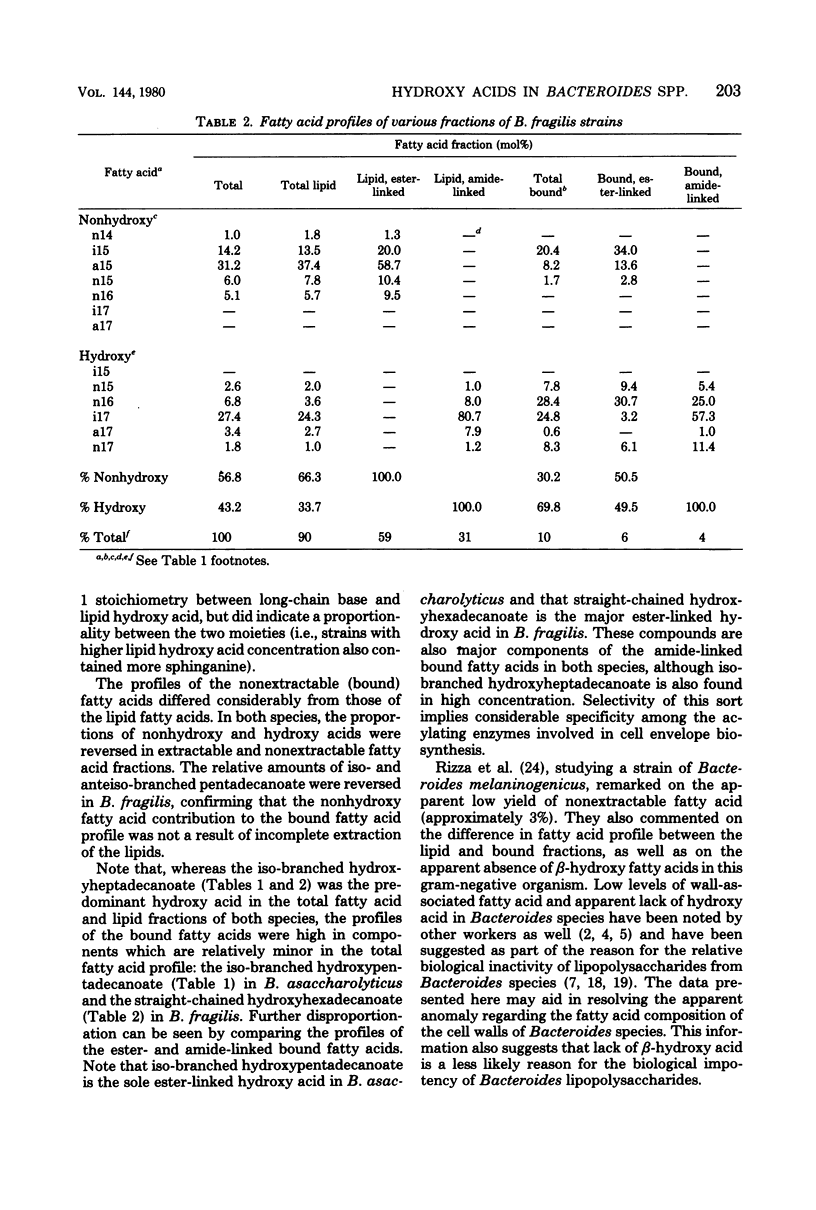
Two strains of Bacteroides asaccharolyticus and two strains of Bacteroides fragilis were analyzed for total fatty acid, total lipid fatty acid, and total bound fatty acid profiles. Extracted lipids and defatted cell residues were subjected to sequential alkaline and acid methanolyses to distinguish ester- and amide-linked fatty acids in each fraction. In the lipid fractions, all the ester-linked fatty acids were nonhydroxylated, whereas all of the amide-linked fatty acids were hydroxylated. In the nonextractable fractions, both hydroxy and nonhydroxy fatty acids were found in both ester and amide linkage, although hydroxy acids predominated. The fatty acid profiles of the bound fractions differed widely from those of the lipid fractions. Bound fatty acid represented approximately 10% of the total cellular fatty acids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANSELL G. B., SPANNER S. THE ALKALINE HYDROLYSIS OF THE ETHANOLAMINE PLASMALOGEN OF BRAIN TISSUE. J Neurochem. 1963 Dec;10:941–945. doi: 10.1111/j.1471-4159.1963.tb11921.x. [DOI] [PubMed] [Google Scholar]
- Bzdega J., Meisel-Mikolajczyk F., Kubica J. Fatty acids in the endotoxins of various Bacteroides fragilis subspecies. Bull Acad Pol Sci Biol. 1977;25(1):21–25. [PubMed] [Google Scholar]
- Hofstad T. Chemical characteristics of Bacteroides melaninogenicus endotoxin. Arch Oral Biol. 1968 Sep;13(9):1149–1155. doi: 10.1016/0003-9969(68)90067-8. [DOI] [PubMed] [Google Scholar]
- Hofstad T., Kristoffersen T. Chemical characteristics of endotoxin from Bacteroides fragilis NCTC 9343. J Gen Microbiol. 1970 Apr;61(1):15–19. doi: 10.1099/00221287-61-1-15. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Doundowlakis J., Takacs B. J. Phospholipid composition of gliding bacteria: oral isolates of Capnocytophaga compared with Sporocytophaga. Infect Immun. 1979 Oct;26(1):305–310. doi: 10.1128/iai.26.1.305-310.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D. L., Onderdonk A. B., Polk B. F., Bartlett J. G. Surface antigens as virulence factors in infection with Bacteroides fragilis. Rev Infect Dis. 1979 Mar-Apr;1(2):278–290. doi: 10.1093/clinids/1.2.278. [DOI] [PubMed] [Google Scholar]
- Knoche H. W., Shively J. M. The structure of an ornithine-containing lipid from Thiobacillus thiooxidans. J Biol Chem. 1972 Jan 10;247(1):170–178. [PubMed] [Google Scholar]
- Kunsman J. E., Caldwell D. R. Comparison of the sphingolipid content of rumen Bacteroides species. Appl Microbiol. 1974 Dec;28(6):1088–1089. doi: 10.1128/am.28.6.1088-1089.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunsman J. E. Characterization of the lipids of six strains of Bacteroides ruminicola. J Bacteriol. 1973 Mar;113(3):1121–1126. doi: 10.1128/jb.113.3.1121-1126.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBach J. P., White D. C. Identification of ceramide phosphorylethanolamine and ceramide phosphorylglycerol in the lipids of an anaerobic bacterium. J Lipid Res. 1969 Sep;10(5):528–534. [PubMed] [Google Scholar]
- Lambe D. W., Jr Characterization of a polyvalent conjugate of Bacterroides fragilis by fluorescent antibody staining. Am J Clin Pathol. 1979 Jan;71(1):97–101. doi: 10.1093/ajcp/71.1.97. [DOI] [PubMed] [Google Scholar]
- Lambe D. W., Jr Determination of Bacteroides melaninogenicus serogroups by fluorescent antibody staining. Appl Microbiol. 1974 Oct;28(4):561–567. doi: 10.1128/am.28.4.561-567.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe D. W., Jr, Jerris R. C. Description of a polyvalent conjugate and a new serogroup of Bacteroides melaninogenicus by fluorescent antibody staining. J Clin Microbiol. 1976 May;3(5):506–512. doi: 10.1128/jcm.3.5.506-512.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe D. W., Jr, Moroz D. A. Serogrouping of Bacteroides fragilis subsp. fragilis by the agglutination test. J Clin Microbiol. 1976 Jun;3(6):586–592. doi: 10.1128/jcm.3.6.586-592.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makula R. A., Finnerty W. R. Isolation and characterization of an ornithine-containing lipid from Desulfovibrio gigas. J Bacteriol. 1975 Aug;123(2):523–529. doi: 10.1128/jb.123.2.523-529.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansheim B. J., Onderdonk A. B., Kasper D. L. Immunochemical and biologic studies of the lipopolysaccharide of Bacteroides melaninogenicus subspecies asaccharolyticus. J Immunol. 1978 Jan;120(1):72–78. [PubMed] [Google Scholar]
- Mansheim B. J., Onderdonk A. B., Kasper D. L. Immunochemical characterization of surface antigens of Bacteroides melaninogenicus. Rev Infect Dis. 1979 Mar-Apr;1(2):263–277. doi: 10.1093/clinids/1.2.263. [DOI] [PubMed] [Google Scholar]
- Mayberry W. R. Hydroxy fatty acids in Bacteroides species: D-(--)-3-hydroxy-15-methylhexadecanoate and its homologs. J Bacteriol. 1980 Aug;143(2):582–587. doi: 10.1128/jb.143.2.582-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberry W. R., Smith P. F., Langworthy T. A. Heptose-containing pentaglycosyl diglyceride among the lipids of Acholeplasma modicum. J Bacteriol. 1974 Jun;118(3):898–904. doi: 10.1128/jb.118.3.898-904.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberry W. R., Smith P. F., Langworthy T. A., Plackett P. Identification of the amide-linked fatty acids of Acholeplasma axanthum S743 as D(-)3-hydroxyhexadecanoate and its homologues. J Bacteriol. 1973 Dec;116(3):1091–1095. doi: 10.1128/jb.116.3.1091-1095.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plackett P., Smith P. F., Mayberry W. R. Lipids of a sterol-nonrequiring Mycoplasma. J Bacteriol. 1970 Nov;104(2):798–807. doi: 10.1128/jb.104.2.798-807.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza V., Tucker A. N., White D. C. Lipids of Bacteroides melaninogenicus. J Bacteriol. 1970 Jan;101(1):84–91. doi: 10.1128/jb.101.1.84-91.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W., Dittmar K., Wilmes R. Sphingolipid metabolism in Bacteroideaceae. Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):715–725. doi: 10.1515/bchm2.1975.356.s1.715. [DOI] [PubMed] [Google Scholar]
- Thiele O. W., Schwinn G. Bakterielle Ornithinlipide. Z Allg Mikrobiol. 1974;14(5):435–443. doi: 10.1002/jobm.3630140509. [DOI] [PubMed] [Google Scholar]
- White D. C., Tucker A. N., Sweeley C. C. Characterization of the iso-branched sphinganines from the ceramide phospholipids of Bacteroides melaninogenicus. Biochim Biophys Acta. 1969 Dec 17;187(4):527–532. doi: 10.1016/0005-2760(69)90050-2. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G. Composition and structure of the ornithine-containing lipid from Pseudomonas rubescens. Biochim Biophys Acta. 1972 May 23;270(1):1–17. doi: 10.1016/0005-2760(72)90171-3. [DOI] [PubMed] [Google Scholar]



