Abstract
Objective
Disease relapses are common for patients with anti-neutrophil cytoplasm antibody associated vasculitis (AAV). The role of low-dose glucocorticoids (GC) in relapse prevention is controversial. We undertook a systematic review and meta-analysis to determine if GC target doses influence relapses of AAV.
Methods
Medline, EMBASE and Cochrane databases were searched for observational studies and randomized controlled trials of treatment of AAV that included a predefined GC treatment plan. The association of GC target dose with the proportion of relapses in studies was assessed using meta-regression and multi-level generalized linear modeling.
Results
Thirteen studies (983 patients) were identified for inclusion. There were no studies directly comparing GC regimens. We classified 288 patients as having a non-zero GC target dose by study end and 695 patients as having a zero GC target dose by study end. The pooled proportion of patients with a relapse was 36% (95% confidence interval [CI] 25 to 47%). GC regimen was the most significant variable explaining the variability between the proportions of patients with relapses. The proportion of patients with a relapse was 14% (95% CI 10 to 19%) in non-zero GC target dose and 43% (95% CI 33 to 52%) in zero GC target dose studies. Differences other than GC regimens exist between studies that complicate the comparability of trials and isolation of the variability in relapses due to GC target alone.
Conclusions
Studies with longer courses of GC in AAV are associated with fewer relapses. These results have implications for study design and outcome assessment in clinical trials of AAV.
Keywords: anti-neutrophil cytoplasm antibodies, vasculitis, prednisolone, prednisone, Wegener’s granulomatosis
The initial treatment of Wegener’s granulomatosis (WG), microscopic polyangiitis (MPA) and renal limited vasculitis[anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV)] with an immunosuppressive medication and glucocorticoids (GC) has become the standard of care. Compared to historical cohorts, these medications have dramatically improved patient survival (1–6). Patients successfully treated for AAV continue, however, to have high rates of relapse associated with the accrual of organ damage and exposure to toxic medications (7). Optimal treatment strategies for patients with AAV remain to be defined.
Studies in the last twenty years have addressed the use of immunosuppressive medications in AAV (8–15). Unlike immunosuppressive medications, the use of GC has not been rigorously evaluated. There is little evidence to guide the use of GC and there is considerable practice pattern variation, especially after the induction of remission. Of particular debate is whether low-dose GC contributes to maintaining remission of AAV. Some support the use of long-term, low-dose GC claiming improved disease control, a subsequent reduction in the exposure to toxic immunosuppressive medications, fewer periods of exposure to high-dose GC, and a reduction in the accumulation of disease-related scarring. Others argue that the use of long-term, low-dose GC is ineffective at reducing relapses and exposes patients to the potential toxicity of high cumulative doses of GC. Thus, the efficacy of long-term, low-dose GC for the treatment of AAV to prevent relapses or reduce treatment-related toxicity is a matter of continued debate (16).
No randomized controlled trials (RCTs) have compared the effects of using long-term, low-dose GC to other treatment regimens in AAV. We explored the effect of different GC regimens on relapse rates among patients with AAV by conducting a systematic review and meta-analysis of studies of AAV in which GC were used as part of an induction regimen.
Materials and Methods
Data Sources
Electronic databases of medical literature (Medline, EMBASE and Cochrane Central Library) were searched using the OVID search engine. Contemporary cohorts of patients with AAV are of the most interest so we limited our search from January 1995 to December 2008. Our search strategy combined the use of two separate search strings (Supplementary Table 1). The first string was designed to capture all studies with small vessel vasculitis by combining terms for: anti-neutrophil cytoplasm antibody, Wegener’s granulomatosis, microscopic polyangiitis, microscopic polyarteritis, polyarteritis nodosa, or vasculitis. The second string was designed to include all studies with GC use by combining the following terms: glucocorticoid, corticosteroid, prednisone, or prednisolone. All search terms were used as both keywords and database thesaurus terms and used the OVID “explode” option. We augmented our search strategy by reviewing the reference lists of relevant articles and contacting experts in the field.
Study Selection
Studies were assessed for eligibility in a two-stage procedure. In the first stage, all identified abstracts were reviewed. Those that met the inclusion criteria, or those for which there was uncertainty as to eligibility, were selected for full text review. Selected articles were reviewed by two investigators (MW and DJ) in the second stage and evaluated on inclusion and exclusion criteria. Inclusion criteria were: 1) prospectively studied patients with AAV (Wegener’s granulomatosis, microscopic polyangiitis and/or renal limited vasculitis but NOT Churg-Strauss Syndrome); 2) a treatment regimen included GC; 3) GC treatment was protocol driven; and 4) relapses were reported. Exclusion criteria were: 1) case series; 2) study follow-up less than 18 months; 3) outcomes for patients with AAV not reported separately from patients without AAV. Studies were eligible whether published in full or as abstracts, and irrespective of language.
Data Extraction and Quality Assessment
Data was abstracted to standardized forms from all studies eligible studies. Disagreement was resolved by consensus. Abstracted data included study design, details of the treatment protocol, definitions of remission and relapse, baseline patient data, and the occurrence of first relapses. For studies with incomplete data or where uncertainties existed, authors were contacted for clarification. The quality of RCTs was assessed on the basis of the description of randomization, blinding and withdrawals using the Jadad score with 0 representing poorest quality and 5 highest (17). The quality of cohort studies was assessed using four items from the Downs and Black checklist: by whom and when groups were accrued, description of withdrawals/drop-outs and adjustment for confounding variables (18).
Analysis
The primary outcome was the proportion of patients with a relapse during the study period. Relapses were defined as in the original articles based on clinical and laboratory assessment of disease activity. Studies that attempted to fully withdraw GC at any point in the study were classified as “zero GC target dose” studies while those that did not attempt to withdraw GC during the study period were classified as “non-zero GC target dose” studies. A priori, zero GC target dose studies were further classified by whether they targeted the discontinuation of GC before 12 months (early zero GC target dose) or after 12 months (late zero GC target dose).
To generate an overall proportion of patients with a relapse, the primary analysis pooled the proportion of patients with relapse from all studies. RCTs were included by pooling the intervention and control arms of the study to form a single cohort. A random-effects model was utilized to estimate the proportion of patients with relapse according to the methods of Der Simonian and Laird (19). The degree of heterogeneity between trials was assessed using the Q statistic and the I2 statistic was used to describe the degree of variability between point estimates that was due to heterogeneity. Meta-regression was used to examine factors that possibly contributed to the variability between studies. These factors included classification of the GC target dose, inclusion of newly diagnosed patients only, inclusion of only patients with WG patients, the use of cyclophosphamide as the primary medication for induction of remission, the use of methotrexate for the maintenance of remission, the withdrawal of immunosuppressive medications, and the inclusion of patients with renal involvement.
A secondary analysis was undertaken using each limb of a RCT as a separate cohort. Generalized multi-level models were constructed to account for the lack of independence of limbs from the same RCT while generating estimates of the effect of the non-GC treatments, timing of treatment withdrawal, inclusion of only patients with WG, inclusion of prevalent patients and GC withdrawal. These methods estimate regression coefficients at the overall study and treatment limb levels and have been used in meta-analyses to account for complex patterns of heterogeneity (20–22).
We did not test for publication bias due to the small number of eligible studies for this analysis. All statistical analyses were performed using Stata version 10 (Statacorp, College Station, TX).
Results
Study Selection
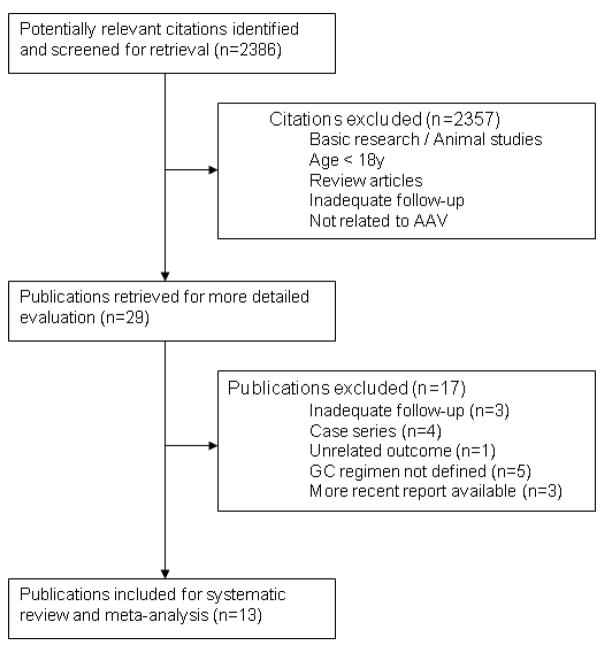
A total of 2386 citations were identified of which 29 were considered for full text review (Figure 1 and Appendix 1). Of the 29 studies, five prospective observational studies and eight RCTs comparing non-GC treatments were identified for analysis. Ten studies were published as full manuscripts (13–15;23–28), while three were published in abstract form (29–31). The authors of all studies published in abstract form made full, final data available and three studies were published in a peer-reviewed journal during the preparation of this manuscript (32–34). No RCTs directly comparing GC treatment regimens were identified. Data from patients from one study were reported in 1999 with extended follow-up data published later (12;35;36).
Figure 1.
Flow diagram of study selection process.
Study Characteristics
Only patients who entered remission and therefore able to experience a relapse were included. Nine-hundred, eighty-three patients from 13 studies were included for analysis: 776 patients were from eight RCTs (13–15;23;24;29;31;37) and 207 patients were from four observational studies (12;25–27;38). Study characteristics are summarized in Table 1. Oral GC therapy consisted of either prednisone or prednisolone. The study by DeGroot and colleagues compared four regimens of which only two met our inclusion/exclusion criteria and were included (26). Study durations varied between 16 and 32 months of follow-up with a median follow-up of 20 months. The expected time from study start to the target GC dose in studies with a non-zero GC target dose ranged from 12 to 22 months while those with a zero target ranged from 6 to 27 months.
Table 1.
Characteristics of studies of ANCA-associated vasculitis included for analysis.
| Study | Design | Patients in Remission | Median Follow-up (months) | Induction Treatment | Maintenance Treatment | GC target dose | Time to GC target dose (months) | Patients with Relapse (%) |
|---|---|---|---|---|---|---|---|---|
| Sneller (1995) | Cohort | 30 | 19 | MTX | MTX | 0 | 7 | 11 (37) |
| DeGroot (1996) | Cohort | 22/11 | 16/20 | CYC | MTX | 0/5 | 27/22 | 3 (14)/1 (9) |
| Guillevin (1997) | RCT | 42 | NR | CYC | CYC | 0 | 22† | 15 (36) |
| Reinhold-Keller (2002) | Cohort | 71 | 25 | CYC | MTX | 0 | 10† | 26 (37) |
| Guillevin (2003) | RCT | 38 | 32 | CYC | None | 0 | 10† | 13 (34) |
| Jayne (2003) | RCT | 144 | 18 | CYC | CYC/AZA | 7.5 | 12 | 21 (14) |
| Langford (2003) | Cohort | 42 | 32 | CYC | MTX | 0 | 8 | 22 (52) |
| DeGroot (2005) | RCT | 87 | 18 | CYC/MTX | AZA/MTX | 0 | 12 | 52 (60) |
| Pagnoux (2005) | RCT | 126 | 36 | CYC | AZA/MTX | 0 | 18 | 44 (35) |
| WGET (2005) | RCT | 164 | 22 | CYC/MTX | MTX | 0 | 6 | 104 (63) |
| Metzler (2007) | RCT | 54 | 21 | CYC | MTX/LEF | 0 | 7† | 20 (37) |
| Stassen (2007) | Cohort | 31 | 19 | MMF | MMF | 0 | 8† | 19 (61) |
| DeGroot (2007)* | RCT | 133 | 18 | CYC | AZA | 5 | 12 | 20 (15) |
Based on updated data from 2004 abstract and details from author;
estimated time based on described regimen and 70 kg starting weigh
RCT = randomized controlled trial; WGET = Wegener’s Granulomatosis Etanercept Trial; GC = glucocorticoid; MTX = methotrexate; CYC = cyclophosphamide; AZA = azathiopirine; LEF = leflunomide; MMF = mycophenolate mofetil
Patient characteristics were comparable with respect to the mean age and gender mix of patients (Table 2). Seven studies included only patients with WG while five studies included both WG and MPA. One study included patients with MPA or classical polyarteritis nodosa but only the results of the MPA group were included in this analysis (24). All studies included at least some patients with renal involvement and seven studies reported serum creatinine (mean creatinine range 106 – 255 μmol/L) (13–15;23;24;29;39). Seven studies included only patients with a new diagnosis of AAV.
Table 2.
Characteristics of patients in studies of ANCA-associated vasculitis included for analysis.
| Study | Patients Enrolled | Patients in Remission | Age (mean) | Female (%) | WG/MPA (%/%) | Renal Involvement (%) | New Diagnosis (%) |
|---|---|---|---|---|---|---|---|
| Sneller (1995) | 42 | 30 | 35 | 52 | 100/0 | 52 | 46 |
| DeGroot (1996) | 22/11 | 22/11 | 45 | 39 | 100/0 | NR | 100 |
| Guillevin (1997) | 50 | 42 | 54 | 40 | 100/0 | 74 | 100 |
| Reinhold-Keller (2002) | 71 | 71 | 49 | 42 | 100/0 | NR | 75 |
| Guillevin (2003) | 47 | 56 | 55 | 47 | 0/100 | 83 | 100 |
| Jayne (2003) | 155 | 145 | 58 | 53 | 61/39 | 94 | 100 |
| Langford (2003) | 42 | 42 | 38 | 36 | 100/0 | 60 | 57 |
| DeGroot (2005) | 95 | 87 | 53 | 54 | 94/6 | 28 | 100 |
| Pagnoux (2005) | 159 | 126 | 58 | 52 | 76/24 | 77 | 100 |
| WGET (2005) | 181 | 164 | 50 | 40 | 100/0 | 54 | 45 |
| Metzler (2007) | 54 | 54 | 55 | 41 | 100/0 | NR | 100 |
| Stassen (2007) | 32 | 31 | 52 | 52 | 91/9 | 47 | 3 |
| DeGroot (2007) | 149 | 133 | 62 | 44 | 42/58 | NR | 100 |
WG = Wegener’s granulomatosis; MPA = microscopic polyangittis; WGET = Wegener’s Granulomatosis Etancercept Trial
Three studies with a total of 288 patients had non-zero GC target doses (15;26;29). Eleven studies with a total of 695 patients had zero GC target dose within the study period (12–14;23–27;31;40). The study by DeGroot et al (1996) contributed one cohort to each group. Studies in the zero GC target dose group were subdivided: three studies with a zero GC target dose longer than 12 months (178 patients) (23;26;31) and eight with a zero GC target before 12 months (517 patients) (12–14;24;25;27;41;42). Definitions of remission and relapse were broadly similar between studies and no study used an achieved dose of GC as part of their definition of remission or relapse (see Supplementary Table 2). Of the eight RCTs, four did not find differences in the occurrence of relapses between treatment arms (13;15;29;31).
Study Quality
Study quality was mixed. One RCT obtained a score of 3 (randomized, blinded and accounted for loss to follow-up) and all others a score of 2 out of 5. All observational cohort studies selected patients from the same population and adequately accounted for patient drop-outs but the study by De Groot et al studied patients from different time periods (26).
Meta-Analysis of Studies
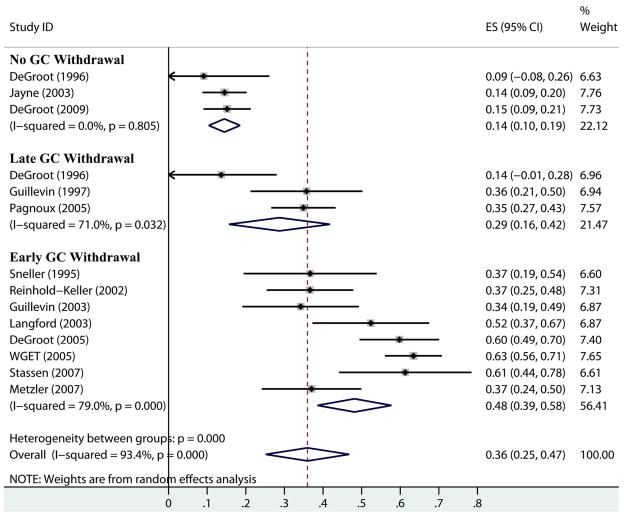
When using each RCT as a single cohort, the overall pooled estimate of the proportion of patients experiencing a relapse was 36% (95% CI 25 – 47%) (Figure 2). However, the variability between studies was greater than expected by chance alone with significant heterogeneity detected (Q=196; p<0.001; with an associated I2 of 93%).
Figure 2.
Forest plot of proportion of patients with a relapse in studies of ANCA-associated vasculitis sorted by glucocorticoid (GC) target dose using random effects model. “No GC withdrawal” studies targeted 5 – 7.5 mg prednisone/day; “Late GC withdrawal” studies targeted withdrawal after 12 months; “Early GC withdrawal” studies targeted withdrawal before 12 months. Horizontal lines and diamonds represent 95% confidence intervals of individual studies and pooled group estimates respectively; dotted line represents overall pooled estimate.
Meta-regression demonstrated the use of a non-zero GC target dose as a significant source of heterogeneity (p=0.001) and resulted in an 11% reduction in heterogeneity (residual I2 82%). Factors not found to be significant included: the inclusion of patients with prior relapses (p=0.07), the inclusion of patients with MPA (p=0.65), the discontinuation of immunosuppressive medication (p=0.48), the use of methotrexate as either an induction or maintenance therapy (p=0.46), the proportion of patients with renal involvement (p=0.27) or the duration of follow-up (p=0.46) (Table 3). Meta-analysis of the subgroup of studies with a nonzero GC target dose estimated 15% of patients experience a relapse (95% CI 10 to 19%). Significant heterogeneity was not detected (Q=0.4; p=0.81; I2=0%). Meta-analysis of the subgroup of studies with a zero GC target dose estimated 42% of patients experience a relapse (95% CI 32 to 52%) but significant heterogeneity was still present (Q=72; p<0.001; I2=86%).
Table 3.
Proportion of patients with a relapses of AAV by subgroup categories. P-values obtained from mixed-effects meta-regression.
| Subgroup | Number of Studies | Relapses, % (95% CI) | I2 | p-value |
|---|---|---|---|---|
| All | 14 | 34 (23–46) | 94 | NA |
| GC Target | 0.001 | |||
| Zero | 11 | 41 (31–52) | 85% | |
| Non-Zero | 3 | 14 (10–19) | 0 | |
| GC Duration | ||||
| Early WD | 8 | 48 (37–58) | 82 | <0.001 |
| Late WD | 3 | 27 (16–39) | 64 | 0.13 |
| No WD | 3 | 14 (10–19) | 0 | Reference |
| Inclusion of MPA | ||||
| Yes | 5 | 35 (18–52) | 95 | 0.65 |
| No | 9 | 35 (20–48) | 89 | |
| Stopped Immunosuppression | ||||
| Yes | 9 | 37 (23–51) | 90 | 0.48 |
| No | 5 | 30 (18–42) | 90 | |
| Used MTX | ||||
| Yes | 9 | 37 (23–50) | 91 | 0.46 |
| No | 5 | 30 (17–43) | 91 | |
| Included Relapsing Patients | ||||
| Yes | 5 | 50 (34–66) | 85 | 0.07 |
| No | 9 | 28 (17–39) | 90 | |
| Follow-up | 13 | NA | NA | 0.46 |
WD=withdrawal; MPA= microscopic polyangiitis; NA=not applicable
Meta-regression was also performed classifying studies as using a non-zero GC target dose, a late zero GC target dose (after 12 months), or an early zero GC target dose (before 12 months). The late zero GC target dose group (relapses in 27%; 95% CI 16 to 39%) was not significantly different than the non-zero group (relapses in 14%; 95% CI 10 to 19%; p=0.13) but the early zero GC target dose group (relapses in 48%; 95% CI 38 to 58%) had significantly more relapses than the non-zero group (p<0.001). This grouping of GC target doses resulted in a 23% reduction in heterogeneity (residual I2 70%). The results of meta-regression for other variables were not altered significantly in this analysis. Grouping studies that continued GC for at least 12 months (non-zero GC target dose and late zero GC target dose) demonstrated a 20% risk of relapse (95% CI 12 to 28%) compared to early zero GC target dose studies with a relapse risk of 48% (95% CI 37 to 58%).
Meta-analysis of Individual RCT Limbs
In univariable models, non-zero GC target dose was associated with relapses (p<0.001) as was the withdrawal of immunosuppressive medications (p=0.01), and the inclusion of relapsing patients (p=0.01). However, in multivariable models, only the use of a non-zero GC target dose remained significantly associated with the proportion of patients with a relapse (p=0.004) compared with withdrawal of immunosuppressive medications (p=0.52) and relapsing patients (p=0.11).
Regrouping studies as non-zero GC target dose, late zero GC target dose, and early zero GC target dose demonstrated a significant difference between non-zero GC target dose studies and early zero GC target dose studies but not between non-zero GC studies and late zero GC studies. These analyses were consistent between all analyses.
A sensitivity analysis utilizing the median duration of follow-up to estimate the rate of relapses did not alter the results of the primary or secondary analyses: zero GC target dose studies continued to demonstrate more relapses than non-zero GC target dose studies after correction for the follow-up duration. A sensitivity analysis using only RCTs also did not significantly alter the results of the meta-analysis.
Discussion
This meta-analysis compares the proportion of patients with a relapse of AAV across RCTs and observational studies with a total of 983 patients. The proportions of patients with a relapse of AAV varied significantly between studies. A significant component of this variability may be related to the GC target dose. The proportion of relapses in patients in studies that had GC target dose of zero was approximately 3 fold higher than in patients in studies with a non-zero target.
The results of our study have several important implications. The potential impact of GC dosing should be considered when comparing the results of different studies and when comparing the outcomes of treatment arms within a given study. Small differences in the management of GC between treatment groups may produce significant differences in the risk of relapse. Additionally, in the design of future trials of AAV the potential impact of GC schedules on relapse rates must be considered as they may substantially alter sample size estimations. Strict, protocol driven glucocorticoid use is necessary to prevent the potentially confounding effects of small GC dose separations.
Our study also suggests that early withdrawal of GC is associated with more relapses of AAV and implies that low-dose GC for greater than 12 months would provide a demonstrable benefit to patients. However, the nature of the relapses captured in studies included for analysis are incompletely reported (e.g. severe versus non-severe) making the importance of the observed relapses difficult to interpret. The excess relapses in zero GC regimens may be minor and not associated with increased mortality or a reduction in quality of life. Most relapses of AAV are, however, associated with escalation of the GC dose and often a concomitant increase in immunosuppressive medications (13). Alternatively, there is a substantial number of patients with AAV who can achieve a zero-GC dose without subsequent relapse. The benefits associated with longer GC treatment may therefore be restricted to patients at high risk of a relapse.
Further complicating the evaluation of the risk-benefit ratio of using longer courses of low-dose GC is the inability to accurately assess the occurrence of adverse events in regimens that mandate extended courses of GC. Comparisons are confounded by differences in the severity of disease and immunosuppressive regimens used between cohorts as well as the difficulty in correctly identifying adverse events due to GC treatment alone. Furthermore, some adverse events attributed to GC treatment, such as an increase in fracture risk, increased risk of cardiovascular disease and long-term effects of weight gain, likely late occurring and are unlikely to be captured in the standard follow-up time periods used in the included trials.
Our study has limitations to consider. A study-level analysis is open to the influence of many confounding variables, the effects of which are difficult to assess. The use of meta-regression is a low-powered statistical test to assess the effects of variables on the overall results. Our finding that some of the parameters tested were not significant may be the result of inadequate statistical power. However, this issue makes our finding regarding the influence of study GC target dose even more intriguing. Moreover, several variables, such as immunosuppressive medication dosing, can only be roughly classified at the study level. Additionally, although the follow-up times for these studies were similar, due to limited time-to-event published data we were unable to generate hazard ratio data to more thoroughly control for the impact of follow-up time. The included studies also did not use a uniform definition of relapse. We chose to include any recorded first relapse in the included studies so that all relapses presumably represent the loss of disease control in patients that had entered remission.
Our study has several notable strengths. We utilized a comprehensive review of clinical trials in AAV and additional data was obtained directly from investigators. We used advanced statistical techniques to focus on key factors that may affect relapses in each study. The results of our study are strengthened by their consistency across sensitivity analyses and our findings appear more plausible given the stepwise increase in relapses with decreasing GC treatment times when the studies were classified by early, late or non-zero GC targets. Our study is also consistent with the findings of Boomsma and colleagues that the withdrawal of immunosuppressive medications is associated with an increased risk of a relapse of AAV (43). Our study addresses, with the best available evidence, a question of high importance to both clinicians treating AAV and investigators studying AAV.
Long-term use of GC after the induction of remission in AAV may significantly alter disease activity. The potential differences in relapse rates between different GC tapering regimens must be taken into consideration in the deisgn of clinical trials of AAV. Given the potentially large difference in relapses between protocols utilizing longer durations of low-dose glucocorticoids and protocols with early discontinuation, an RCT of appropriate power and duration to address this question is needed.
Supplementary Material
Acknowledgments
We wish to thank the authors of studies who provided information about relapses and the details of their studies and in particular, Drs. Christian Pagnoux, Loic Guillevin, Claudia Metzler, and Wolfgang Gross.
Dr. Walsh is supported through post-doctoral fellowship grants from the Kidney Research Scientist Core Education National Training program and the Alberta Heritage Foundation for Medical Research. Dr. Merkel was also supported by a Mid-Career Development Award in Clinical Investigation (NIH-NIAMS: K24 AR02224). This work was supported in part by the Vasculitis Clinical Research Consortium (www.RareDiseasesNetwork.org/vcrc) through a grant from the National Institutes of Health (National Center for Research Resources and the National Institute of Arthritis and Musculoskeletal and Skin Diseases) (NIH-NCRR: U54 RR019497) and by NIH AR47785.
Footnotes
Authors Contributions and Conflicts of Interest
I, Michael Walsh, declare that I participated in the design, data gathering, data analysis, manuscript preparation and revision of this study. I have no conflicts of interest to declare.
I, Peter Merkel, declare that I participated in the design, manuscript preparation and revisions of this study. I have no conflicts of interest to declare.
I, Alfred Mahr, declare that I participated in the design, manuscript preparation and revisions of this study. I have no conflicts of interest to declare.
I, David Jayne, declare that I participated in the design, data gathering, manuscript preparation and revisions of this study. I have no conflicts of interest to declare.
Reference List
- 1.Collagen Diseases and Hypersensitivity Panel (MRC) Treatment of polyarteritis nodosa with cortisone: results after three years. Br Med J. 1960;1399:1400. [PMC free article] [PubMed] [Google Scholar]
- 2.Frohnert PF, Sheps SG. Long-term folow-up study of periarteritis nodosa. Am J Med. 1967;43:8–11. doi: 10.1016/0002-9343(67)90144-1. [DOI] [PubMed] [Google Scholar]
- 3.Fauci AS, Wolff SM, Johnson JS. Effect of cyclophosphamide upon the immune response in Wegener’s granulomatosis. N Engl J Med. 1971;285(27):1493–6. doi: 10.1056/NEJM197112302852701. [DOI] [PubMed] [Google Scholar]
- 4.Novack SN, Pearson CM. Cyclophosphamide therapy in Wegener’s granulomatosis. N Engl J Med. 1971;284(17):938–42. doi: 10.1056/NEJM197104292841703. [DOI] [PubMed] [Google Scholar]
- 5.Leib ES, Restivo C, Paulus HE. Immunosuppressive and corticosteroid therapy of polyarteritis nodosa. Am J Med. 1979;67:941–5. doi: 10.1016/0002-9343(79)90634-x. [DOI] [PubMed] [Google Scholar]
- 6.Nachman PH, Hogan SL, Jennette JC, Falk RJ, Nachman PH, Hogan SL, et al. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7(1):33–9. doi: 10.1681/ASN.V7133. [DOI] [PubMed] [Google Scholar]
- 7.Seo P, Min YI, Holbrook JT, Hoffman GS, Merkel PA, Spiera R, et al. Damage caused by Wegener’s granulomatosis and its treatment: prospective data from the Wegener’s Granulomatosis Etanercept Trial (WGET) Arthritis Rheum. 2005;52(7):2168–78. doi: 10.1002/art.21117. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman GS, Leavitt RY, Fleisher TA, Minor JR, Fauci AS. Treatment of Wegener’s granulomatosis with intermittent high-dose intravenous cyclophosphamide. The American Journal of Medicine. 1990;89(4):403–10. doi: 10.1016/0002-9343(90)90367-m. [DOI] [PubMed] [Google Scholar]
- 9.Adu D, Pall A, Luqmani RA, Richards NT, Howie AJ, Emery P, et al. Controlled trial of pulse versus continuous prednisolone and cyclophosphamide in the treatment of systemic vasculitis. QJM. 1997;90(6):401–9. doi: 10.1093/qjmed/90.6.401. [DOI] [PubMed] [Google Scholar]
- 10.Haubitz M, Schellong S, Gobel U, Schurek HJ, Schaumann D, Koch KM, et al. Intravenous pulse administration of cyclophosphamide versus daily oral treatment in patients with antineutrophil cytoplasmic antibody-associated vasculitis and renal involvement: a prospective, randomized study. [see comment] Arthritis & Rheumatism. 1998;41(10):1835–44. doi: 10.1002/1529-0131(199810)41:10<1835::AID-ART16>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Birck R, Warnatz K, Lorenz HM, Choi M, Haubitz M, Grunke M, et al. 15-Deoxyspergualin in patients with refractory ANCA-associated systemic vasculitis: a six-month open-label trial to evaluate safety and efficacy. J Am Soc Nephrol. 2003;14(2):440–7. doi: 10.1097/01.asn.0000048716.42876.14. [DOI] [PubMed] [Google Scholar]
- 12.Langford CA, Talar-Williams C, Barron KS, Sneller MC. Use of a cyclophosphamide-induction methotrexate-maintenance regimen for the treatment of Wegener’s granulomatosis: extended follow-up and rate of relapse. American Journal of Medicine. 2003;114(6):463–9. doi: 10.1016/s0002-9343(03)00077-9. [DOI] [PubMed] [Google Scholar]
- 13.Wegener’s Granulomatosis Etanercept Trial (WGET) Research Group. Etanercept plus standard therapy for Wegener’s granulomatosis. New England Journal of Medicine. 2005;352(4):351–61. doi: 10.1056/NEJMoa041884. [DOI] [PubMed] [Google Scholar]
- 14.DeGroot K, Rasmussen N, Bacon PA, Tervaert JW, Feighery C, Gregorini G, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis & Rheumatism. 2005;52(8):2461–9. doi: 10.1002/art.21142. [DOI] [PubMed] [Google Scholar]
- 15.Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JWC, Dadoniene J, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. New England Journal of Medicine. 2003;349(1):03. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 16.OMERACT 7. J Rheumatol; Proceedings of the International Consensus Conference on Outcome Measures in Rheumatology Clinical Trials; May 8–12, 2004; Asilomar, California, USA. 2005. pp. 2447–95. [PubMed] [Google Scholar]
- 17.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 18.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egger M, Smith GD, Altman D. Systematic Reviews in Health Care: Meta-analysis in context. London: BMJ Publishing Group; 2001. [Google Scholar]
- 20.Frost C, Clarke R, Beacon H. Use of hierarchical models for meta-analysis: experience in the metabolic ward studies of diet and blood cholesterol. Stat Med. 1999;18(13):1657–76. doi: 10.1002/(sici)1097-0258(19990715)18:13<1657::aid-sim155>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20(19):2865–84. doi: 10.1002/sim.942. [DOI] [PubMed] [Google Scholar]
- 22.Stein PD, Hull RD, Patel KC, Olson RE, Ghali WA, Brant R, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med. 2004;140(8):589–602. doi: 10.7326/0003-4819-140-8-200404200-00005. [DOI] [PubMed] [Google Scholar]
- 23.Guillevin L, Cordier JF, Lhote F, Cohen P, Jarrousse B, Royer I, et al. A prospective, multicenter, randomized trial comparing steroids and pulse cyclophosphamide versus steroids and oral cyclophosphamide in the treatment of generalized Wegener’s granulomatosis. [see comment] Arthritis & Rheumatism. 1997;40(12):2187–98. doi: 10.1002/art.1780401213. [DOI] [PubMed] [Google Scholar]
- 24.Guillevin L, Cohen P, Mahr A, Arene JP, Mouthon L, Puechal X, et al. Treatment of polyarteritis nodosa and microscopic polyangiitis with poor prognosis factors: a prospective trial comparing glucocorticoids and six or twelve cyclophosphamide pulses in sixty-five patients. Arthritis & Rheumatism. 2003;49(1):93–100. doi: 10.1002/art.10922. [DOI] [PubMed] [Google Scholar]
- 25.Sneller MC, Hoffman GS, Talar-Williams C, Kerr GS, Hallahan CW, Fauci AS. An analysis of forty-two Wegener’s granulomatosis patients treated with methotrexate and prednisone. Arthritis & Rheumatism. 1995;38(5):608–13. doi: 10.1002/art.1780380505. [DOI] [PubMed] [Google Scholar]
- 26.DeGroot K, Reinhold-Keller E, Tatsis E, Paulsen J, Heller M, Nolle B, et al. Therapy for the maintenance of remission in sixty-five patients with generalized Wegener’s granulomatosis: Methotrexate versus trimethoprim/sulfarnethoxazole. Arthritis & Rheumatism. 1996;39(12) doi: 10.1002/art.1780391215. [DOI] [PubMed] [Google Scholar]
- 27.Reinhold-Keller E, Fink CO, Herlyn K, Gross WL, De GK. High rate of renal relapse in 71 patients with Wegener’s granulomatosis under maintenance of remission with low-dose methotrexate. Arthritis & Rheumatism. 2002;47(3):326–32. doi: 10.1002/art.10459. [DOI] [PubMed] [Google Scholar]
- 28.Stassen PM, Cohen Tervaert JW, Stegeman CA. Induction of remission in active anti-neutrophil cytoplasmic antibody-associated vasculitis with mycophenolate mofetil in patients who cannot be treated with cyclophosphamide. Ann Rheum Dis. 2007;66(6):798–802. doi: 10.1136/ard.2006.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Groot K, Jayne D, Tesar V, Savage C. Randomised controlled trial of daily oral versus pulse cyclophosphamide for induction of remission in ANCA-associated systemic vasculitis. Kidney & Blood Pressure Research. 2005;28:195. [Google Scholar]
- 30.Metzler C, Wagner-Bastmeyer R, Gross WL, Reinhold-Keller E. Leflunomide versus methotrexate for maintenance of remission in Wegener’s granulomatosis - unexpected high relapse-rate under oral methotrexate. Ann Rheum Dis. 2005;64(Suppl_3):262. [Google Scholar]
- 31.Pagnoux C, Mahr A, Cohen P, Hamidou M, Ruivard M, Puechal X, et al. Corticosteroids and pulse cyclophosphamide followed by maintenance therapy with methotrexate or azathioprine: a prospective multicenter randomized trial (WEGENT) Ann Rheum Dis. 2005;64(Suppl_3):246. [Google Scholar]
- 32.Metzler C, Miehle N, Manger K, Iking-Konert C, De GK, Hellmich B, et al. Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener’s granulomatosis. Rheumatology (Oxford) 2007;46(7):1087–91. doi: 10.1093/rheumatology/kem029. [DOI] [PubMed] [Google Scholar]
- 33.De GK, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150(10):670–80. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 34.Pagnoux C, Mahr A, Hamidou MA, Boffa JJ, Ruivard M, Ducroix JP, et al. Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med. 2008;359(26):2790–803. doi: 10.1056/NEJMoa0802311. [DOI] [PubMed] [Google Scholar]
- 35.Langford CA, Talar-Williams C, Barron KS, Sneller MC. A staged approach to the treatment of Wegener’s granulomatosis: induction of remission with glucocorticoids and daily cyclophosphamide switching to methotrexate for remission maintenance. Arthritis & Rheumatism. 1999;42(12):2666–73. doi: 10.1002/1529-0131(199912)42:12<2666::AID-ANR24>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 36.Langford CA, Talar-Williams C, Sneller MC. Use of methotrexate and glucocorticoids in the treatment of Wegener’s granulomatosis. Long-term renal outcome in patients with glomerulonephritis. Arthritis & Rheumatism. 2000;43(8):1836–40. doi: 10.1002/1529-0131(200008)43:8<1836::AID-ANR20>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 37.Metzler C, Miehle N, Manger K, Iking-Konert C, De GK, Hellmich B, et al. Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener’s granulomatosis. Rheumatology (Oxford) 2007;46(7):1087–91. doi: 10.1093/rheumatology/kem029. [DOI] [PubMed] [Google Scholar]
- 38.Stassen PM, Cohen Tervaert JW, Stegeman CA. Induction of remission in active anti-neutrophil cytoplasmic antibody-associated vasculitis with mycophenolate mofetil in patients who cannot be treated with cyclophosphamide. Ann Rheum Dis. 2007;66(6):798–802. doi: 10.1136/ard.2006.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stassen PM, Cohen Tervaert JW, Stegeman CA. Induction of remission in active anti-neutrophil cytoplasmic antibody-associated vasculitis with mycophenolate mofetil in patients who cannot be treated with cyclophosphamide. Ann Rheum Dis. 2007;66(6):798–802. doi: 10.1136/ard.2006.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stassen PM, Cohen Tervaert JW, Stegeman CA. Induction of remission in active anti-neutrophil cytoplasmic antibody-associated vasculitis with mycophenolate mofetil in patients who cannot be treated with cyclophosphamide. Ann Rheum Dis. 2007;66(6):798–802. doi: 10.1136/ard.2006.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metzler C, Miehle N, Manger K, Iking-Konert C, De GK, Hellmich B, et al. Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener’s granulomatosis. Rheumatology (Oxford) 2007;46(7):1087–91. doi: 10.1093/rheumatology/kem029. [DOI] [PubMed] [Google Scholar]
- 42.Stassen PM, Cohen Tervaert JW, Stegeman CA. Induction of remission in active anti-neutrophil cytoplasmic antibody-associated vasculitis with mycophenolate mofetil in patients who cannot be treated with cyclophosphamide. Ann Rheum Dis. 2007;66(6):798–802. doi: 10.1136/ard.2006.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boomsma MM, Stegeman CA, van der Leij MJ, Oost W, Hermans J, Kallenberg CG, et al. Prediction of relapses in Wegener’s granulomatosis by measurement of antineutrophil cytoplasmic antibody levels: a prospective study. Arthritis Rheum. 2000;43(9):2025–33. doi: 10.1002/1529-0131(200009)43:9<2025::AID-ANR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.