Abstract
Background
Kaposi sarcoma-associated herpesvirus (KSHV) is the causal agent for Kaposi sarcoma (KS) and multicentric Castleman disease (MCD) in HIV-infected patients. Patients with KSHV-MCD develop fevers, wasting, hypoalbuminemia, cytopenias, and hyponatremia that are related to overproduction of KSHV-encoded vial interleukin (IL)-6 (vIL-6) and human IL-6.
Methods
We identified 6 HIV-infected patients with KS or serological evidence of KSHV infection who had severe inflammatory MCD-like symptoms but in whom we could not diagnose MCD, and hypothesized that these symptoms resulted from vIL-6 overproduction. Serum vIL-6 levels were assessed in these 6 patients and compared to 8 control patients with symptomatic KSHV-MCD and 32 control patients with KS. KSHV viral load, serum human IL-6 (hIL-6), and human IL-10 were also evaluated.
Results
Patients with inflammatory MCD-like symptoms but without MCD had elevated vIL-6 levels comparable to patients with symptomatic KSHV-MCD and significantly greater than control patients with KS (P = 0.0026). Elevated hIL-6, IL-10, and KSHV viral loads were also comparable to patients with symptomatic KSHV-MCD and significantly greater than those with KS.
Conclusions
A subset of patients with HIV and KSHV co-infection, but without MCD, can develop severe systemic inflammatory symptoms associated with elevated levels of KSHV vIL-6, IL-6, and KSHV viral loads. Excess lytic activation of KSHV, production of the lytic gene product vIL6, and associated immunologic dysregulation may underlie the pathophysiology of these symptoms. This IL-6-related inflammatory syndrome is important to consider in critically ill patients with HIV and KSHV co-infection.
Keywords: viral interleukin-6, human herpesvirus 8, Kaposi sarcoma, multicentric Castleman disease, interleukin-6
Introduction
Multicentric Castleman disease (MCD) is a B-cell lymphoproliferative disorder characterized by lymphadenopathy and inflammatory manifestations including fevers, malaise, wasting, hypoalbuminemia, cytopenias, and hyponatremia [1]. These symptoms are attributable to inflammatory cytokine overproduction, especially IL-6 [2]. In HIV infected patients, MCD is usually caused by Kaposi sarcoma-associated herpesvirus (KSHV), also called human herpesvirus-8 (HHV-8) [3–6]. KSHV is also the causative agent for Kaposi sarcoma (KS) and primary effusion lymphoma (PEL) [7, 8].
The KSHV genome is notable for molecularly pirated genes with homology to human genes, including vIL-6, which exerts immunologic effects similar to hIL-6 although it is about 1000-fold less active [9–12]. vIL-6 is a lytic gene, and an unusual feature of KSHV-MCD, compared to KS and PEL, is that the pathologic plasmablasts frequently express lytic KSHV proteins [5, 6]. Patients with KSHV-MCD have elevated serum vIL-6, and mice expressing vIL-6 or hIL-6 manifest certain abnormalities similar to KSHV-MCD [2, 13, 14]. Therefore, KSHV vIL-6 is considered an important factor causing KSHV-MCD symptoms. KSHV-MCD patients also have elevated serum hIL-6, which may contribute to symptoms, and IL-10 whose role is less clear [2, 15].
Over the past several years, we observed several HIV-infected patients with either KS or serological evidence of KSHV infection who had severe inflammatory MCD-like symptoms and laboratory abnormalities, but in whom we could not diagnose MCD. We hypothesized that overproduction of cytokines, particularly vIL-6 from KSHV-infected cells, was the principal cause of these clinical abnormalities. Using an enzyme-linked immunosorbent assay (ELISA) for vIL-6, we compared serum vIL-6 levels in 6 such cases to control patients with symptomatic KSHV-MCD or HIV-associated KS. Serum hIL-6, IL-10, KSHV viral load, and other parameters were measured to further evaluate the immunologic and virologic milieu in cases and controls.
Patients, materials and methods
Patient Selection
A retrospective review of 143 patients seen between 1993 and 2006 in the HIV and AIDS Malignancy Branch adult clinic with HIV and known KSHV co-infection (either KS or serum antibodies to KSHV) identified 6 patients with unexplained MCD-like inflammatory symptoms in whom we were unable to diagnose MCD. All 6 were seen after 2000. These patients had unexplained fevers, with at least 3 other clinical, biochemical or radiographic findings suggestive of MCD including wasting, edema, effusion, anemia, thrombocytopenia, cytopenias, hypoalbuminemia, hyponatremia, elevated inflammatory markers, elevated PTT, adenopathy, and splenomegaly. Brief case histories are provided (Supplement). In each case, MCD had been considered. Efforts to diagnose MCD or related diseases included three lymph node biopsies, one lung biopsy, three bone marrow biopsies, one thoracentesis, and one pericardiocentesis. Pathologic evaluation generally revealed reactive lymphoid or plasmacytoid cells in these tissues, except one lymph node in which KS spindle cells were observed. However, no diagnosis of MCD could be made. These patients were designated as having “MCD-like syndrome”. None developed MCD during follow-up of 3–60 months (median 10 months). One patient had an autopsy in which MCD was not found. Microbiological assessment was performed in each case. Although several patients had HIV viremia or bacterial infections [possible Lyme disease in a subsequent evaluation done elsewhere (1), S. aureus (2), clinical cellulitis (1)], these were not felt to fully account for the symptoms.
To explore the hypothesis that this MCD-like syndrome was caused at least in part by overproduction of IL-6, particularly vIL-6, we assayed serum vIL-6, serum hIL-6, and other immunologic and virologic parameters on stored serum, plasma, and peripheral blood mononuclear cells (PBMC) obtained at the first time point each patient showed the abnormal clinical profile and samples were available. In one patient, PBMC for KSHV viral load was obtained 2 days before the serum for vIL-6 and human cytokines. As controls, we selected 8 HIV-infected patients with symptomatic KSHV-MCD and 32 with HIV-KS, who had stored clinical samples obtained 1987–2006 (all but one obtained 1996 or later). KS controls were sub-categorized as severe (>50 lesions and/or visceral disease) or mild (<50 lesions, no visceral disease). Importantly, cases and controls were selected without knowledge of KSHV viral load, vIL-6 levels, or hIL-6 levels. All patients were enrolled on NCI Institutional Review Board approved protocols that allowed such testing after obtaining informed consent.
Viral IL-6 Assay
We measured vIL-6 by modifying a sandwich ELISA previously described [14, 16]. Using a different mouse monoclonal antibody (clone v6m 31.2.4) to coat 96-well plates, we incubated diluted serum sample overnight at 4° C, then incubated with rabbit polyclonal anti-vIL-6 antibody, followed by affinity-purified human serum protein-absorbed goat antirabbit IgG (H+L) antibody conjugated to horseradish peroxidase (Bio-Rad, Hercules, CA) diluted 1:5000 in PBS-T/BSA. SureBlue TMB Microwell Peroxidase Substrate (KPL, Inc., Gaithersburg, MD) was then added to the wells for 10 minutes, followed by stop solution (1 N H/Cl). Plates were read at 450 nm with correction at 630 nm. Standard curves were generated, and vIL-6 levels calculated as previously described [16]. We utilized a new monoclonal antibody in this study because the previously utilized antibody [14, 16] sometimes bound to a component in normal serum from patients not known to be KSHV infected. These modifications reduced false-positive detection of vIL-6. Fifty-five serum samples from normal donors not known to be infected with HIV or KSHV were tested, with the upper 95% confidence limit in these samples (2850 pg/mL) set as the cut-off for elevated vIL-6. This cut-off, substantially higher than that used in previous studies [14, 16], further increased assay specificity. This assay did not detect hIL-6 added to serum at concentrations up to 10,000 pg/mL.
KSHV quantitative real-time PCR
DNA was extracted from peripheral blood mononuclear cells (PBMC) using the QIAamp DNA blood mini kit (Qiagen, Valencia, CA). DNA quality and concentration was assessed by optical density using Nanodrop1000 (Thermo Scientific, Wilmington, DE). DNA concentration was adjusted to 250 ng per 10 μL for two quantitative real-time PCR assays developed using TaqMan® (Applied Biosystems, Foster City, CA). Negative control wells were run in triplicate on each assay plate. KSHV DNA was detected using previously reported primers for the K6 gene region [17]. The number of cellular equivalents was determined using a quantitative assay for human endogenous retrovirus 3 (ERV-3) [18]. Samples were tested in triplicate for both assays, averaged, and reported as viral DNA copies per million PBMCs [19].
KSHV Serology
In some cases, plasma was tested for anti-KSHV antibodies using enzyme immunoassays specific for the latency associated nuclear antigen (LANA) and a lytic structural glycoprotein, K8.1, using previously described methods [20].
Other assays
Serum hIL-6, IL-10 and 5 other cytokines [IL-1β, IL-8, IL-12 p70, interferon gamma (IFNγ), and tumor necrosis factor alpha (TNFα)] were evaluated using the MSD 96-Well Multiarray® Proinflammatory 7-plex Assay (Meso-Scale Discovery, Gaithersburg, MD) and the Sector® Imager. Using this assay, mean serum hIL-6 level in healthy donors is 2.3 pg/mL (SD = 1.1 pg/mL) [21]. Cross-reactivity of vIL-6 was tested in the Meso-Scale IL-6 assay by diluting vIL-6 in a protein containing kit diluent. The Meso-Scale hIL-6 assay did not detect vIL-6 at concentrations ranging from 0.6 pg/ml to 20,000 pg/ml. Results were confirmed with vIL-6 diluted in pooled normal donor serum, using serum with albumin or IL-6 as negative and positive controls. Analysis of IL12p70 and IFNγ was performed excluding values from two patients (MCD-like patient 3, one KS control), who were receiving IL12 on a research protocol, which would lead to an expected elevation of these two cytokines. CD4 counts were assessed by fluorescent-activated cell sorting. Plasma HIV-1 mRNA was measured by quantitative RNA polymerase chain reaction using Roche Amplicor® HIV-1 Monitoring Kits (Roche Diagnostic Systems, Branchburg, NJ).
Statistical Analysis
Patients were categorized as having MCD, MCD-like syndrome, severe KS, or mild KS. We hypothesized that vIL-6 would be elevated in the MCD-like syndrome group compared to KS controls. We also analyzed KSHV viral load, hIL-6 and hIL-10. Fisher’s exact test was used to compare elevated vIL-6 (>2850 pg/mL) and detectable HIV viral load between groups. Other cytokine and KSHV viral load comparisons were performed using an exact form of the Wilcoxon rank-sum test. Principal comparisons were between the MCD-like syndrome group and either the combined KS control group or the MCD group. Other than comparison of vIL-6 between MCD-like patients and those with KS, statistical analyses were considered exploratory, with no formal correction for multiple comparisons. All p-values ≤ 0.01 are interpreted as statistically significant, while 0.01 < p ≤ 0.05 indicate strong trends and p > 0.05 is considered not significant.
Results
Patient characteristics
Patient characteristics are shown in Table 1. Patients in both MCD and MCD-like syndrome groups had low median sodium, albumin, hemoglobin and platelets. Plasma HIV was detectable in 4 of 5 patients with MCD-like syndrome in which it was measured at the time vIL-6 levels were obtained. HIV was detectable in 2 of 7 MCD patients assayed and 22 of the 35 KS patients assayed. There was no significant difference in HIV viral load between MCD-like syndrome patients and either MCD (p=0.24) or the combined KS patients (p=1.0, Fisher’s exact test). CD4 counts were comparable across all 4 groups (p=0.87, Kruskal-Wallis test).
Table 1.
Patient characteristics
| MCD | MCD-like Syndrome | Severe KS | Mild KS | |
|---|---|---|---|---|
| Number of Patients | 8 | 6 | 24 | 8 |
| Age | 41 [34 – 47] | 38 [29 – 52] | 36 [27 – 46] | 36 [30 – 49] |
| Prescribed cART | 7 (87.5%) | 4 (66.7 %) | 21 (87.5%) | 5 (62.5%) |
| CD4 (cells/μL) | 189 [21 – 364] | 255 [28 – 492] | 228 [12 – 1060] | 287 [52 – 572] |
| HIV Viral Load (copies/mL) | <50 [<50 – 664] | 4650 [<50 – 1.1×106] | 930 [<50 – 1.3 ×106] | <50 [<50 – 27,500] |
| Sodium (mEq/L) | 130 [127 – 136] | 132 [127 – 139] | 138 [133 – 142] | 137 [133 – 138] |
| Albumin (g/dL) | 2.7 [1.7 – 3.9] | 2.6 [2.0 – 3.9] | 4 [3.0 – 4.8] | 4.2 [3.3 – 5.4] |
| White Blood Cells (K/μL) | 5.7 [2.7 – 10.1] | 4.5 [2.3 – 6.4] | 4.2 [2.0 – 10.7] | 4.3 [3.2 – 7.2] |
| Hemoglobin (g/dL) | 10.2 [7.4 –12.7] | 8.7 [7.0 – 14.8] | 13.6 [9 – 15.5] | 13.7 [9.8 – 15.9] |
| Platelets (K/μL)* | 126 [11 – 262] | 158 [62 – 231] | 253 [117 – 504] | 265 [194 – 300] |
| Median Follow-up (months) | 36 [4 – 91] | 10 [3 – 60] | 75 [3 – 148] | 34 [6 – 128] |
| Deaths | 1 (12.5%) | 3 (50%) | 6 (25%) | 1 (12.5%) |
Patient laboratory and other variables are listed with median [range]. Other parameters are number of patients (%).
Normal Range for Platelets: Men: 161–347 (K/μL), Women: 173–369 (K/μL)
Serum vIL-6 levels
Serum vIL-6 was elevated (>2850 pg/mL) in all patients with MCD flares (median 8150 pg/mL, range 4069 to 12,931 pg/mL) (Figure 1). Serum vIL-6 was also elevated in the original serum sample in 5 of the 6 patients with MCD-like symptoms (median 5678 pg/mL, range <2850 to 39,835 pg/ml) and was not statistically different from those in the MCD group (p=0.43, Fisher’s exact test). One patient with MCD-like symptoms but initially undetectable vIL-6 had an elevated level (3307 pg/ml) three weeks later (Table 2). By contrast, only 5 of the 32 KS control patients had detectable vIL-6. vIL-6 was significantly elevated in the MCD-like syndrome group compared to the combined KS control groups (p = 0.0026, Fishers exact test). These results demonstrate that patients with KSHV infection or KS, selected for otherwise unexplained MCD-like inflammatory symptoms, had high serum vIL-6 levels comparable to patients with an MCD flare. Interestingly, closer evaluation of clinical records showed all 5 KS controls with elevated vIL-6 had visceral KS, and 4 of these 5 KS controls had clinical abnormalities suggestive of MCD (hemoglobin <10.0 g/dL in 2, platelets <150K/μL in 1, and albumin < 3.1 g/dL in 1), although none had severe inflammatory symptoms or had been identified as being MCD-like prior to the measurement of vIL-6.
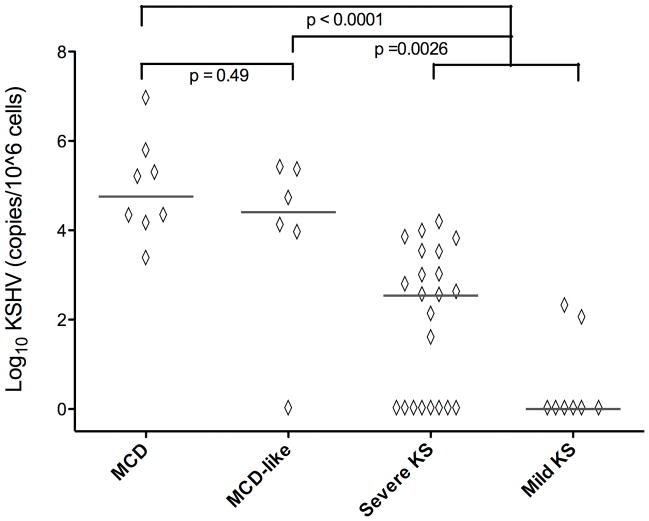
Figure 1. Comparisons of Serum vIL-6 between MCD, MCD-like and KS Control Groups.
Comparisons of vIL-6 between MCD (n=8) MCD-like (n=6) Severe KS (n= 24) and Mild KS (n=8) groups were performed using Fischer’s exact test (<2850 vs. ≥ 2850 pg/ml). Values shown are log10 transformed pg/ml, p-values are two-sided. ----------- = median - -- - -- - = lower limit of detection.
Table 2.
Clinical course of patients with MCD-like symptoms and no MCD
| Pt | KS | CD4 (cells/μL) | Infections at time of evaluation | vIL-6* (pg/ml) | Peak vIL-6 (pg/ml) | hIL-6** (pg/ml) | KSHV viral load (copies/106 cells) | Clinical Course |
|---|---|---|---|---|---|---|---|---|
| 1 | + | 324 | S. aureus and yeast in tracheal aspirate | 4,254 | 4,254 | 41.5 | 248,485 | Patient treated with combination chemotherapy, antibiotics and additional therapy with AZT and valganciclovir for suspected MCD-like syndrome. Fevers persisted with intermittently elevated vIL-6. Tracheal aspirate cultures persistently grew S. aureus and yeast. Patient developed thrombocytopenia leading to diffuse alveolar hemorrhage and death from respiratory distress. |
| 2 | − | 492 | Possible Lyme disease | 15,372 | 15,372 | 2.1 | <1 | Patient had high antibody titer to KSHV K8.1, but lymph node biopsy failed to reveal MCD. Symptoms and elevated vIL-6 persisted during 2.5 month evaluation. Patient later treated for possible Lyme disease at another hospital, with prolonged antibiotics. At 4.8 years (last contact) patient well on cART, and had not developed MCD. |
| 3 | + | 118 | 7,102 | 7,102 | 93.5 | 51,111 | Patient treated with liposomal doxorubicin and IL-12 on clinical study. He had persistent fevers and elevated vIL-6. Chemotherapy stopped after 3 months due to progressive KS, and the patient died shortly thereafter. | |
| 4 | + | 255 | Clinical KS associated cellulitis | <2,850 | 3,307 | 27.12 | 222,000 | Patient with cellulitis at time of evaluation was initially treated with antibiotics and liposomal doxorubicin, with improvement in inflammatory symptoms. Patient had elevated vIL-6 in a serum sample obtained three weeks after initial evaluation. Patient had improvement KS over 5 months; no subsequent MCD over 13.5 months follow-up. |
| 5 | + | 28 | None initially | 39,835 | 39,835 | 11.5 | 12,632 | Patient was enrolled on a trial of liposomal doxorubicin and IL-12. First cycle complicated by S. aureus bacteremia and fungal cholecystitis. Serum vIL-6 persistently elevated throughout his clinical course. The patient had progressive KS despite 4 cycles of liposomal doxorubicin. The patient opted for hospice care and died at 7.5 months. Autopsy did not reveal MCD. |
| 6 | − | 562 | Chronic hepatitis B | 3,894 | 10,489 | 205.7 | 8,697 | Patient had waxing and waning vIL-6 over first 2 months after starting cART and receiving a brief course of valganciclovir, with improvement in clinical symptoms. vIL-6 decreased to <2850 and remained undetectable through month 9. Patient then discontinued cART and was lost to follow-up for 8 months. Patient readmitted with a large pericardial effusion, but no evidence of primary effusion lymphoma, and undetectable vIL-6. Patient was restarted on cART with no subsequent MCD over 3.5 years follow-up. |
Value when initially evaluated. Lower limit of detection 2,850 pg/ml
Value when initially evaluated. Normal value in healthy donors 2.3 +/− 1.1 pg/ml IL-6
KSHV Viral Load
KSHV viral load was assessed in all but 2 KS patients (who had inadequate stored material). All but 1 with MCD-like syndrome had quantifiable KSHV (median 31,900 copies/106 cells), with levels comparable to symptomatic MCD patients (median 87,400 copies/106 cells, p = 0.49) (Figure 2). The MCD-like syndrome group had significantly more KSHV viral copies than the combined KS groups (median 73 copies/106 cells, p=0.0026). Interestingly, patients with severe KS had substantially more KSHV than those with mild KS (p = 0.02).
Figure 2. Comparisons of KSHV Viral Load between MCD, MCD-like and KS Control Groups.
Comparisons of PBMC-associated KSHV viral load between MCD (n=8) MCD-like (n=6) Severe KS (n= 22) and Mild KS (n=8) groups were performed using an exact form of the Wilcoxon rank-sum test. Values are log10 transformed copies/106 cells, p- values are two-sided. ----------- = median.
Serum hIL-6, IL-10, and other cytokines
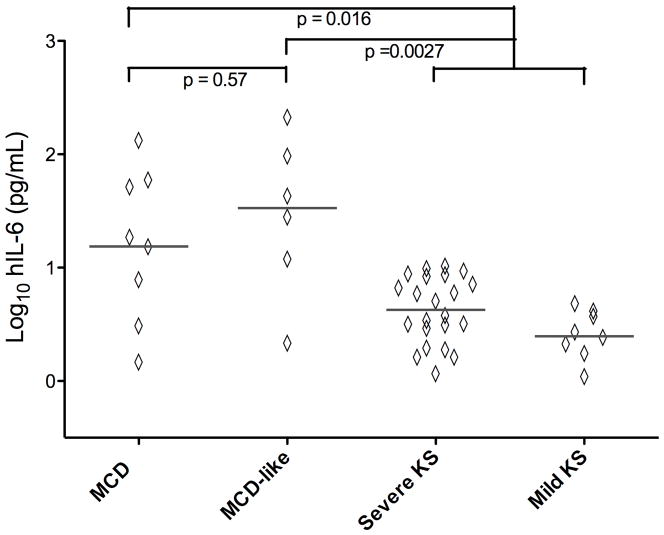
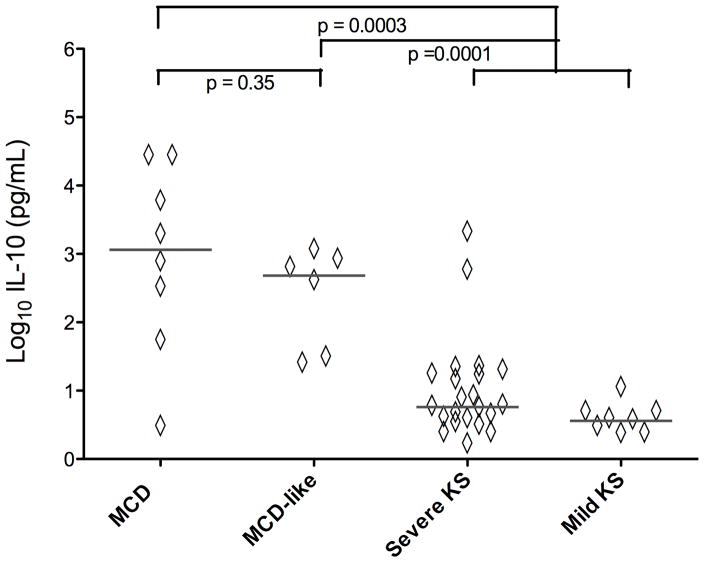
Serum hIL-6 was also elevated in MCD-like syndrome patients (median 34.3 pg/ml) and comparable to those with KSHV-MCD flares (median 16.4 pg/ml, p = 0.57) (Figure 3). The hIL-6 levels in the MCD-like syndrome group were substantially greater than the combined KS group (median 3.5 pg/ml, p = 0.0027). Similarly, IL-10 was elevated in the MCD-like syndrome group (median 494 pg/ml), with levels comparable to the KSHV-MCD group (median 1275 pg/ml, p = 0.35) and significantly greater than the combined KS group (median 4.7 pg/ml, p = 0.0001). The only other cytokines with levels modestly increased in the MCD-like syndrome group compared to the combined KS groups, with a trend towards statistical significance, were IL-1β (median 1.51 pg/mL vs. median 0.48 pg/mL, p = 0.011) and IFN-γ (median 2.62 pg/ml vs. 1.04 pg/ml, p = 0.046) (Supplemental Figures 1–5). There were no significant differences between the MCD-like syndrome group and the combined KS group in the levels of IL-8, IL-12 p70, or TNFα.
Figure 3. Comparisons of hIL6 between MCD, MCD-like and KS Control Groups.
Comparisons of hIL-6 between MCD (n=8) MCD-like (n=6) Severe KS (n= 22) and Mild KS (n=8) groups were performed using an exact form of the Wilcoxon rank-sum test. Values are log10 transformed pg/ml, p-values are two-sided. ----------- = median.
Follow-up of MCD-like syndrome patients
The course of MCD-like syndrome patients is described in Table 2 and the Supplemental Material. None subsequently developed MCD. Three patients (patients 1, 3, and 5), each with extensive progressive KS, had persistent MCD-like symptoms and elevated vIL-6 and died during a follow-up period ranging from 3 to 60 months. Causes of death were alveolar hemorrhage, progressive KS, and progressive KS complicated by infections. The other 3 patients (patients 2, 4, and 6), had resolution of their MCD-like symptoms and elevated vIL-6 when last seen after 4.8 years, 13.5 months, and 3.5 years respectively. In addition to cART, patient 2 was treated for possible Lyme disease, patient 4 was treated with antibiotics for cellulitis and liposomal doxorubicin for severe KS, and patient 6 was treated with cART and valganciclovir for possible MCD-like syndrome.
Discussion
KSHV-MCD is characterized histologically by KSHV-infected plasmablastic cells in the mantle zone of lymphoid organs [22]. These cells express the KSHV antigen, LANA, are restricted for expression of lambda light chains, and often express KSHV vIL-6 and other KSHV lytic genes [5, 6, 23]. The clinical profile of patients with MCD is dominated by systemic inflammatory symptoms that are believed to be due to excessive production of KSHV vIL-6 by the plasmablasts, as well as overproduction of hIL-6 and possibly other cytokines such as hIL-10.
We report here 6 patients with KSHV infection but without apparent MCD who exhibited symptomatology similar to MCD without other obvious etiology. Five of these patients had detectable serum vIL-6 when first tested (the 6th had elevated vIL-6 subsequently) with vIL-6 in this group comparable to a control group with symptomatic KSHV-MCD. These patients also had elevated hIL-6 comparable to patients with KSHV-MCD flares, as well as increased IL-10.
Using a somewhat different vIL-6 assay, some patients with KS (but without MCD or MCD-like symptoms) have previously been reported to have detectable serum vIL-6 [16], and patients with HIV infection may have some elevations in serum hIL-6 and IL-10 [14, 24, 25]. However, the present study demonstrates that serum vIL-6, hIL-6, and IL-10 levels, as well as KSHV viral load in patients with MCD-like symptoms were significantly greater than an HIV-KS control group without inflammatory syndromes at time of evaluation, and similar to a group with symptomatic KSHV-MCD. Notably, we utilized a more specific assay for vIL-6, employing a substantially higher cut-off. Based on these findings, vIL-6 and hIL-6 overproduction appears likely to be responsible for at least part of the symptomatology in these MCD-like patients. Overproduction of IL-10 or other cytokines may also contribute. Our conservative cut-off for detection of vIL-6 (2850 pg/mL) does not exclude the possibility that patients could have vIL-6-induced symptomtology even with vIL-6 levels below the limit of detection.
The question must be asked if these patients had MCD. We were unable to diagnose MCD despite careful evaluation, and none subsequently developed MCD. If these patients represented undiagnosed MCD, it would suggest that MCD frequently evades detection and that the incidence of KSHV-MCD is higher than generally appreciated [1, 26]. A related question is whether increased cytokine levels and MCD-like symptoms may have resulted from other infectious processes. Four of the 6 patients had evidence of some bacterial infection and their symptoms may have resulted in part from these infectious processes. However, increased vIL-6 levels suggest at a minimum that in patients with extensive KSHV involvement, other infections may trigger release of vIL-6 from KSHV-infected cells that may cause or contribute to excessive inflammatory symptoms. Lytic activation of KSHV-infected cells through toll-like receptor signaling is one possible mechanism, although further study is needed to clarify this issue [27]. Finally, one of the MCD-like syndrome patients (patient 4) had what appeared to be worsening of KS as part of immune reconstitution inflammatory syndrome (IRIS) a month after starting cART [28], and two others (patients 1 and 3) were within their first year of cART. It is possible that KSHV activation as part of IRIS can contribute to MCD-like syndrome in some patients.
All but one of the 6 patients with MCD-like syndrome had a high KSHV burden, manifested by extensive KS (3 patients) and/or a high viral load of PBMC KSHV (4 patients). Also, all 5 KS control patients with detectable vIL-6 had advanced KS with visceral involvement. vIL-6 is a lytic gene, and factors that may activate production of KSHV lytic genes in these patients include underlying cytokine dysregulation from uncontrolled HIV or other infections, poor immunologic control, or tissue hypoxia [24, 29–32]. KSHV activation in these patients may also have contributed to overproduction of hIL-6 and IL-10 [33–35].
Three MCD-like patients had KS that progressed despite chemotherapy and contributed to their deaths. IL-6 can induce the growth of KS-derived spindle cells [36, 37], and we have noted that in patients with KS and KSHV-MCD, KS is difficult to control until MCD is brought into clinical remission (R.L. and R.Y., unpublished observation). Substantial overproduction of vIL-6, hIL-6, and other cytokines in these patients likely contributes to tumor growth and resistance to therapy.
This study provides evidence that some patients with a high burden of KSHV may manifest a symptom complex related to IL-6 overproduction even though they do not have MCD. The patients reported here share a common physiologic mechanism for their symptomatology (elevated vIL6, hIL-6, and possibly other cytokines directly or indirectly produced by KSHV), but are heterogeneous with regard to mechanism of lytic activation of KSHV and cytokine excess. Physicians should be alert to the possibility that pathological vIL-6 (and hIL-6) overproduction in some HIV/KSHV co-infected patients, especially those with a large burden of KS or KSHV, may contribute to severe inflammatory symptomatology. This syndrome is particularly worth considering in patients with advanced KS and MCD-like symptoms whose KS progresses despite chemotherapy. It may also possibly mimic or exacerbate manifestations of sepsis in patients with KS.
Additional studies are needed to better understand the pathogenesis of elevated vIL-6 and other cytokines in such patients, and to develop diagnostic criteria and treatment strategies. Several approaches have been reported to have utility in MCD, including rituximab, interferon alpha, and ganciclovir [1, 38, 39], but it is unclear how these approaches will work in MCD-like syndrome. Agents like rituximab, for example, that target B cells may not be effective against vIL-6 produced by KS spindle cells. As we learn more about this syndrome, novel therapeutic approaches that address activation of KSHV lytic genes or target vIL-6 and hIL-6 overproduction [31, 38, 40]may be worth evaluating.
Supplementary Material
Figure 4. Comparisons of IL-10 between MCD, MCD-like and KS Control Groups.
Comparisons of IL-10 between MCD (n=8) MCD-like (n=6) Severe KS (n= 22) and Mild KS (n=8) groups were performed using an exact form of the Wilcoxon rank-sum test. Values are log10 transformed pg/ml, p-values are two-sided. ----------- = median.
Acknowledgments
The authors thank the patients who participated on this study. The authors also thank the clinical staff of the HIV and AIDS Malignancy Branch, the Medical Oncology Branch, and the NIH Clinical Center for their help.
Financial Support. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute. Additional funding comes from the National Cancer Institute, NIH, under Contract No. HHSN261200800001E.
Footnotes
Potential Conflicts of Interest.
G.T. is a co-inventor on a patent describing the measurement of KSHV vIL-6. This invention was made when G.T. was an employee of the US Government under 45 Code of Federal Regulations Part 7. All rights, title, and interest to this patent have been assigned to the U.S. Department of Health and Human Services. The government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (P.L. 99-502). R.Y is the spouse of G.T. All other authors: no conflicts.
References
- 1.Waterston A, Bower M. Fifty years of multicentric Castleman’s disease. Acta Oncol. 2004;43(8):698–704. doi: 10.1080/02841860410002752. [DOI] [PubMed] [Google Scholar]
- 2.Brandt SJ, Bodine DM, Dunbar CE, Nienhuis AW. Dysregulated interleukin-6 expression produces a syndrome resembling Castleman’s syndrome. J Clin Invest. 1990;86:592–9. doi: 10.1172/JCI114749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995 Aug 15;86(4):1276–80. [PubMed] [Google Scholar]
- 4.Boshoff C, Weiss R. AIDS-related malignancies. Nat Rev Cancer. 2002 May;2(5):373–82. doi: 10.1038/nrc797. [DOI] [PubMed] [Google Scholar]
- 5.Staskus KA, Sun R, Miller G, et al. Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. J Virol. 1999;73(5):4181–7. doi: 10.1128/jvi.73.5.4181-4187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parravicini C, Chandran B, Corbellino M, et al. Differential viral protein expression in Kaposi’s sarcoma-associated herpesvirus-infected diseases: Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. Am J Pathol. 2000 Mar;156(3):743–9. doi: 10.1016/S0002-9440(10)64940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332(18):1186–91. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 8.Chang Y, Cesarman E, Pessin M, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–9. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 9.Moore PS, Boshoff C, Weiss RA, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996 Dec 6;274(5293):1739–44. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 10.Boshoff C, Endo Y, Collins PD, et al. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278(5336):290–4. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee M, Osborne J, Bestetti G, Chang Y, Moore PS. Viral IL-6-induced cell proliferation and immune evasion of interferon activity. Science. 2002 Nov 15;298(5597):1432–5. doi: 10.1126/science.1074883. [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995 Aug 15;86(4):1243–54. [PubMed] [Google Scholar]
- 13.Aoki Y, Jaffe ES, Chang Y, et al. Angiogenesis and hematopoiesis induced by Kaposi’s sarcoma-associated herpesvirus-encoded interleukin-6. Blood. 1999 Jun 15;93(12):4034–43. [PubMed] [Google Scholar]
- 14.Aoki Y, Yarchoan R, Wyvill K, Okamoto S, Little RF, Tosato G. Detection of viral interleukin-6 in Kaposi sarcoma-associated herpesvirus-linked disorders. Blood. 2001 Apr 1;97(7):2173–6. doi: 10.1182/blood.v97.7.2173. [DOI] [PubMed] [Google Scholar]
- 15.Oksenhendler E, Carcelain G, Aoki Y, et al. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric castleman disease in HIV-infected patients. Blood. 2000 Sep 15;96(6):2069–73. [PubMed] [Google Scholar]
- 16.Aoki Y, Tosato G, Fonville TW, Pittaluga S. Serum viral interleukin-6 in AIDS-related multicentric Castleman disease. Blood. 2001 Apr 15;97(8):2526–7. doi: 10.1182/blood.v97.8.2526. [DOI] [PubMed] [Google Scholar]
- 17.de Sanjose S, Marshall V, Sola J, et al. Prevalence of Kaposi’s sarcoma-associated herpesvirus infection in sex workers and women from the general population in Spain. Int J Cancer. 2002 Mar 1;98(1):155–8. doi: 10.1002/ijc.10190. [DOI] [PubMed] [Google Scholar]
- 18.Yuan CC, Miley W, Waters D. A quantification of human cells using an ERV-3 real time PCR assay. J Virol Methods. 2001 Feb;91(2):109–17. doi: 10.1016/s0166-0934(00)00244-5. [DOI] [PubMed] [Google Scholar]
- 19.Whitby D, Marshall VA, Bagni RK, et al. Reactivation of Kaposi’s sarcoma-associated herpesvirus by natural products from Kaposi’s sarcoma endemic regions. Int J Cancer. 2007 Jan 15;120(2):321–8. doi: 10.1002/ijc.22205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malope BI, Pfeiffer RM, Mbisa G, et al. Transmission of Kaposi sarcoma-associated herpesvirus between mothers and children in a South African population. J Acquir Immune Defic Syndr. 2007 Mar 1;44(3):351–5. doi: 10.1097/QAI.0b013e31802f12ea. [DOI] [PubMed] [Google Scholar]
- 21.Toedter G, Hayden K, Wagner C, Brodmerkel C. Simultaneous detection of eight analytes in human serum by two commercially available platforms for multiplex cytokine analysis. Clin Vaccine Immunol. 2008 Jan;15(1):42–8. doi: 10.1128/CVI.00211-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oksenhendler E, Boulanger E, Galicier L, et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood. 2002 Apr 1;99(7):2331–6. doi: 10.1182/blood.v99.7.2331. [DOI] [PubMed] [Google Scholar]
- 23.Dupin N, Diss TL, Kellam P, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000 Feb 15;95(4):1406–12. [PubMed] [Google Scholar]
- 24.Breen EC, Rezai AR, Nakajima K, et al. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990;144(2):480–4. [PubMed] [Google Scholar]
- 25.Stylianou E, Aukrust P, Kvale D, Muller F, Froland SS. IL-10 in HIV infection: increasing serum IL-10 levels with disease progression--down-regulatory effect of potent anti-retroviral therapy. Clin Exp Immunol. 1999 Apr;116(1):115–20. doi: 10.1046/j.1365-2249.1999.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powles T, Stebbing J, Bazeos A, et al. The role of immune suppression and HHV-8 in the increasing incidence of HIV-associated multicentric Castleman’s disease. Ann Oncol. 2009 Apr;20(4):775–9. doi: 10.1093/annonc/mdn697. [DOI] [PubMed] [Google Scholar]
- 27.Gregory SM, West JA, Dillon PJ, Hilscher C, Dittmer DP, Damania B. Toll-like receptor signaling controls reactivation of KSHV from latency. Proc Natl Acad Sci U S A. 2009 Jul 14;106(28):11725–30. doi: 10.1073/pnas.0905316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leidner RS, Aboulafia DM. Recrudescent Kaposi’s sarcoma after initiation of HAART: a manifestation of immune reconstitution syndrome. AIDS Patient Care STDS. 2005 Oct;19(10):635–44. doi: 10.1089/apc.2005.19.635. [DOI] [PubMed] [Google Scholar]
- 29.Mercader M, Taddeo B, Panella JR, Chandran B, Nickoloff BJ, Foreman KE. Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells. Am J Pathol. 2000 Jun;156(6):1961–71. doi: 10.1016/S0002-9440(10)65069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang J, Renne R, Dittmer D, Ganem D. Inflammatory cytokines and the reactivation of Kaposi’s sarcoma-associated herpesvirus lytic replication. Virology. 2000 Jan 5;266(1):17–25. doi: 10.1006/viro.1999.0077. [DOI] [PubMed] [Google Scholar]
- 31.Davis DA, Singer KE, Reynolds IP, Haque M, Yarchoan R. Hypoxia enhances the phosphorylation and cytotoxicity of ganciclovir and zidovudine in Kaposi’s sarcoma-associated herpesvirus infected cells. Cancer Res. 2007 Jul 15;67(14):7003–10. doi: 10.1158/0008-5472.CAN-07-0939. [DOI] [PubMed] [Google Scholar]
- 32.Haque M, Davis DA, Wang V, Widmer I, Yarchoan R. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) contains hypoxia response elements: relevance to lytic induction by hypoxia. J Virol. 2003 Jun;77(12):6761–8. doi: 10.1128/JVI.77.12.6761-6768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones KD, Aoki Y, Chang Y, Moore PS, Yarchoan R, Tosato G. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi’s sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood. 1999 Oct 15;94(8):2871–9. [PubMed] [Google Scholar]
- 34.Schwarz M, Murphy PM. Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-kappa B and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J Immunol. 2001 Jul 1;167(1):505–13. doi: 10.4049/jimmunol.167.1.505. [DOI] [PubMed] [Google Scholar]
- 35.Qin Z, Kearney P, Plaisance K, Parsons CH. Pivotal Advance: Kaposi’s sarcoma-associated herpesvirus (KSHV)-encoded microRNA specifically induce IL-6 and IL-10 secretion by macrophages and monocytes. J Leukoc Biol. 2009 Aug 20; doi: 10.1189/jlb.0409251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ensoli B, Nakamura S, Salahuddin SZ, et al. AIDS-Kaposi’s sarcoma-derived cells express cytokines with autocrine and paracrine growth effects. Science. 1989;243:223–6. doi: 10.1126/science.2643161. [DOI] [PubMed] [Google Scholar]
- 37.Miles SA, Rezai AR, Salazar-Gonzalez JF, et al. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc Natl Acad Sci USA. 1990;87:4068–72. doi: 10.1073/pnas.87.11.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casper C, Nichols WG, Huang ML, Corey L, Wald A. Remission of HHV-8 and HIV-associated multicentric Castleman disease with ganciclovir treatment. Blood. 2004 Mar 1;103(5):1632–4. doi: 10.1182/blood-2003-05-1721. [DOI] [PubMed] [Google Scholar]
- 39.Bower M, Powles T, Williams S, et al. Brief communication: rituximab in HIV-associated multicentric Castleman disease. Ann Intern Med. 2007 Dec 18;147(12):836–9. doi: 10.7326/0003-4819-147-12-200712180-00003. [DOI] [PubMed] [Google Scholar]
- 40.Uldrick T, O’Mahoney D, Aleman K, et al. HIgh-dose Zidovudine + Valganciclovir in the Treatment of Kaposi’s Sarcoma-associated Herpesvirus-associated Multicentric Castleman’s Disease: A Targeted Therapy Using Antiviral Drugs that Are Activated to Toxic Moieties by Kaposi’s Sarcoma-associated Herpesvirus Lytic Genes. 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.