Abstract
Background
A candidate vaccine consisting of human immunodeficiency virus type 1 (HIV-1) subunit gp120 protein (AIDSVAX™ B/B) was found previously to be non-protective despite strong antibody responses against the vaccine antigens. We assessed the magnitude and breadth of neutralizing antibody responses in this trial.
Methods
Neutralizing antibodies were measured against highly sensitive (tier 1) and moderately sensitive (tier 2) strains of HIV-1 subtype B in two independent assays. Vaccine recipients were stratified by gender, race and high versus low behavioral risk of HIV-1 acquisition.
Results
Most vaccine recipients mounted potent neutralizing antibody responses against HIV-1MN and a subset of other tier 1 viruses. Occasional weak neutralizing activity was detected against tier 2 viruses. The response against tier 1 and tier 2 viruses was significantly stronger in women than in men. Race and behavioral risk of HIV-1 acquisition had no significant effect on the response. Prior vaccination had little effect on the neutralizing antibody response that arose post infection.
Conclusions
Weak overall neutralizing antibody responses against tier 2 viruses is consistent with a lack of protection in this trial. The magnitude and breadth of neutralization reported here should be useful for identifying improved vaccines.
Keywords: HIV, vaccines, antibodies
INTRODUCTION
Efforts to develop an effective HIV-1 vaccine have emphasized an ability to elicit virus-specific CD8+ T cells and neutralizing antibodies (NAbs) (1–3). Genetic variability has given rise to multiple genetic subtypes of HIV-1 that exhibit a wide spectrum of antigenic diversity within and between subtypes(4–8)and pose major obstacles for vaccine development. Most variability occurs in the surface gp120 and transmembrane gp41 envelope (Env) glycoproteins that mediate virus entry and serve as the sole targets for NAbs (9–13). HIV-1 evades many NAbs by altering primary recognition sequences and by masking epitopes with N-linked glycans and other conformational and steric constraints that limit antibody access (10, 14, 15). An ideal vaccine may need to overcome these evasion strategies and elicit NAbs against a wide range of circulating variants. Although it is not clear how to achieve this goal, evidence suggests the virus has vulnerabilities, and that broadly cross-reactive NAb induction is indeed possible (16, 17).
Various Env-containing vaccine candidates have elicited NAbs in phase 1 and 2 human clinical trials (3). The antibodies often neutralize T cell line adapted strains and other highly neutralization-sensitivity strains but they do not neutralize most circulating strains of HIV-1(18–20). T cell line adapted strains and a subset of circulating strains that exhibit a high level of neutralization susceptibility are classified as tier 1 viruses (21). The tier 1 phenotype is associated with spontaneous epitope exposure in the sequence-variable cysteine-cysteine loops and in the conserved coreceptor binding domain of gp120 (22–24). Most circulating strains have evolved under immune pressure to conceal these epitopes, resulting in an overall lower level of neutralization susceptibility that is classified as a tier 2 phenotype (21). Whether a certain level of neutralizing activity against tier 1 and tier 2 viruses will predict protection against HIV-1 is not known; however, it is generally agreed that neutralization of tier 2 viruses should be a priority for vaccines (25–28).
Many previous evaluations of vaccine-elicited NAb responses against tier 2 viruses utilized poorly defined virologic reagents and substandard assay methodologies. New high throughput assay technologies are now available that utilize engineered cell lines and reporter genes for highly sensitive, quantitative and reproducible results (14, 29). These new assays have been optimized and validated and utilize well-characterized Env-pseudotyped viruses, including transmitted/founder viruses from sexually acquired infections that are thought to be important targets for vaccination (30–35).
Here we assessed the NAb response in the Vax004 efficacy trial of a candidate HIV-1 gp120 vaccine (AIDSVAX B/B) that was evaluated on the basis of eliciting NAbs (36, 37). Strong antibody responses were detected previously by ELISA and by neutralization of HIV-1MN (38); however, the vaccine did not prevent the acquisition of infection, nor did it impact viral loads in participants who acquired infection after vaccination (39, 40). A similar bivalent gp120 vaccine (AIDSVAX B/E) (41) was ineffective in an efficacy trial in Bangkok intravenous drug users despite comparable antibody responses (42). Lack of efficacy in both trials precluded an assessment of NAbs as a correlate of protection. However, a recent trial of a prime-boost regimen that included AIDSVAX B/E provided modest evidence for a reduced rate of HIV-1 infection(43), which in the future may afford such assessments. Vax004 is the first opportunity to quantify the magnitude and breadth of a non-protective NAb response in human efficacy trials of HIV-1 vaccines, providing a useful reference for future vaccine evaluations.
VOLUNTEERS, MATERIALS AND METHODS
Clinical trial design
The Vax004 trial design was described previously (38–40). The vaccine consisted of two gp120 proteins derived from HIV-1 subtype B strains MN and GNE8. Vax004 and the present study were conducted in accordance with the Declaration of Helsinki and local institutional review board requirements. Written informed consent was obtained from all subjects.
Serologic specimens and stratification
Peripheral blood for plasma was collected in Vacutainer® CPT™ tubes containing sodium citrate as anticoagulant (Becton-Dickinson). Peripheral blood for serum was collected without an anticoagulant. Plasma and sera were stored at −80°C, thawed and heat-inactivated at 56°C for 1 hour prior to assay. Vaccine recipients were stratified by gender, race and high versus low risk of acquiring HIV-1 infection, selected randomly within each group. Low and higher risk groups were defined based on a behavioral risk score variable constructed from baseline questionnaire data, which was used in the primary analyses of Vax004(38, 39). The low risk group consists of participants with lowest risk score 0, and the higher risk group those with risk score ≥ 4. For the nonwhite and female strata, there were not enough available participants with risk score ≥ 4, and in these cases the higher risk group includes some participants with risk scores 1–3.
Viruses
HIV-1 subtype B reference strains 6535.3, QH0692.42, SC422661.8, PVO.4, TRO.11, AC10.0.29, RHPA4259.7, THRO4156.18, REJO4541.67, TRJO4551.58, WITO4160.33 and CAAN5342.A2 closely approximate transmitted/founder viruses from sexually acquired infections (30). Additional subtype B viruses from sexually acquired infections included WEAU-d15.410.787, BB1006-11.C3.1601, BB1054-07.TC4.1499, BB1056-10.TA11.1826, BB1012-11.TC21, 6240.08.TA.4622, 6244.13.B5.4576, 62357.14.D3.4589, which are considered authentic transmitted/early founder viruses (34). Tier 1 viruses included HIV-1MN, SF162.LS, Bal.26, BZ167.12, Bx08.16, SS1196.1, MW965.26 and 92BR025.9. All tier 1 viruses are subtype B except MW965.26 and 92BR025.9, which are subtype C. HIV-1MN was used as an uncloned stock. All other viruses were used as Env-pseudotyped viruses containing a single full-length gp160 clone of the designated strain.
Additional viruses were derived by random sampling from 13 vaccine recipients and 14 placebo recipients within 6 months of infection from Vax004 subjects who received at least 4 inoculations prior to infection. These viruses were used as cloned quasi species of plasma-derived Env-pseudotyped viruses (29).
Neutralization assays
Neutralization was measured with blinded samples in 96-well culture plates by using firefly luciferase (Luc) reporter gene expression to quantify infection. One assay (30, 31) was performed in a HeLa cell line (TZM-bl, also known as JC53-BL) that expresses CD4, CCR5 and CXCR4 (44) and contains a Luc reporter gene (45). Unless otherwise specified, plasma samples were assayed at eight dilutions starting at 1:10. Nab titers were calculated as the sample dilution conferring a 50% reduction in relative luminescence units (RLU) relative to virus control wells after subtraction of background RLU in cell control wells. An additional set of assays tested a 1:10 dilution of serum rather than plasma to avoid the mild toxicity of anticoagulant. Results in these latter assays were calculated as percent reduction in RLU in wells containing post-immunization serum relative to the RLU in wells containing corresponding pre-immune serum from the same subject. HIV-1MN was prepared in H9 cells. Env-pseudotyped viruses were prepared by co-transfecting 293T/17 cells (American Type Culture Collection) with an Env-expressing plasmid plus an Env-defective backbone plasmid (pSG3Δenv) as described (30, 31).
A second assay (29, 35) utilized an astroglioma cell line engineered to express viral fusion receptors (U87.CD4.CCR5.CXCR4). Plasma samples were assayed at eight dilutions starting at 1:10. Nab titers were calculated as the sample dilution conferring a 50% reduction in RLU relative to virus control wells after subtraction of background RLU in cell control wells. Env plasmid libraries were cloned from either infected cell cultures, env expression vectors (tier 1 and 2 reference panels) or plasmas from HIV-infected trial participants. Viral stocks were prepared by cotransfecting HEK293 cells with env plasmid libraries along with an HIV genomic vector containing a Luc indicator gene in place of env.
Statistical methods
Assessment of neutralization of individual isolates
Boxplots were used to graphically display distributions of log10 NAb titers to individual isolates. NAb responses to an individual isolate were summarized by the percentage of subjects who had a positive response(“positive response rate”), and by the geometric mean titer (GMT) of NAbs (“titers of NAbs”) within the subgroup of subjects with a positive response (responders). Positive response rates were compared between groups by 95% confidence intervals(CI) about the difference in positive response rates, and by a Fisher’s exact test for different rates. Titers of NAbs among responders were compared between groups by 95% CIs about the ratio of GMTs. Equality of the overall distribution of log10 NAb titers between two groups was tested as described (46), using 10,000 permutated data-sets to compute a p-value. The false discovery rate (FDR) was used to determine tests that remained statistically significant after adjustment for the multiple hypothesis tests. The FDR method was performed at level 0.05.
Assessment of magnitude and breadth of neutralization of a panel of isolates
A magnitude-breadth (M-B) curve was used to describe the magnitude(NAb titer) and breadth (number of isolates neutralized) of an individual plasma sample assayed against a panel of tier 2 HIV-1 isolates (47). Based on NAb titers to m isolates, the x-axis of an M-B plot is the threshold of neutralization that is considered positive, whereas the y-axis is the percent of the m targets neutralized. The area under the curve (AUC) of a M-B curve provides an overall summary of the M-B profile, and equals the average log10 NAb titer over the m targets. The Mann Whitney test was used to compare the AUC of M-B curve between groups, which provides an overall test for different aggregate NAb responses. Wilcoxon signed rank tests were used to compare within-subject differences in the AUC of M-B plots between two distinct panels of HIV-1 isolates, which determined whether one panel was more easily neutralized than the other. All p-values are 2-sided.
RESULTS
Pre-infection NAb responses
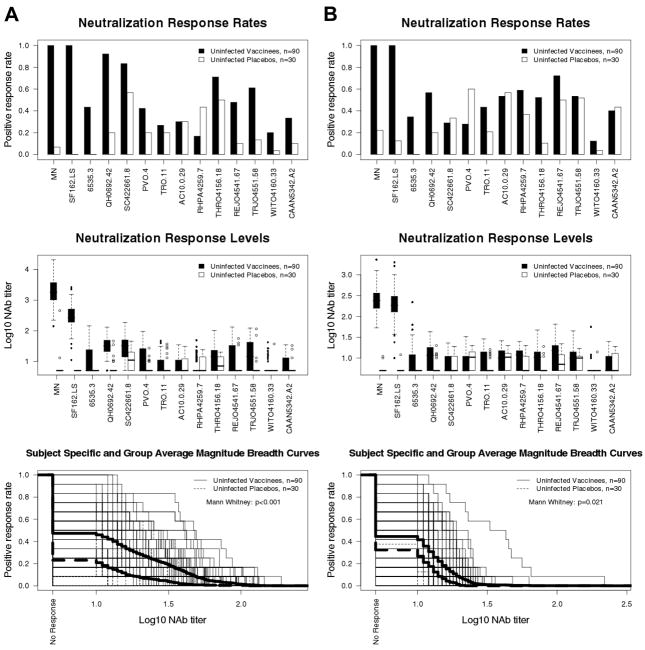
Plasma samples from 2 weeks post fourth inoculation (90 vaccine recipients and 30 placebo recipients who were uninfected at the time of blood draw) were assessed in two independent assays; this time point corresponds to peak vaccine-elicited antibody responses (38). High titer NAbs were detected against HIV-1MN and SF162.LS in most vaccine recipients in both assays (Fig. 1A and B). Sporadic weak neutralizing activity was detected against tier 2 reference strains in both assays (Fig. 1A and B). Positive response rates (frequency of results ≥1:10 plasma dilution) and titers of NAbs against the tier 2 reference viruses were significantly higher for vaccine than placebo recipients for 9 of 12 viruses in the TZM-bl assay and for 6 of 12 viruses in the U87.CD4.CCR5.CXCR4 assay. False positive results (i.e., higher responses in placebo than vaccine recipients) were obtained with RHPA4259.7 in the TZM-bl assay and with PVO.4 in the U87.CD4.CCR5.CXCR4 assay. Because of the low plasma dilutions tested, occasional false positive neutralization was not unexpected. Overall positive response rates against tier 2 viruses were 47% (range 17–92%) and 23% (range 0–57%) for vaccine and placebo recipients, respectively, in the TZM-bl assay. Corresponding positive response rates in the U87.CD4.CCR5.CXCR4 assay were 44% (range 12–72%) and 32% (range 0–60%), respectively. Therefore net positive response rates for vaccine recipients (subtracting positive response rates for placebo recipients) were 24% in the TZM-bl assay and 12% in the U87.CD4.CCR5.CXCR4 assay. Neutralization of tier 2 reference strains was significantly greater for vaccine compared to placebo recipients in both assays when magnitude and breadth of neutralization were considered in aggregate.
Fig. 1.
Comparison of pre-infection NAb responses among vaccine and placebo recipients as measured with tier 1 and tier 2 reference strains. NAbs in plasma samples from 90 randomly selected vaccine recipients and 30 randomly selected placebo recipients, all of whom who were uninfected at the time of blood draw (2 weeks post fourth inoculation), were assessed against HIV-1MN, SF162.LS and a panel of 12 subtype B tier 2 reference strains. Positive response rates (frequency of positive results at ≥1:10 plasma dilution), titers of NAbs and M-B curves were derived from results obtained in the TZM-bl (A) and U87.CD4.CCR5.CXCR4 (B) assays. For the box plots of NAb titers (middle panel), 25% of values lie below the box, 25% lie above the box, and 50% lie below the horizontal line (the median) inside the box. Vertical lines above the box extend to a distance 50% greater than the height of the box; points beyond this are unusually high values (outliers). Subject-specific and group averages in M-B plots are shown as light and heavy lines, respectively, and are for the tier 2 viruses only.
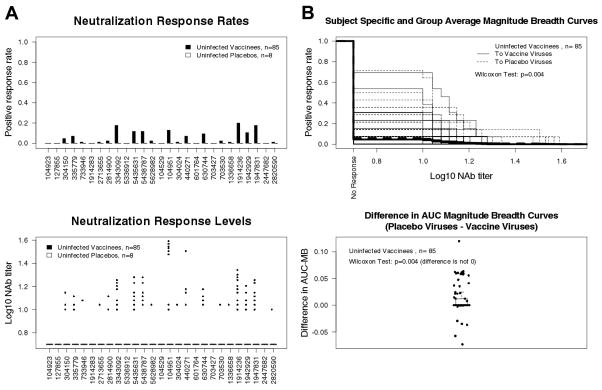
Pre-infection plasmas from vaccine recipients exhibited weak neutralizing activity against early viruses from 13 vaccine and 14 placebo recipients (Fig. 2). Pooling over the 27 isolates, overall positive response rates were 5% for vaccine and 0% for placebo recipients (Mann Whitney p = 0.05). When M-B curves were compared, vaccine-elicited antibodies were more likely to neutralize viruses from placebo recipients than viruses from vaccine recipients (p= 0.004, Fig. 2B). The magnitude of this latter difference was small, with 54 vaccine recipients having equal AUC for the two sets of viruses, 23 having greater AUC for placebo viruses, and 8 having smaller AUC for placebo viruses; thus the result may be of little biological importance. Results with post-infection plasmas from placebo recipients (i.e., natural NAb response to infection) showed that viruses from infected placebo recipients were intrinsically slightly more sensitive to neutralization (data not shown, p-value = 0.013).
Fig. 2.
Comparison of pre-infection NAb responses among vaccine and placebo recipients as measured with viruses from trial participants. Plasma samples in Figure 1 were assessed for neutralizing activity against viruses from 27 trial participants obtained at the earliest available post-infection time point. A. Neutralization response rates and the titers of NAbs. The first 13 viruses from the left are from vaccine recipients and the second 14 viruses are from placebo recipients. B. M-B curves to the vaccine recipient isolate panel and to the placebo recipient isolate panel (top) and differences in AUC of M-B curves for the placebo and vaccine isolate panels (bottom). Subject-specific and group averages in M-B plots are shown as light and heavy lines, respectively. All results in A and B were obtained in the U87.CD4.CCR5.CXCR4 assay. Parallel assessments in the TZM-bl assay were not performed.
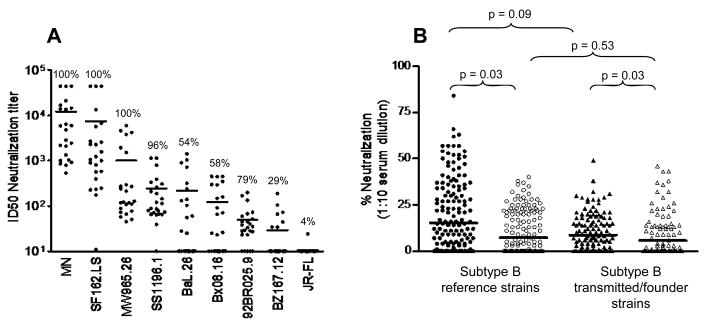
Plasmas from a subset of vaccine and placebo recipients in Figure 1 were assessed for neutralization breadth against a larger panel of tier 1 viruses and one additional prototypic tier 2 virus (JR-FL) in the TZM-bl assay (Fig. 3A). Plasmas from placebo recipients were mostly negative. Plasma from all vaccine recipients neutralized HIV-1MN and SF162.LS, with GMTs of 4931 and 1431, respectively. Moderate to low levels of NAbs were detected against tier 1 viruses MW965.26, SS1196.1, Bal.26, Bx08.16, 92BR025.9 and BZ167.12, with GMTs of 263, 134, 48, 44, 34 and 17, respectively. Plasma from a single vaccine recipient neutralized JR-FL (titer = 24).
Fig. 3.
Breadth of pre-infection NAbs against tier 1 and tier 2 viruses among vaccine and placebo recipients. A. Plasma samples from 24 randomly selected vaccine recipients and 5 placebo recipients (2 weeks post fourth immunization, prior to infection) among the same 120 trial participants in Figure 1 were assayed against HIV-1MN, SF162.LS, six additional tier 1 viruses and one prototypic tier 2 virus (JR-FL) in the TZM-bl assay. Plasma samples were assayed at eight dilutions starting at 1:20. NAb titers <20 were assigned a value of 10. Results are shown for vaccine recipients only. Results with placebo recipient plasmas were low (SS1196.1, four samples with NAb titers of 29–59; MW965.26, one sample with a NAb titer of 31) or negative (all remaining tests). Positive response rate (% of values ≥50 neutralization) is shown above each scatter plot. B. Serum samples from additional vaccine and placebo recipients (n=20 each) were tested for neutralizing activity at a 1:10 dilution in the TZM-bl assay against the 12 subtype B tier 2 reference strains (same as Fig. 1A, excluding tier 1 viruses MN and SF162.LS). Many of these same samples (16 vaccine and 17 placebo recipients) were also assayed against 8 tier 2 transmitted/founder clade B strains (WEAU-d15.410.787, BB1006-11.C3.1601, BB1054-07.TC4.1499, BB1056-10.TA11.1826, BB1012-11.TC21, 6240.08.TA.4622, 6244.13.B5.4576, 62357.14.D3.4589); sufficient quantities were not available for all samples to be assayed against this latter panel of viruses. Serum samples prior to the first inoculation (pre-immune) and 2 weeks post fourth inoculation (prior to infection) were assayed in triplicate on the same assay plate. Percent neutralization was calculated by dividing the average RLU of pre-immune serum by the average RLU of post-immune serum, subtracting this result from 1 and multiplying by 100. For each subject and each tier 2 panel (12 reference viruses and 8 transmitted/founder viruses), the average of the percent neutralization values across the isolates in the panel was computed. These averages were compared between the vaccine and placebo groups for each panel with Mann Whitney tests, and were compared between the two panels with a paired data Wilcoxon signed-rank test. Solid symbols, vaccine recipients; open symbols, placebo recipients.
Additional assays were performed with serum rather than plasma, and compared a 1:10 dilution of post-immune serum (2 weeks post 4 thin oculation) to a 1:10 dilution of corresponding pre-immune serum from additional randomly sampled vaccine and placebo recipients who were uninfected at the time of blood draw. This method automatically adjusts for non-specific activity in corresponding pre-immune serum and thus may be a more stringent measure of true neutralization. Sera were assayed against the 12 tier 2 reference strains and 8 authentic tier 2 transmitted/founder viruses. Subjects were randomly sampled to comprise an equal distribution of African Americans and whites of both genders; this number was not adequate for statistical comparisons between races and genders. Vaccine recipients exhibited weak but statistically significant neutralizing activity against both sets of viruses (Fig. 3B). Transmitted/early founder viruses were slightly less sensitive to neutralization compared to the tier 2 reference viruses but this difference was not significant (p =0.09 for vaccine recipients and p = 0.53 for placebo recipients; testing procedure described in figure legend). Overall positive response rates (≥50% neutralization) against the tier 2 reference viruses were 8.3% for vaccine recipients and 0% for placebo recipients. Although all results with transmitted/early founder viruses were below 50% neutralization, and values ≥50% neutralization was used to calculate positive response rates, positive deflections <50% neutralization might predict stronger potency at lower serum dilutions.
Association between neutralization of HIV-1MN and of tier 2 viruses
Titers of NAbs against HIV-1MN in vaccine recipients were positively correlated with titers against four tier 2 strains in the U87.CD4.CCR5.CXCR4 assay (6535.3, THRO4165.18, REJO4541.67, PVO.4; Spearman r >0.20), one of which was significant after FDR adjustment (6535.3, r = 0.40). HIV-1MN NAb titers were weakly positively correlated with AUC of M-B curves against the 12 tier 2 reference viruses (r= 0.24 and p= 0.025 TZM-bl assay; r= 0.15 and p= 0.16 U87.CD4.CCR5.CXCR4 assay). No significant correlation was seen between HIV-1MN NAb titers and neutralization of viruses from trial participants.
Comparison of post-infection NAb responses among vaccine and placebo recipients
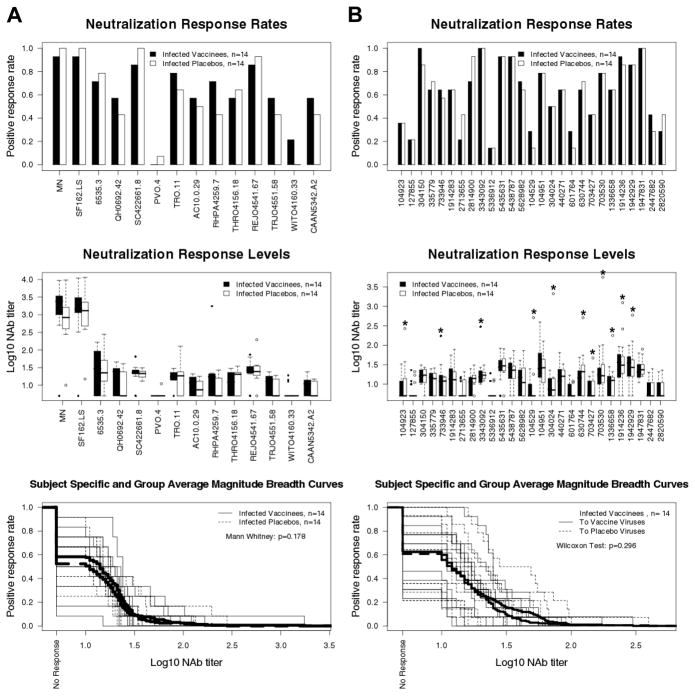
NAbs were assessed in plasma from 14 vaccine recipients and 14 placebo recipients 12–24 months after diagnosis of infection(prior to antiretroviral therapy). All subjects received 4 inoculations of either the vaccine or placebo prior to diagnosis. Results in the TZM-bl assay were published previously (47). Results in the U87.CD4.CCR5.CXCR4 assay are shown in Figure 4. Titers of post-infection NAbs against HIV-1MN were significantly higher for vaccine recipients than placebo recipients in both assays, suggesting the vaccine augmented the response to HIV-1MN. No significant difference was seen between vaccine and placebo recipients for NAbs against SF162.LS, the 12 tier 2 reference strains and the 27 viruses from trial subjects. Assays with viruses from infected trial participants included autologous plasma/virus combinations from 2 vaccine and 8 placebo recipients that yielded considerably stronger neutralization than heterologous combinations. Differences among vaccine and placebo recipients were non-significant regardless of whether autologous combinations were included in the statistical analysis.
Fig. 4.
Comparison of post-infection NAb responses among vaccine and placebo recipients. NAbs were assessed in plasma samples from 14 vaccine recipients and 14 placebo recipients 12–24 months after diagnosis of infection. All subjects were antiretroviral therapy naïve at the time of plasma collection. A. Assays with MN, SF162.LS and the subtype B reference panel of tier 2 viruses. B. Assays with viruses from trial participants. In the top two diagrams, the first 13 viruses from the left are from vaccine recipients and the second 14 viruses are from placebo recipients. Autologous virus/plasma combinations in the middle diagram (Neutralization Response Levels) are indicated by an asterisk. All results in A and B were obtained in the U87.CD4.CCR5.CXCR4 assay. Subject-specific and group averages in M-B plots are shown as light and heavy lines, respectively, and are for the tier 2 viruses only.
Comparison of NAb responses among pre-infection vaccine recipients and post infection placebo recipients
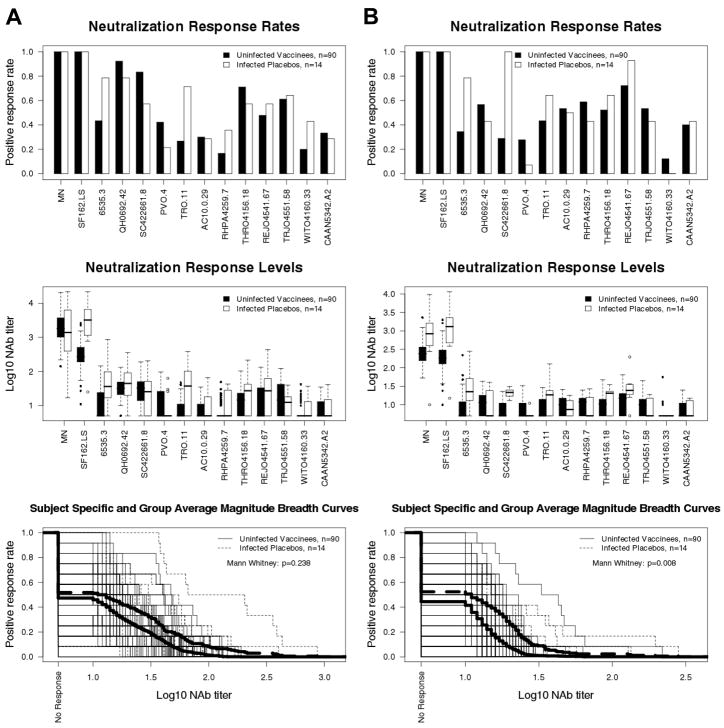
Peak vaccine-elicited NAb responses in 90 trial participants (2 weeks post fourth inoculation) were compared to the early response that arose post-infection in 14 placebo recipients (1–2 years post diagnosis). Results are shown in Figure 5. Titers of NAbs against HIV-1MN were similar in both cases, whereas titers against SF162.LS were significantly elevated in infected placebo recipients (GMT 2451 vs 288, p <0.001 in TZM-bl assay; GMT 1006 vs 184, p <0.001 in U87.CD4.CCR5.CXCR4 assay). M-B curves in the TZM-bl assay showed that responses against the tier 2 reference strains were similar among the two groups (p = 0.24), whereas a small but significantly elevated response was seen in infected placebo recipients using the U87.CD4.CCR5.CXCR4 assay: p <0.001 for all 39 tier 2 viruses (data not shown); p= 0.008 for the tier 2 reference strains shown in Fig. 5. Thus, the vaccine-elicited response did not exceed the response that arose after 1–2 years of infection in the absence of vaccination.
Fig. 5.
Comparison of pre-infection NAb responses in vaccine recipients to post-infection NAb responses in placebo recipients. Plasma from 90 vaccine recipients (2 weeks post fourth inoculation) and 14 placebo recipients (1–2 years post diagnosis) were assayed against MN, SF162.LS and the subtype B reference panel of tier 2 viruses. A. TZM-bl assay. B. U87.CD4.CCR5.CXCR4 assay. Subject-specific and group averages in M-B plots are shown as light and heavy lines, respectively, and are for the tier 2 viruses only.
Influence of demographic factors on the pre-infection neutralizing antibody response in vaccine recipients
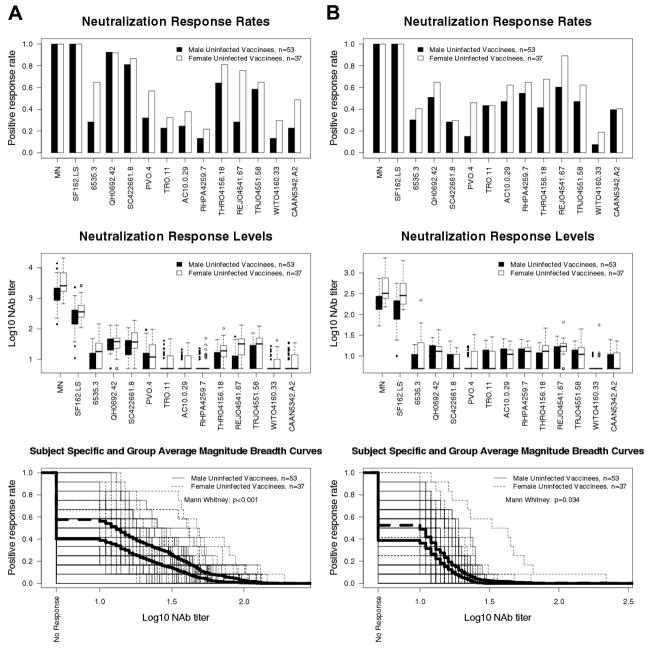
NAbs in the 90 vaccine recipients (2 weeks post fourth inoculation, prior to infection) were compared among genders, race (African American and whites) and low versus high risk behavior groups. Results in both assays demonstrated higher titers of NAb against HIV-1MN and SF162.LS in women than in men (approximate 2-fold increase in GMT, p <0.008). Additionally, M-B curves showed higher aggregate responses to the 12 tier 2 reference viruses in women compared to men (p <0.001 TZM-bl assay; p =0.034 U87.CD4.CCR5.CXCR4 assay)(Fig. 6). A non-significant trend toward higher M-B curves also was seen for women when all 39 tier 2 viruses were considered in aggregate (p =0.073 U87.CD4.CCR5.CXCR4 assay). Race and risk behavior level had no significant effect.
Fig. 6.
Comparison of pre-infection NAb responses among men and women vaccine recipients (n=90 evaluated in Fig. 1) as measured with the tier 1 and tier 2 reference strains evaluated in Fig. 1. Positive response rates (frequency of positive results at ≥1:10 plasma dilution), titers of NAbs and M-B curves were derived from results obtained in the TZM-bl (A) and U87.CD4.CCR5.CXCR4 (B) assays. Subject-specific and group averages in M-B plots are shown as light and heavy lines, respectively, and are for the tier 2 viruses only.
Comparison of tier 2 reference strains and viruses from trial participants
The tier 2 reference strains were more susceptible to non-specific neutralization (p= 0.004 for pre-infection placebo samples) and to specific neutralization (p <0.001 for pre-infection vaccine samples) (tests as for Fig. 2B) than viruses from trial participants. Having a positive response to the reference panel was predictive of having a positive response to the trial participant panel for post-infection vaccine and placebo samples (odds ratio 8.17, p = 0.019) but not for pre-infection vaccine samples. Because viruses from trial participants were only assayed in U87.CD4.CCR5.CXCR4 cells, where slightly elevated NAb responses were detected post-infection, the magnitude of vaccine-elicited NAb response against tier 2 viruses might border the magnitude required to achieve reproducible results in the two independent assays.
DISCUSSION
We confirm that most vaccine recipients in Vax004 possessed moderate to high titers of NAbs against HIV-1MN. Moderate neutralizing activity was often detected against other tier 1 strains but only occasional weak neutralizing activity was detected against tier 2 strains. Prior vaccination augmented the NAb response against HIV-1MN post-infection but had little measurable effect on the post-infection NAb response against tier 2 viruses. Overall the vaccine-elicited NAb response was no better than the relatively weak response that arose after 1–2 years of infection in the absence of vaccination. Relatively weak NAb responses against tier 2 strains is consistent with the lack of protection in this trial.
Vaccine-elicited NAb responses against tier 2 viruses, albeit weak, were statistically significant (compared to placebo) against tier 2 Env-pseudotyped reference strains and against pseudoviruses containing a more recent set of authentic transmitted/founder Envs, suggesting that the reference panel detects NAbs of interest for vaccines. Both sets of pseudoviruses contained single Env clones whereas pseudoviruses containing Env from trial participants were a quasi species. Greater genetic complexity of the Env quasi species might account for observed differences in non-specific activity and neutralization-sensitivity when assayed in U87.CD4.CCR5.CXCR4 cells. In both cases, neutralization of tier 2 viruses was poorly predicted by NAbs against the HIV-1MN, thus reinforcing the importance of including tier 2 viruses when assessing vaccine-elicited NAbs. Additionally, vaccine recipient plasma appeared more likely to neutralize Env quasi species from infected placebo recipients than from infected vaccine recipients (p= 0.004), although the small magnitude of this possible effect suggests little if any biological significance. Beyond Vax004, for efficacy trials with evidence for positive vaccine efficacy, a larger effect of this kind could indicate that some circulating viruses are more sensitive to vaccine-elicited NAbs that blocked their transmission to exposed vaccine recipients. We encourage similar assessments of NAbs, combined with complementary genetic analyses of the viruses (49), in RV144 and future trials where measurable protection is achieved.
Our results are consistent with a previous report (38) showing significantly elevated titers of NAbs against HIV-1MN in women than in men (2-times higher GMT in both assays) and no significant difference in the response between high and low behavioral risk groups in Vax004. We also observed significantly stronger responses in women than in men for NAbs against SF162.LS and tier 2 reference strains. Contrary to previous reports (38, 48, 50), we found no significant difference in NAb responses between African Americans and whites. Our results lend support to a possible effect of gender on the NAb response to certain HIV-1 vaccines. Additional studies are needed to delineate the nature of this effect.
Modest protection in the recent efficacy trial in Thailand (RV144) will provide additional opportunities to learn more about the requirements for effective vaccination against HIV-1. One way to improve the efficacy of current HIV-1 vaccines may be to elicit stronger NAb responses against tier 2 strains of the virus. The magnitude and breadth of neutralization reported here for a non-protective vaccine should serve as a useful reference to identify improved vaccine designs.
Acknowledgments
We thank George Shaw, Beatrice Hahn and the Center for HIV/AIDS Vaccine Immunology (CHAVI) for contributing the transmitted/founder HIV-1 Envs. We also thank Michael Peterson and David Jobes for assistance in coordinating the study. In addition, we acknowledge the excellent technical assistance of Alicia Gaitan, Barbara Sokolik-Wolak, Hongmei Gao and Kelli Greene.
Financial Support: HIV Vaccine Trials Network and the National Institutes of Health (AI46705)
Footnotes
Presented in part: AIDS Vaccine 2005, Montreal, Quebec, Canada, 6–9 September 2005.
Potential conflicts of interest: M.Gurwith., F.S., and P.W.B are former employees of VaxGen; P.G. and S.G.S. received consulting fees from VaxGen in the past.
References
- 1.Letvin NL. Progress and obstacles in the development of an AIDS vaccine. Nat Rev Immunol. 2006;6:930–9. doi: 10.1038/nri1959. [DOI] [PubMed] [Google Scholar]
- 2.McMichael AJ. HIV vaccines. Annu Rev Immunol. 2006;24:227–55. doi: 10.1146/annurev.immunol.24.021605.090605. [DOI] [PubMed] [Google Scholar]
- 3.Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2009 doi: 10.1146/annurev-immunol-030409-101256. in press. [DOI] [PubMed] [Google Scholar]
- 4.Korber B, Gaschen B, Yusim K, Thakallapally R, Kesmir C, Detours V. Evolutionary and immunological implications of contemporary HIV-1 variation. Br Med Bull. 2001;58:19–42. doi: 10.1093/bmb/58.1.19. [DOI] [PubMed] [Google Scholar]
- 5.McCutchan FE. Understanding the genetic diversity of HIV-1. AIDS. 2000;14(Suppl 3):S31–44. [PubMed] [Google Scholar]
- 6.Moore JP, Cao Y, Leu J, Qin L, Korber B, Ho DD. Inter-and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J Virol. 1996;70:427–44. doi: 10.1128/jvi.70.1.427-444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mascola JR, Louwagie J, McCutchan FE, Fischer CL, Hegerich PA, Wagner KF, et al. Two antigenically distinct subtypes of human immunodeficiency virus type 1: viral genotype predicts neutralization serotype. J Infect Dis. 1994;169:48–54. doi: 10.1093/infdis/169.1.48. [DOI] [PubMed] [Google Scholar]
- 8.Binley JM, Wrin T, Korber B, Zwick MB, Wang M, et al. Comprehensive crossclade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78:13232–52. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaschen B, Taylor J, Yusim K, Foley B, Lang D, Novitsky V, et al. Diversity considerations in HIV-1 vaccine selection. Science. 2002;296:2354–2360. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–8. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Lipchina I, Cocklin S, Chaiken I, Sodroski J. Antibody binding is a dominant determinant of the efficiency of human immunodeficiency virus type 1 neutralization. J Virol. 2006;80:11404–11408. doi: 10.1128/JVI.01102-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crooks ET, Moore PL, Richman D, Robinson J, Crooks JA, Franti M, et al. Characterizing anti-HIV monoclonal antibodies and immune sera by defining the mechanism of neutralization. Human Antibodies. 2005;14:101–113. [PMC free article] [PubMed] [Google Scholar]
- 13.Moore PL, Crooks ET, Porter L, Porter L, Zhu P, Cayanan CS, et al. The nature of non- functional envelope proteins on the surface of human immunodeficiency virus type 1. J Virol. 2006;80:2515–2528. doi: 10.1128/JVI.80.5.2515-2528.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, et al. Antibody neutralization and escape. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 15.Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 16.Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med. 2009;15:866–870. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- 17.Walker LM, Phogat SK, Chan-Hui P-Y, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Kaminski S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR Protocol G Principal Investigators. Broad and potent neutralizing antibodies from an African donor reveal new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mascola JR, Snyder SW, Weislow OS, Belay SM, Belshe RB, Schwartz DH, et al. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 19.Bures R, Gaitan A, Zhu T, Graziosi C, McGrath KM, Tartaglia J, et al. Immunization with recombinant canarypox vectors expressing membrane-anchored gp120 followed by gp160 protein boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 2000;16:2019–2035. doi: 10.1089/088922200750054756. [DOI] [PubMed] [Google Scholar]
- 20.Belshe RB, Gorse GJ, Mulligan MJ, Evans TG, Keefer MC, Excler J-L, et al. Induction of immune responses to HIV-1 canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. AIDS. 1998;12:2407–2415. doi: 10.1097/00002030-199818000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, et al. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessments of neutralizing antibodies. J Virol. 2010;84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bou-Habib DC, Roderiquez G, Oravecz T, Berman PW, Lusso P, Norcross MA. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis KL, Gray ES, Moore PL, Decker JM, Salomon A, Montefiori DC, et al. High titer HIV-1V3-specific antibodies with broad reactivity but low neutralizing potency in acute infection and following vaccination. Virology. 2009;387:414–426. doi: 10.1016/j.virol.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Decker JM, Bibollet-Ruche F, Wei X, Wang S, Levy DN, Wang W, et al. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J Exp Med. 2005;201:1407–1419. doi: 10.1084/jem.20042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews TJ. Dilemma of neutralization resistance of HIV-1 filed isolates and vaccine development. AIDS Res Hum Retroviruses. 1994;10:631–632. doi: 10.1089/aid.1994.10.631. [DOI] [PubMed] [Google Scholar]
- 26.Moore JP, Burton DR. Urgently needed: a filter for the HIV-1 vaccine pipeline. Nat Med. 2004;10:769–771. doi: 10.1038/nm0804-769. [DOI] [PubMed] [Google Scholar]
- 27.Douek DC, Kwong PD, Nabel GJ. The rational design of an AIDS vaccine. Cell. 2006;124:677–681. doi: 10.1016/j.cell.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, et al. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5:233–236. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 29.Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Nat Acad Sci USA. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79 :10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Salazar-Gonzalez JF, Derdeyn CA, Morris L, Williamson C, Robinson JE, et al. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in southern Africa. J Virol. 2006;80:11776–11790. doi: 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulkarni SS, Lapedes A, Tang H, Gnanakaran S, Daniels MG, Zhang M, et al. Highly complex neutralization determinants on a monophyletic lineage of newly transmitted subtype C human immunodeficiency virus type 1 env clones from India. Virology. 2009;385:505–520. doi: 10.1016/j.virol.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blish CA, Nedellec R, Mandaliya K, Mosier DE, Overbaugh J. HIV-1 subtype A envelope variants from early in infection have variable sensitivity to neutralization and to inhibitors of viral entry. AIDS. 2007;21:693–702. doi: 10.1097/QAD.0b013e32805e8727. [DOI] [PubMed] [Google Scholar]
- 34.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci (USA) 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweighart B, Liu Y, Huang W, Chappey C, Lie YS, Petropoulos CJ, Wrin T. Development of an HIV-1 reference panel of subtype B envelope clones isolated from the plasma of recently infected individuals. J Acqui Immune Defic Syndr. 2007;46:1–11. doi: 10.1097/QAI.0b013e318074eb5a. [DOI] [PubMed] [Google Scholar]
- 36.Berman PW. Development of bivalent rgp120 vaccines to prevent HIV type 1 infection. AIDS Res Hum Retroviruses. 1998;14:S277–S289. [PubMed] [Google Scholar]
- 37.Francis DP, Gregory T, McElrath MJ, Belshe RB, Gorse GJ, Migasena S, et al. Advancing AIDSVAX™ to phase 3. Safety, immunogenicity, and plans for phase 3. AIDS Res Hum Retroviruses. 1998;14:S325–S331. [PubMed] [Google Scholar]
- 38.Gilbert PB, Peterson ML, Follmann D, Hudgens MG, Francis DP, Gurwith M, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis. 2005;191:666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 39.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF, et al. Placebo-controlled phase 3 trial of recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert PB, Ackers ML, Berman PW, Francis DP, Popovic V, Hu DJ, et al. HIV-1 virologic and immunologic progression and initiation of antiretroviral therapy among HIV-1-infected subjects in a trial of the efficacy of recombinant glycoprotein 120 vaccine. J Infect Dis. 2005;192:974–983. doi: 10.1086/432734. [DOI] [PubMed] [Google Scholar]
- 41.Berman PW, Huang W, Riddle L, Gray AM, Wrin T, Vennari J, et al. Development of bivalent (B/E) vaccines able to neutralize CCR5-dependent viruses from the United States and Thailand. Virol. 1999;265:109. doi: 10.1006/viro.1999.0031. [DOI] [PubMed] [Google Scholar]
- 42.Pitisuttithum P, Gilbert PB, Gurwith M, Heyward W, Martin M, van Griensven F, et al. Randomized, placebo-controlled efficacy trial of a bivalent rgp120 HIV-1 vaccine among injecting drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 43.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 44.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infection by macrophage tropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lachenbruch PA. Comparison of two-part models with competitors. Statistics in Medicine. 2001;20:1215–1234. doi: 10.1002/sim.790. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y, Gilbert PB, Montefiori DC, Self SG. Simultaneous evaluation of the magnitude and breadth of a left-and right-censored multivariate response, with application to HIV vaccine development. Statistics Biopharm Res. 2009;1:81–91. doi: 10.1198/sbr.2009.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montefiori DC, Metch B, McElrath MJ, Self S, Weinhold KJ, Corey L. Demographic factors that influence the neutralizing antibody response in recipients of recombinant HIV-1 gp120 vaccines. J Infect Dis. 2004;190:1962–1969. doi: 10.1086/425518. [DOI] [PubMed] [Google Scholar]
- 49.Pérez-Losada M, Jobes DV, Sinangil F, Crandall KA, Posada D, Berman PW. Phylodynamics of HIV-1 from a phase III AIDS vaccine trial in North America. MBE Journal. 2010 doi: 10.1093/molbev/msp254. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pérez-Losada M, Posada D, Arenas M, Jobes DV, Sinangil F, Berman PW, Crandall KA. Ethnic differences in the adaptation rate of HIV gp120 from a vaccine trial. Retrovirology. 2009;6:67–69. doi: 10.1186/1742-4690-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]