Abstract
This study describes the development of emerging intonation in six children who had received a cochlear implant (CI) before the age of three years. At the time their implant was activated, the children ranged in age from 11 to 37 months. Spontaneous longitudinal speech samples were recorded from 30-minute sessions in which the child interacted with his or her mother. Data were collected 2 months before activation of each child's CI and at monthly intervals after activation for 6 months. The findings were compared to the typical pattern of early intonation development in children with normal hearing (NH). The results suggested that young CI recipients progress through stages similar to those observed in children with NH. However, the intonation development of children with a CI reflects a marked interaction between chronological age at implantation and amount of CI experience. That is, after 2 months of CI-assisted hearing experience, the older children demonstrated a later stage of intonation development than younger children. These preliminary results support the idea that children acquire some foundations or prerequisites of intonation production through maturation, as measured by chronological age, even without robust auditory experience.
Intonation refers to distinctive patterns of vocal melody (Crystal, 1991; Cruttenden, 1997). The melodies of speech are related to virtually all levels of verbal communication, including emotional expression, pragmatics, and syntactic structure. On the acoustic level, melody patterns result from linguistically significant changes in the fundamental frequency (f0) of the voice. The physiological correlate of f0 is the rate of vibration of the vocal folds. Finally, on the psychological level, “tone” refers to the functional organization of pitch patterns in the phonological system of speakers and listeners.
Recent research in infant speech has supported the hypothesis that intonation is an early-developing system. A strong form of the hypothesis, in fact, suggests that children acquire the intonational system before the onset of meaningful speech (e.g., Locke, 1983). Although this strong claim has not been substantiated to-date, the evidence does suggest that children attain significant milestones in the acquisition of intonation during the first year of life. To investigate the typical stages of intonation development in prelinguistic and early meaningful speech, Snow (2006, 2007) studied the production of rising and falling pitch patterns in English-speaking children with normal hearing (NH) between the ages of 6 and 23 months. The dependent variable was “accent range,” that is, how much pitch changed within a tone contour. When infants consistently produced rising or falling utterance-final tones with a width of pitch change that is comparable to that of older children and adults, this was taken as evidence that the infants had acquired those tones.
The most striking feature of the observed developmental pattern in Snow's (2006) study is its U-shaped configuration. In the first year of life, from 6 to 10 months, children produce a wide accent range, in rising and falling contours, that is comparable to that of older preschool children. Then, at about 10 months, a sharp discontinuity occurs in which children restrict the width of pitch change to a narrow range. Although this is ostensibly a regression, Snow argued that the decline at 10 months represents a developmental advance, arguably the first measurable step in children's acquisition of intonation as an intentional, linguistic system. To support this conclusion, Snow pointed out that the hypothesized advance in intonation coincides with the onset of pragmatics (Bates, Camaioni, & Volterra, 1975), one of the prelinguistic foundations of intonation.
The youngest infants in Snow's (2006) crossectional study were 6 months old. Thus, the study did not document the development of intonation in infants with NH between birth and 6 months of age. However, large-scale studies of infant vocalizations have provided a preliminary sketch of f0 patterns that infants produce during the first weeks and months of life. For example, Capute, Palmer, Shapiro, et al. (1986) reported that a significant milestone occurs at about 2 months in association with the onset of cooing. Vocalizations emerging at this stage constitute the beginning of intonation because cooing demonstrates for the first time melody patterns that are relatively adult like and that clearly convey affective states (MacNeilage & Davis, 1990). The nearly simultaneous beginning of social smiling attests further to the robust development of emotional experience in the first weeks of life. In fact, this remarkably early association between affect, facial expression, and pitch of the voice constitutes the fundamental prelinguistic underpinning of intonation.
Based on these observations of early intonation-affective behaviors, an initial increase in children's accent range plausibly accompanies the onset of cooing at 1½ –2 months. Thus, the observed and inferred stages of normal intonation development reflect two discontinuities in the prelinguistic period: an initial increase in average pitch change at 2 months (Capute et al., 1986; MacNeilage & Davis, 1990) and a sharp decrease at about 10 months (Snow, 2006)
The early development of intonation in the first year of life seems to reflect infants' special sensitivity to f0 patterns in the input. Fernald & Kuhl (1987) concluded that infants at 4 months of age not only perceive f0 patterns but preferentially distinguish the expanded and emotionally expressive f0 contours of “motherese” from the less salient contours of adult-directed speech. Because auditory experience in the first months of life is a critical prerequisite for f0 perception and recognition, children with compromised hearing are likely to be at risk for atypical intonation development. For young children with CIs, hearing is compromised in the child's pre- and post-implant experience. Children who eventually receive a CI often had some auditory experience via amplification before implantation. However, for the participants in the present study, this relatively weak exposure to audition via hearing aids was not sufficient to support language acquisition, and so the parents opted for implantation. After more robust hearing via a CI becomes available, audition remains compromised by limitations imposed by the prosthetic device itself, as discussed next in a brief review of the literature.
F0 perception and production in children with a CI
Perceptual studies have indicated that the acoustic signal is degraded in CIs relative to normal hearing (NH). In particular, f0 tends to be weakly represented in CIs (Peng, Tomblin, Spencer, & Hurtig, 2007; Kuo, Rosen, & Faulkner, 2008). To investigate the limited representation of f0 in CIs, Lin, Lee, Huang, & Peng (2007) asked adult Mandarin speakers with NH to identify tones in monosyllabic word productions that acoustically simulated CIs with a different number of channels. They found that continuous increases in the number of simulated channels (from 8 to 16, for example) improved the listeners' lexical tone perception but not phoneme recognition. As a result of the severe and selective constraints imposed by the number of channels, f0 might not be strongly represented by the implant. In fact, CI users might rely on other cues, especially temporal patterns – the least effective cues in lexical tone perception (Kuo et al., 2008). Users of CIs also seem to rely substantially on temporal cues in the perception of intonational pitch patterns (Chatterjee & Peng, 2008). Other studies are consistent with these conclusions. For example, children with a CI have difficulty using f0 information to identify Cantonese lexical tones (Ciocca, Francis, Aisha, & Wong, 2002). Using a picture-identification task, Ciocca et al. asked children with CIs between the ages of 4 and 8 years to identify different Cantonese tones in the target word /ji/. The early-deafened CI listeners performed poorly compared to a 6-year-old child with a moderate hearing impairment. Similarly, in direct comparisons between CI devices and hearing aids (HAs), groups of children with different devices and degrees of hearing loss demonstrated a similar rank order of perceptual skills across a variety of word and prosodic patterns, CI use did not lead to an advantage over HA use in the perception of suprasegmental features (Most & Peled, 2007).
If the acoustic representation of f0 in CI devices is poorer than normal hearing, the tone production of children with a CI might also be adversely affected. In Cantonese, for example, a group of 3 children acquired lexical pitch patterns corresponding to the tonal inventory more slowly than they acquired the vowel inventory (Barry, Blamey, Lee, & Cheung, 2000), suggesting that f0 patterns may be more difficult to acquire than speech sounds. In studies of intonation production, Peng, Tomblin, Spencer, & Hurtig (2007) elicited imitated productions of a rising contour (yes-no question) from children with a CI. According to the judgments of adults with NH, the children with a CI did not produce the contour accurately. On the other hand, some studies have reported that children with CIs demonstrated normal “phrasing and pitch” (Lenden & Flipsen, 2007), based on the Prosody-Voice Screening Profile (Shriberg, Kwiatkowski, & Rasmussen, 1990). Because the relatively sparse evidence about production is mixed, children with CIs might be at risk for atypical intonation development. On the other hand, their greatly increased access to auditory information might lead to typical or nearly typical patterns of intonation development. The principal aim of this study is to evaluate the effects of age at implantation and amount of robust hearing experience on the development of intonation in children with CIs.
Snow's (2006) study of prelinguistic intonation development in children with NH suggested that intonation depends not only on listening experience but also on early developing cognitive, motoric, social-affective, pragmatic, and gestural communication skills. In fact, the most far-reaching implication of the study was that intonation grows out of a developmental progression leading from non-linguistic experience (affect) to prelinguistic communication (pragmatics) and finally linguistic experience (the onset of syntax). Most importantly, the primitive stages devoted to affect and pragmatics are mediated by nonlinguistic or even nonverbal modalities. Thus, the development of affective and pragmatic precursors to intonation may not necessarily depend on linguistic input or auditory experience. If these prelinguistic skills are crucial for intonation development, as we hypothesize, children with delays only in audition might experience lesser or transient delays in the perception and production of intonation than hearing status alone would predict. Thus, the expected severity and persistence of intonation delays in children with CIs depends on the role of hearing experience versus chronological age. At present, however, the role of each of these factors and the extent of a possible interaction between them is unknown.
To investigate this issue, this study focused on the development of intonation in 6 deaf children who received a cochlear implant by the age of three. The study was designed to compare the emerging intonation of these 6 children with the recently documented pattern of development for children with NH. The principal aim of the research was to determine whether early intonation milestones are predicted by amount of CI-assisted auditory experience, chronological age, or an interaction between audition and maturation. Specifically the study addressed the following theoretical and applied questions:
Does the acquisition of intonation in children with a CI proceed through the same stages of development that have been observed in children with NH during the first 10 months of life?
Does the acquisition of intonation in children with a CI show an interaction between age and hearing experience?
If children are implanted after the age of two years, do they demonstrate a continuing pattern of delayed development or do they begin to “catch up” with children who had been implanted at a younger age?
Methods
Participants
Spontaneous utterances from four girls and two boys were examined for this retrospective study of changes in fundamental frequency range following cochlear implantation. Each participating child was implanted by the age of 36 months (range of 10 to 36 months) and had hearing parents. The children were selected from a group of seven children who participated in a study of prelinguistic vocal development (Ertmer, Young, & Nathani, 2007). The child who was not selected from the Ertmer et al. (2007) study was identified as having learning difficulties in addition to profound hearing loss. In some sessions, this child also did not have any utterances meeting the selection criteria for intonation analysis. Except for hearing loss, the selected participants for the current study had no other physical or learning disabilities. Table 1 contains audiological and CI information for each child. Individual children are referenced with a code that indicates their gender and their chronological age at implantation. For example, M-36 received his CI at 36 months. F-30 received hers at 30 months.
Table 1.
Audiometric and cochlear implant information for each participant.
| Child (gender-age at implantation in months) | Age at Identification (Length of hearing aid trial) | Device (processing strategy) | Mean Better-ear Unaided Hearing Thresholds Pre-implantation (dB HL) | Mean Aided Hearing Thresholds (dB HL) First 6 Months of CI Use | Age Range During Pre-implant and First 6 Post-Implantation Sessions (months) |
|---|---|---|---|---|---|
| M-36 | 32 months (4 months) | Clarion Multi-strategy (CIS) | NR | 27 | 35 – 42 |
| F-30 | 12 months (17 months) | Nucleus 24 (ACE) | 93 | 26 | 29 – 37 |
| F-28 | 23 months (5 months) | Clarion Multi-strategy (CIS) | NR | 25 | 27 – 35 |
| F-20 | 10 months (9 months) | Clarion Multi-strategy (CIS) | 93 | 29 | 19 – 27 |
| F-18 | 15 months (2 months) | Nucleus 24 (ACE) | 103 | 39* | 17 – 25 |
| M-10 | 2 days (7 months) | Clarion Multi-strategy (CIS) | NA | 25 | 9 – 17 |
CIS = Continuous Interleaved Sampling, ACE = Advanced Combination Encoder; NR = No response at maximum audiometer output levels; NA = Not available; child could not be conditioned for behavioral testing. Auditory Brainstem Response testing predicted severe-profound bilateral hearing impairment
6-month PTA not available, PTA from 12-month assessment.
All of the participants had a hearing aid trial and received family-centered intervention for 1–2 hours per week after their hearing losses were identified and continued to receive intervention services once their CIs were activated. Three children (M-36, F-28, and F-20) began attending a full-day oral education preschool program shortly after their third birthdays. Of the three remaining children, F-30 received 2 hours of individual Auditory-Verbal therapy weekly before and after implantation. F-18 participated in twice weekly, intervention sessions through the Parent-Infant Communication Development program at Purdue University. The youngest recipient (M-10) received 1-hour/week family-centered intervention at the home during the first two years of CI use. Three families had begun to use manual signs on a limited basis before the start of the study (M-36, F-28, and F-18); the remaining families used oral communication only. The families of M-36 and F-28 discontinued signing upon enrollment in an oral preschool. F-18's family and interventionists focused mainly on the development of spoken language but used manual signs intermittently to supplement speech after implant activation. Family-centered communication intervention was provided by speech-language pathologists and teachers of deaf children and focused primarily on stimulating auditory, speech, and vocabulary development.
The Adaptive Behaviors and Motor Skills subtests of the Battelle Developmental Inventory (BDI; Newborg, Stock, & Wnek, 1984) were administered prior to enrollment in the Ertmer et al. (2007) study. These subtests were used to assess child development in areas not directly influenced by hearing and oral language ability. All children scored within or above two standard deviations of the mean for their ages on the selected subtests. The Symbolic Play Test (SPT; Lowe & Costello, 1988) was given to determine whether the nonverbal play skills were comparable to chronological ages. Only one child scored below age-level on the SPT: M-36 achieved a score of 25 months when he was 30 months old. The remaining SPT age scores ranged from 0 to 11.9 months above CA. M-10 did not complete the SPT due to scheduling conflicts. Mothers also completed a health and developmental history for their child prior to the start of the study. No additional learning or physical disabilities were reported for any of the participants in the current study and each child was eventually enrolled in mainstream elementary classrooms. The functioning of each child's CI was monitored through daily checks and parent and teacher observations as well as regularly scheduled mapping sessions at implant centers. The children did not experience any periods of prolonged CI malfunction during the study.
It should be noted that F-30 wore bilateral hearing aids longer than the other children (approximately 17 months) before receiving a cochlear implant. Following implant surgery, she used a CI alone for approximately 4 months before being fit with a digital hearing aid (HA) for her non-implanted ear. F-30's speech perception ability was examined after 14 months of CI + HA experience to determine the relative influence of each device. Live-voice, auditory-only administration of the Northwestern University Children's Perception of Speech (NU-Chips; Elliott & Katz, 1980) revealed that she identified 76% of stimulus words in the CI+HA condition, and 84% of stimulus words when using her CI alone. These scores suggest that she was reliant on the implant signal to recognize spoken words and speech features. The remaining participants did not wear HAs after surgery.
Data Collection
Two half-hour data collection sessions were held within 2 months before implant activation. During these sessions, mothers were instructed to play with their child in their “usual way” using an assortment of familiar toys. Audio-recordings were made by placing boundary/ Pressure Zone Microphones (PZM) within 3 feet of the child. The microphones were connected to either a preamplifier coupled with a Marantz audio cassette recorder (model PMD 221) or directly to a Sony Handicam 8mm camcorder (model CCD-TRV37). Both audio-cassette recorders and camcorders were used during the first year of the study. Subsequent sessions were recorded with camcorders alone to simplify data collection. The quality of the audio signals from both devices was judged to be comparable and acceptable for acoustic analysis. Post-implantation recordings of parent-child interactions were made in the child's home at monthly intervals beginning one month after implant activation. Procedures and equipment for these sessions were the same as those used in pre-implant sessions. A speech-language pathologist/data collector (SLP) was present throughout each recording session. Because the SLP was the child's clinician, the data collection context included only everyday home setting and the participation of parents and other familiar adults. In sum, the experimenters did not feel that there was any reason to question the representativeness of the children's recorded vocalizations. For the current investigation, utterances from the two pre-implantation sessions and the first 6 post-implantation sessions (post-implantation months 1 – 6) were analyzed.
Data Analysis
Audio recordings of participant utterances were chosen for analysis in the following ways in the Ertmer et al. (2007) study. A minimum of two judges viewed each half-hour audio-video recording and counted the number of utterances in each 10-minute segment. The judges were undergraduate and graduate students who had completed coursework in phonetics and were trained to identify utterances using the following criteria: an utterance is a vocalization or a group of vocalizations separated from all others by either audible ingressive breaths or a perceived silence of 1 second or longer (Lynch, Oller, & Steffens, 1989). Each 30-minute parent-child recording was segmented into 10-minute sections to identify the time-period in which the children produced the most vocalizations. The first 65 utterances from the 10-minute segment with the most utterances were selected for analysis. These utterances were then digitized using a Kay Elemetrics Computerized Speech Laboratory (Model 4300) and a sampling rate of 20 kHz. Of the 65 digital sound files, the first 50 consecutive utterances that were of adequate quality for auditory perceptual judgments (i.e., sufficient in loudness to be classified and without excessive talk-over or background noise), were included in an analysis of vocal development by Ertmer et al. (2007). For each of the participants in the current study, the same 50-utterance samples from two pre-implantation and the first six post-implantation sessions that were analyzed by Ertmer et al. provided the corpus for investigating changes in fundamental frequency range.
To select utterances for intonation analysis, a graduate student in Audiology was trained to identify and select speech-like utterances from the corpus of 50 utterances per child per session. At minimum, speech-like vocalizations were required to contain a voiced vocalic element (Stoel-Gammon, 1989). In addition, all utterances were assigned to linguistic categories that might influence intonation production. For example, the analyst judged each utterance to be either meaningful or non-meaningful. Meaningful utterances (i.e., a single-word utterance or longer vocalization containing at least one word) were identified on the basis of three sources of information: 1) the utterance contained two or more phonemes and the same number of syllables as the presumed adult target word, 2) the word was appropriate for the context, and 3) the parent responded to the utterance in a meaningful way. Both meaningful and non-meaningful utterances were analyzed. Imitations were also transcribed but imitated words are not reported in this paper.
All utterances were also assigned to one of three syllable structure levels based on Stoel-Gammon (1989) and Oller (1980). Level 1 utterances corresponded to a vowel with no consonants (V) or a CV syllable with glottal consonants. Level 2 utterances contained a CV syllable with a supralaryngeal consonant. Level 3 utterances contained a CVC syllable with supralaryngeal consonants. The three syllable types will be designated here as V, CV, and CVC. Instrumental Analysis
Monosyllabic utterances were selected for acoustic analysis from the transcribed samples, excluding those that were very faint, expressed a low level of emotion, or were excessively noisy (e.g., Snow, 1994). For example, utterances were discarded that were perceived to be marginally voiced and less than half of the generated f0 estimates were voiced (0 values). The selection criteria were based on two contextual categories: syllable type with 3 levels (V, CV, and CVC) and linguistic function with 2 levels (non-meaningful vs. meaningful). Thus, there were 6 combinations of categories and levels (“utterance types”). A maximum of ten consecutive utterances in each utterance type was selected for analysis. Thus, the maximum possible size of the acoustically analyzed sample for each child and recording session was 60 monosyllabic utterances.
The utterances meeting the selection criteria were digitized with a 20 kHz sampling rate and the duration and f0 contour of each utterance was measured using the Computerized Speech Laboratory (CSL) signal analysis system from Pentax-Kay Elemetrics. In accordance with procedures described by Allen & Hawkins (1980), the analyzed portion of each syllable was the vocalic nucleus. The beginning and ending boundaries of each vocalic nucleus were set by inspection of wide-band spectrograms. Each boundary began or ended a portion of clear periodicity (i.e., f0 and 2 or more harmonics showed a steady state). The syllable duration was calculated as the difference between the boundaries.
The pitch extraction algorithms of CSL generated the fundamental frequency (f0) contour between the syllable boundaries. Portions of the signal that the automatic routines failed to analyze correctly were edited. That is, the analyst flagged f0 data points that reflected large and abrupt departures from surrounding data points, and these points were deleted from further processing. The analysis program computed the minimum, maximum, mean, and standard deviation of the f0 values for each monosyllabic utterance. The contour was described as “falling” if the maximum f0 preceded the minimum f0 and “rising” if the maximum f0 followed the minimum f0. “Accent range” was calculated as the logarithmic difference between the maximum and minimum f0, expressed in cents (1 octave = 1200 cents). This measure permitted the frequency data to be expressed in terms of perceptually equivalent units (Burns & Ward, 1982). Accent range in cents was calculated by the formula (1200/log2) (log (max f0/min f0)). The database for acoustic analysis included 298 utterances.
Intra-judge and inter-judge reliabilities were based on 10% of the monosyllabic utterances that were acoustically analyzed for each child. The items selected were spaced at equal proportional intervals across the sample. A second person carried out reliability judgments, also on 10% of the sample. The mean differences between the intra-judge reliability analyses were 3 ms (duration), 4 Hz (max f0), 22 Hz (min f0), and 25 Hz (f0 range). The corresponding results of the inter-judge analyses were as follows: 6 ms (duration), 6 Hz (max f0), 26 Hz (min f0), and 30 Hz (f0 range). For comparison, the corresponding inter-judge reliability data in Snow (2006) were comparable to these results: 12 ms for duration, 10 Hz for minimum f0, 6 Hz for maximum f0, and 4 Hz for f0 range. The reliabilities of the present analyses are consistent with those obtained in previous studies of intonation development in infants and toddlers.
Results
The analysis collapsed the data across falling and rising contour classes and across phonetic classes because these variables did not significantly affect intonation development in 1- and 2-year-olds with normal hearing (Snow, 2006). The data were combined for the two pre-implant sessions because these sessions occurred at approximately the same age. The post-implant data comprised 36 child-sessions (6 monthly sessions for each of 6 children). Eight of these child-sessions contained fewer than 4 utterances meeting the selection criteria for acoustic analysis. For this reason, the 6 original sessions were grouped in pairs for the rest of the analysis. Summarizing, the data were combined for the 2 pre-implant sessions, sessions 3 and 4, 5 and 6, and 7 and 8, resulting in 1 pre-implant session and 3 post-implant sessions.
Each child was assigned to one of two groups based on his or her age at the time of implant activation. The younger children (Group 1) were less than 2 years old and the older children (Group 2) were more than 2 years old at the time of implantation. The results for syllable duration and pitch range by child are shown in Table 2. Group totals are shown for the first 3 children (Group 1: age < 24 months) and the last 3 children (Group 2: age > 24 months).
Table 2.
Means of accent range and duration in monosyllabic tones by child and session (group totals reflect all sessions combined).
| Means | |||||
|---|---|---|---|---|---|
| Child/ Group | Session | Age (months) | Duration (msec) | Pitch Range (cents) | N |
| M-10 | 1 | 9 | 458 | 479 | 10 |
| 2 | 13 | 653 | 672 | 9 | |
| 3 | 15 | 369 | 967 | 5 | |
| 4 | 17 | 327 | 1089 | 15 | |
| all | -- | 441 | 820 | 39 | |
| F-18 | 1 | 17 | 446 | 626 | 20 |
| 2 | 21 | 518 | 773 | 10 | |
| 3 | 23 | 500 | 622 | 26 | |
| 4 | 25 | 314 | 395 | 20 | |
| all | -- | 439 | 583 | 76 | |
| F-20 | 1 | 19 | 475 | 329 | 7 |
| 2 | 23 | 484 | 316 | 8 | |
| 3 | 25 | 362 | 284 | 8 | |
| 4 | 27 | 285 | 333 | 19 | |
| All | -- | 369 | 320 | 42 | |
| < 24 months | All | -- | 421 | 572 | 157 |
| F-28 | 1 | 27 | 356 | 939 | 5 |
| 2 | 31 | 407 | 779 | 9 | |
| 3 | 33 | 1065 | 1237 | 11 | |
| 4 | 35 | 391 | 685 | 14 | |
| all | -- | 580 | 895 | 39 | |
| F-30 | 1 | 29 | 270 | 1060 | 15 |
| 2 | 33 | 300 | 789 | 16 | |
| 3 | 35 | 374 | 640 | 16 | |
| 4 | 37 | 273 | 420 | 15 | |
| all | -- | 305 | 727 | 62 | |
| M-36 | 1 | 35 | 254 | 744 | 8 |
| 2 | 39 | 529 | 514 | 21 | |
| 3 | 41 | 397 | 412 | 8 | |
| 4 | 43 | 359 | 1060 | 3 | |
| All | -- | 435 | 580 | 40 | |
| > 24 months | All | -- | 418 | 732 | 141 |
| All | All | -- | 420 | 647 | 298 |
The data in Table 2 were submitted to an analysis of variance (ANOVA) with a mixed 4×2 factorial design. The dependent variable was accent range or width of the pitch contour (represented by mean scores calculated by child within session). The between-subjects variable was age group (2 levels) and the within-subjects variable was session (4 levels). The ANOVA (see Table 3, Part 1: All 4 sessions) did not reveal any main effects or interactions. The absence of significant findings was probably due to the large variability within and across children.
Table 3.
Summary of statistical tests (dependent variables - Accent range and Duration).
| Part (sessions analyzed) | Accent Range | Duration | |||
|---|---|---|---|---|---|
| Main Effects/Interaction | df | F Statistic | p Value | F Statistic | p Value |
| Part 1: ANOVA (all 4 sessions) | |||||
| Age Group (A) | 1, 4 | 1.607 | 0.274 | 0.056 | 0.825 |
| Implant Experience (E) | 3, 12 | 0.062 | 0.979 | 2.294 | 0.130 |
| A*E | 3, 12 | 0.552 | 0.656 | 2.185 | 0.143 |
| Part 2: ANOVA (First 2 sessions) | |||||
| Age Group (A) | 1, 4 | 3.628 | 0.130 | 13.127 | 0.022 |
| Implant Experience (E) | 1, 4 | 2.518 | 0.188 | 4.831 | 0.093 |
| A*E | 1, 4 | 22.017 | 0.009 | 0.076 | 0.796 |
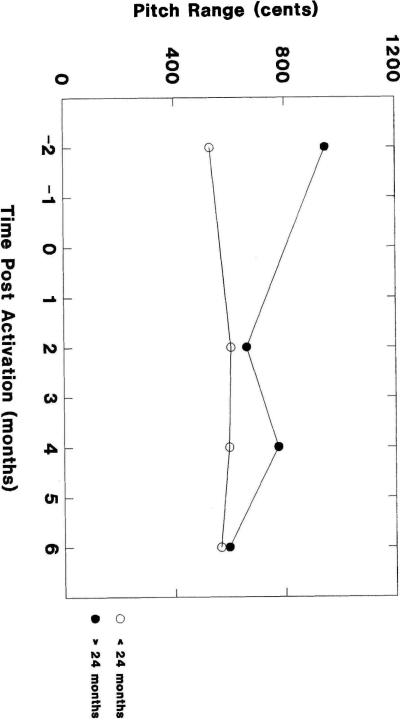
In spite of null findings for main effects, the interaction between age and implant experience approached significance. To illustrate the trend in the direction of an interaction, the individual data from Table 2 are plotted in Figure 1 by age group and session. Inspection of Figure 1 suggested that the interaction was greatest between the pre-implant data collection session (1 month before the child received the implant) and the first post-activation session (1 and 2 months after the implant had been activated). To further investigate this trend, the data for these 2 sessions (the first half of the period of study) were submitted to a second ANOVA with a mixed 2×2 factorial design. Again, the dependent variable was accent range, the between-subjects variable was age group (2 levels), and the within-subjects variable was session (2 levels). The results are summarized in Table 3, Part 2: First 2 sessions.
Figure 1.
Accent range by age group and session.
As in the first ANOVA, the results of the second analysis did not reveal any main effects. However, there was a highly significant interaction between chronological age and implant experience. This interaction indicated that the initial effects of post-implant hearing experience on the children's intonation development were different for younger versus older children.
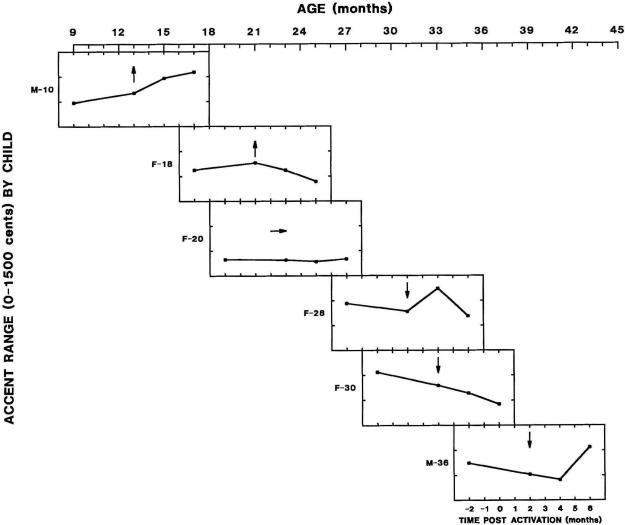
To study this robust interaction effect in more detail, we plotted the individual data profiles for the 6 children by implant experience and chronological age (see Figure 2). Implant experience (the number of months preceding or following activation of the implant) is plotted on the horizontal axis of each individual child's graph. To study the effect of maturation, the children's individual data plots were aligned by chronological age (increasing from left to right).
Figure 2.
Accent range by child, chronological age (months) and implant experience (months).
For each child, an arrow indicates the first session after activation of the child's implant (aggregated data from data collection events at 1 and 2 months post activation). The reader may recall that these two sessions represent the only change that was significant in the ANOVAs as part of the interaction between implant experience and age. Each arrow points directly upward, downward, or horizontally to indicate, respectively, whether the change in accent range between sessions 1 and 2 represented an increase, a decrease, or no change. An increase or decrease was defined as a mean accent range that differed by more than 100 cents or about 6%, the just noticeable difference in pitch change (Rossi, 1978). “No change” was defined as difference that was less than 100 cents. The direction of the arrow in the individual graphs illustrates the relation between implant experience, chronological age, and the type of pitch range development that the child demonstrated.
Inspection of Figure 2 indicates that there was no consistent effect of implant experience on the initial change in accent range after the onset of post-implant hearing. That is, the change was sometimes positive, sometimes negative and, in one instance, no change was observed. Chronological age also did not consistently predict a uniform change in intonation. An initial change toward a narrower accent range, for example, occurred at 31 months, 33 months, and 39 months. Based on visual inspection of the data, these findings are consistent with the statistical results that failed to indicate a significant effect of either chronological age or implant experience.
However, Figure 2 also indicates that chronological age is associated in a categorical way with general phases of intonation development. That is, children who received CIs at 18 months or younger had an initial increase in accent range; children who received CIs after 24 months had a sharp decrease; and one child who was between these age groups demonstrated no change. This pattern accounts for the significant interaction, which indicated that the effects of the first months of implant experience were different for younger versus older children.
Discussion
This study investigated the development of intonation in deaf children who received a cochlear implant between the ages of 10 and 36 months. The findings indicated that neither amount of cochlear implant experience nor chronological age alone predicted the development of intonation. However, there was a robust interaction between implant experience and age. That is, the effects of the first two months of implant experience on intonation varied depending on the child's age. Relative to the pre-implant production data, the youngest children demonstrated an increase in accent range and the oldest children a decrease. One child between the youngest and oldest did not demonstrate any change during the period that spanned the initial transition from pre-implant to post-implant experience.
These qualitatively different changes in intonation correspond nicely to the first three stages of development that we proposed in the introduction for typically developing children. In the first stage, normative data predicted an increase in accent range occurring at 2 or 3 months with the onset of cooing; in the second stage, there is no change for several months; and finally a sharp decrease or regression occurs at 9 or 10 months. At 2 months post-activation, then, the children with CIs match the same developmental milestones as normally hearing children but at different chronological ages. Older children (24 to 36 months) benefited more from the initial months of implant experience than younger children (9 to 24 months). In sum, the effects of early implant experience appear to be contingent on age at implantation.
The important developmental implication is that maturation alone accounts for at least part of the acquisition of intonation. Quantitatively, the relationship between maturation and hearing experience, as predictors of intonation development, is captured by the following formula, where A = Age (mos.), E = Implant or hearing experience (mos.), and k = constant (35): A + 2.5*E = k. For example, a child with normal hearing at 10 months (corresponding to the regression stage) would have a score of 10 + 2.5(10) = 35. A child with a cochlear implant in the current study, with 2 months of implant experience, would be at the same stage of intonation development and would have the same score at 30 months: 30 + 2.5(2) = 35.
This study suggests that amount of implant hearing experience, as expected, is a strong predictor of intonation development (2½ times the effect of chronological age). Consistent with the finding of a significant interaction, chronological age also accounts for some aspects of intonation development, albeit to a lesser extent. This implies that, even before children are implanted and despite limited exposure to speech during hearing aid trials, they are developing other skills that serve as a prelinguistic foundation for intonation, for example, cognitive, social-emotional, pragmatic, gestural, and prelinguistic communication skills. The result is that, once these older children perceive speech through an implant, they make greater short-term gains in intonation development than younger children with the same amount of CI-assisted hearing experience. This offers preliminary support for the claim that the linguistic system of intonation springs from nonlinguistic and early-developing realms of psychological experience as well as access to auditory information (Snow, 2006). In sum, older children might benefit more from the initial months of CI-assisted hearing (and are better able to `close the gap' with normally hearing children) because they have more mature abilities in the areas that serve as nonverbal precursors of intonation, namely cognitive, social-affective, and pragmatic skills. Similar conclusions have been reported in studies of segmental and lexical development in young children with cochlear implants: “Compared to younger age recipients, the older recipients' greater physical, cognitive, and social maturity as well as their more extensive experience with speech intervention appears to have been advantageous for early speech development” (Ertmer et al., 2007, p. 404).
The interpretation of the findings, however, must be tentative. One drawback of this study is the small number of subjects, which casts doubt on the statistical results. However, more important than the inferential findings is the fact that all 6 of the children demonstrated a profile of intonation development that meshes nicely with predictions based on models of intonation in typically developing children.
Second, a possible alternative explanation of the role of maturation is based on the observation that the children had some hearing experience via amplification before implantation. It is plausible that the older group would have more pre-implant hearing experience than the younger group and therefore would have benefited from more exposure to f0 patterns in the input. For each child, the amount of exposure depends on the age at which the hearing loss was identified and the length of the child's hearing aid trial. Inasmuch as these factors varied considerably among the children, we might expect more variability in the results than the data indicated. In fact, the remarkable consistency of the results across all 6 children would not be expected if widely varying amounts of pre-implant hearing experience were a significant predictor. For example, F-30 had a longer hearing aid trial than the other children but her intonation development after implantation was predicted by the same interaction of age and implant experience that accounted for the findings generally. In future research, however, the role of pre-implant hearing experience needs to be carefully evaluated in larger scale studies.
In summary, this study focused on 6 deaf children who received a CI at or before the age of three years. Their intonation production data suggested that children benefited from the first 2 months of implant experience; all 6 children demonstrated a change in intonation behavior corresponding to one of the milestones of intonation development that have been observed or inferred in children with normal hearing during the first year of life. As such, their gains suggest that cochlear implants, although previously shown to provide rather limited information for intonation perception and production (Ciocca et al., 2002; Lin et al., 2007; Peng et al., 2007), nonetheless influence the acquisition of intonation in a positive manner.
The results also showed that the amount of implant experience alone does not predict the child's stage of development. Instead, there is a marked interaction with chronological age at implantation. After the same amount of implant experience, older implant recipients demonstrated a more advanced stage of intonation development than younger children. To account for this robust effect of chronological age, we hypothesize that early developing social-emotional experience and pragmatic skills serve to scaffold the later development of intonation as a linguistic system. Given that these precursors of intonation are nonlinguistic or even nonverbal (hence independent of audition), children might reach some early milestones without delays, long before auditory language experience begins. It is plausible, then, that these prelinguistic achievements will accelerate the acquisition of intonation when young CI recipients are able to perceive pitch contours more clearly in the input.
Among clinical implications, these findings suggest that children implanted early (before 24 months) or late (after 24 months) subsequently demonstrate unmistakable but unequal advances in intonation that favor the older children. In the special case of intonation, the potentially adverse effects of late implantation might be mitigated considerably by the fact that so much of intonation development appears to be related to prelinguistic and nonlinguistic experiences as well as audition. The important long-range question is to determine the effects of early vs. late implantation when the children reach the preschool or early school-age years. At present, the preliminary findings suggest that when children are implanted late, and are therefore delayed at 2 or 3 years, they will “catch up” to a considerable extent with normally hearing children by the age of 4 or 5 years. Further studies with larger numbers of participants are underway to examine this possibility and to investigate other factors (e.g., pre-implant and CI-aided hearing levels) that might influence intonation development following cochlear implantation at a very young age.
ACKNOWLEDGMENTS
This research was funded in part by NIH grants R03DC04226 and R01DC007863 awarded to David Ertmer. We would like to thank Jennifer Slanker for her contributions to the data analysis portions of this research and we thank the children and families who made this study possible.
References
- Allen GD, Hawkins S. Phonological rhythm: Definition and development. In: Yeni-Komshian GH, Kavanagh JF, Ferguson CA, editors. Child phonology. Vol. 1: Production. Academic Press; New York: 1980. pp. 227–256. [Google Scholar]
- Barry J, Blamey P, Lee K, Cheung D. Differentiation in tone production in Cantonese-speaking hearing-impaired children. International Conference on Spoken Language Processing – 2000.2000. pp. 669–672. [Google Scholar]
- Bates E, Camaioni I, Volterra V. The acquisition of performatives prior to speech. Merrill-Palmer Quarterly. 1975;21:205–226. [Google Scholar]
- Burns EM, Ward WD. Intervals, scales, and tuning. In: Deutsch D, editor. The psychology of music. Cambridge University Press; New York: 1982. pp. 241–269. [Google Scholar]
- Capute AJ, Palmer FB, Shapiro BK, Wachtel RC, Schmidt S, Ross A. Clinical linguistic and auditory milestone scale: Prediction of cognition in infancy. Developmental Medicine and Child Neurology. 1986;28:762–771. doi: 10.1111/j.1469-8749.1986.tb03930.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Peng S-C. Processing F0 with cochlear implants: Modulation frequency discrimination and speech intonation recognition. Hearing Research. 2008;235:143–156. doi: 10.1016/j.heares.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocca V, Francis AL, Aisha R, Wong L. The perception of Cantonese lexical tones by early-deafened cochlear implantees. The Journal of the Acoustical Society of America. 2002;111:2250–2256. doi: 10.1121/1.1471897. [DOI] [PubMed] [Google Scholar]
- Cruttenden A. Intonation. Second Edition Cambridge University Press; Cambridge: 1997. [Google Scholar]
- Crystal D. A dictionary of linguistics and phonetics. 3rd edition Blackwell; Oxford: 1991. [Google Scholar]
- Elliott L, Katz D. Development of a new children's test of speech discrimination. Audiotec; St. Louis, MO: 1980. [Google Scholar]
- Ertmer DJ, Young NM, Nathani S. Profiles of vocal development in young cochlear implant recipients. Journal of Speech, Language, and Hearing Research. 2007;50:393–407. doi: 10.1044/1092-4388(2007/028). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald A, Kuhl PK. Acoustic determinants of infant preference for motherese speech. Infant Behavior and Development. 1987;10:279–293. [Google Scholar]
- Kuo Y, Rosen S, Faulkner A. Acoustic cues to tonal contrasts in Mandarin: Implications for cochlear implants. Journal of the Acoustical Society of America. 2008;123:2815–2824. doi: 10.1121/1.2896755. [DOI] [PubMed] [Google Scholar]
- Lenden JM, Flipsen P., Jr. Prosody and voice characteristics of children with cochlear implants. Journal of Communication Disorders. 2007;40:66–81. doi: 10.1016/j.jcomdis.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Lin Y-S, Lee F-P, Huang I-S, Peng S-C. Continuous improvement in Mandarin lexical tone perception as the number of channels increased: A simulation study of cochlear implants. Acta Oto-Laryngologica. 2007;127:505–514. doi: 10.1080/00016480600951434. [DOI] [PubMed] [Google Scholar]
- Locke JL. Phonological acquisition and change. Academic Press; New York: 1983. [Google Scholar]
- Lowe M, Costello AJ. The Symbolic Play Test. NFER-Nelson; London: 1988. [Google Scholar]
- Lynch MP, Oller DK, Steffens M. Development of adult-like vocalizations in a child with congenital absence of cochleas: The case of total deafness. Applied Psycholinguistics. 1989;10:315–333. [Google Scholar]
- MacNeilage PF, Davis BL. Acquisition of speech production: The achievement of segmental independence. In: Hardcastle WI, Marchal A, editors. Speech production and speech modeling. Kluwer; Dordrecht: 1990. [Google Scholar]
- Most T, Peled M. Perception of suprasegmental features of speech by children with cochlear implants and children with hearing aids. Journal of Deaf Studies and Deaf Education. 2007;12:350–361. doi: 10.1093/deafed/enm012. [DOI] [PubMed] [Google Scholar]
- Newborg J, Stock JR, Wnek L. Battelle Developmental Inventory. 1st ed. Riverside Publishing; Itaska, IL: 1984. [Google Scholar]
- Oller DK. The emergence of the sounds of speech in infancy. In: Yeni-Komshian G, Kavanagh JF, Ferguson CA, editors. Child phonology: Vol. 1. Production. Academic Press; New York: 1980. pp. 93–112. [Google Scholar]
- Peng S-C, Tomblin JB, Spencer LJ, Hurtig RR. Imitative production of rising speech intonation in pediatric cochlear implant recipients. Journal of Speech, Language, and Hearing Research. 2007;50:1210–1227. doi: 10.1044/1092-4388(2007/085). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M. Interaction of intensity glides and frequency glissandos. Language and Speech. 1978;21:49–72. doi: 10.1177/002383097802100414. [DOI] [PubMed] [Google Scholar]
- Shriberg LD, Kwiatkowski J, Rasmussen C. The Prosody-Voice Screening Profile. Communication Skill Builders; Tucson, AZ: 1990. [Google Scholar]
- Snow D. Polysyllabic units in the vocalizations of children from 0;6 to 1;1: Intonation groups, tones, and rhythms. Journal of Child Language. 2007;34:765–797. [PubMed] [Google Scholar]
- Snow D. Regression and reorganization of intonation between 6 and 23 months. Child Development. 2006;77:281–296. doi: 10.1111/j.1467-8624.2006.00870.x. [DOI] [PubMed] [Google Scholar]
- Snow D. Phrase-final syllable lengthening and intonation in early child speech. Journal of Speech and Hearing Research. 1994;37:831–40. doi: 10.1044/jshr.3704.831. [DOI] [PubMed] [Google Scholar]
- Stoel-Gammon C. Prespeech and early speech development of two late talkers. First Language. 1989;9:207–224. [Google Scholar]