Abstract
The cyclin E-CDK2 complex accelerates entry into the S phase of the cell cycle and promotes polyploidy, which may contribute to genomic instability in cancer cells. The impact of low-molecular weight isoforms of cyclin E (LMW-E) overexpression on mitotic progression and its link to genomic instability were the focus of this study. Here, we show that full-length cyclin E (EL) and LMW-E overexpression impair the G2-M transition differently by targeting dual-specificity phosphatase Cdc25C activity. We identify Cdc25C as an interaction partner and substrate for cyclin E/CDK2 kinase. Specifically, the cyclin E/CDK2 complex phosphorylates Cdc25C on Ser 214, leading to its premature activation, which coincides with higher cyclin B/CDK1 and polo-like kinase 1 (PLK1) activities in an S phase–enriched population that result in faster mitotic entry. Whereas EL overexpression leads to hyperactivation of Cdc25C, cyclinB/CDK1, and PLK1 in a G2-M–enriched population, LMW-E overexpression causes premature inactivation of Cdc25C and PLK1, leading to faster mitotic exit. In addition, LMW-E–overexpressing cells showed a reduction in the mitotic index in the presence of a spindle poison and faster degradation of cyclin B, suggesting an increased rate of mitotic slippage and adaptation to the spindle checkpoint. Lastly, downregulation of Cdc25C inhibits LMW-E–mediated chromosome missegregation, anaphase bridges, and centrosome amplification. These results suggest that the high levels of LMW-E isoforms found in breast cancer may contribute to cellular transformation and genomic instability by impairing mitotic progression involving Cdc25C.
Keywords: Mitosis, Cdc25C, Cyclin E, LMW-E, PLK1, Cyclin B/CDK1
Introduction
Cyclin E, a regulatory subunit of cyclin-dependent kinase 2 (CDK2) (1), is critical for entry into the S phase in mammalian cells (2-4). Cyclin E induces histone biosynthesis at the G1/S transition and DNA replication and centrosomal duplication during S phase. However, cyclin E deregulation also has a direct effect on mitosis that leads to genomic instability. When full-length cyclin E is overexpressed through inhibition of proteasome-mediated degradation, high levels of cyclin E-associated kinase activity are found in the G2/M phases (5). Additionally, overexpression of full-length cyclin E in U2OS cells resulted in the accumulation of cells with impaired chromosomal alignment and delayed mitotic progression in prometaphase (6). Full-length cyclin E/CDK2 can phosphorylate and inactivate the mitotic ubiquitin ligase APC/CCdh1, thereby increasing cyclin B accumulation in prometaphase and metaphase (6). The regulation of cyclin B expression and its role in activating CDK1 are crucial for controlling the timing of both entry into and exit from mitosis (7). Prior to mitosis, cyclin B-CDK1 complexes are kept in an inactive state by phosphorylation of CDK1 at Thr14 and Tyr15, which is catalyzed by the protein kinases Wee1 and Myt1 (reviewed in (8)). This complex is dephosphorylated by the dual-specificity protein phosphatase Cdc25C. CDK1 activation, upon entry into mitosis, results from simultaneous inhibition of Wee1 and Myt1 and activation of the phosphatase Cdc25C. The phosphorylation of Cdc25C by Polo-like kinase 1 (PLK1) or cyclin B-CDK1 complex is required for its activation, suggesting the presence of a positive feedback loop. Exit from mitosis requires inactivation of cyclin B-CDK1 by APC/Ccdc20-targeted proteosomal degradation of cyclin B, which occurs during metaphase-to-anaphase transition. Any modulation of this finely orchestrated process of activation and inactivation of cyclin B/CDK1 can result in mitotic defects, resulting in altered chromosome segregation and formation of aneuploid cells (9).
In the most aggressive cancers, which metastasize, full-length cyclin E is proteolytically cleaved to its low molecular weight (LMW-E) isoforms from the N-terminal elastase cleavage of the 50-kDa full-length cyclin E1 (termed EL) (10, 11). The LMW-E isoforms are tumor specific, predominantly cytoplasmic (12), and have enhanced biochemical and biological properties that differ from those of EL. The LMW-E isoforms are resistant to inhibition by the CDK inhibitors p21 and p27 (13, 14). Since both EL and LMW-Es are overexpressed in tumors from breast cancer patients (10), we questioned which phenotype, EL or LMW-E predominate. We have recently addressed this question (in the accompanying manuscript) by overexpressing both EL and LMW-E in cells and asking which one can take over the phenotype when length of mitosis is the read out. The result revealed that when EL and LMW-E are co-overexpressed, the LMW-E takes over as shown by the genomic instability and shortening of the period between nuclear envelope breakdown and Anaphase A as compared to EL exopression. In this manuscript we hypothesized that overexpression of either the EL or LMW-E isoforms alters the timing of the M-phase progression (entry and exit) by targeting specific phosphorylation events and upregulating mitotic kinases, leading to polyploidy and genomic instability.
Materials and Methods
Plasmids
Human pEX2T Cdc25C, GST-25(200-256), and GST-25(200-256) S216A were kindly provided by Yolanda Sanchez (15, 16) and human Cdc25C–pCMV-Myc was kindly provided by Jian Kuang. The QuikChange Site-directed Mutagenesis Kit (Stratagene) was used according to the manufacturer's instructions with the following oligonucleotides to substitute human Cdc25C Ser214 to Ala: 5′-cct ata tcg cgc ccc gtc gat gcc aga gaa c-3′and 5′-gtt ctc tgg cat cga cgg ggc gcg ata tag g- 3′.
Cell cultures and transfections
The generation of the EL and LMW-E inducible MCF-7 cell lines are described in the accompanying manuscript. MRC-50 human primary fibroblasts (American Type Culture Collection) derived from normal lung tissue of a 14-week-old male fetus is a normal diploid human cell line with 46,XY karyotype with only a 3.6 % rate of polyploidy and is cultured in DMEM + 10% FCS at 37°C.
Synchronization, cell cycle analysis and measurement of mitotic index
To generate synchronized populations, MCF-7 stable pTRE-EL/LMW-E cells were arrested at the G1/S boundary by aphidicolin block (5 μg/ml) for 24 h. After release, cells were collected, fixed in ice-cold 70% ethanol and stained with propidium iodide for flow cytometry analysis. These experiments were repeated seven times with each cell line and results are provided as western blot analysis (with representative experiments) and flow cytometric and kinase analyses (where the values of all experiments were combined, averaged and statistical analysis on time points showing the significant differences are provided as bar graphs). MRC50 human primary fibroblasts were treated with nocodazole (100 ng/ml), fixed, and stained with propidium iodide for flow cytometry analysis. To measure the mitotic index in live cells, we used HeLa H2B GFP stable cells (17) which were infected with adenovirus EL or LMW-E for 24 hours and then treated with nocodazole (100 ng/ml) for 16 hours and mitotic cells were counted microscopically.
RNA interference
Cdc25C protein was depleted using four different siRNA duplex oligonucleotides (Dharmacon Research). SiRNA transfections were performed using the X-tremeGENE siRNA transfection reagent (Roche). ON-TARGETplus SMARTpool siRNA Cdc25C oligonucleotides (Dharmacon Inc., Lafayette, CO) were made with the following sequences: GAAACUUGGUGGACAGUGA; AGGAAGGGCUUAUGUUUAA; GAGAGAGACACUUCCUUUA; GGGCAAAUUUCUUGGUGAU. Experiments using siRNAs were harvested at 72 hours post transfection unless otherwise specified.
Immunofluorescence (IF) staining
pTRE-EL and pTRE-LMW cells were grown in six-well plates with cover slides, induced or uninduced with doxycycline and then treated with control siRNA or with Cdc25C siRNA. IF staining was performed as described in the accompanying manuscript. Primary antibodies included mouse anti-® tubulin (1:1000; Sigma-Aldrich), rabbit anti-γ tubulin (1:1000; Sigma-Aldrich), rabbit anti-pericentrin (1:500; Abcam), mouse anti-PLK1 (1:200; BD Pharmingen), rabbit anti-Cdc25C (1:100; Santa Cruz Biotechnology).
SDS-PAGE electrophoresis, western blotting, immunoprecipitation, and kinase assay
These assays were performed as previously published (12). The antibodies used were as follows: cyclin A, anti-cyclin E (HE-12), anti-c-Myc, polyclonal anti-cyclin B1, and anti-Cdc25C (all from Santa Cruz Biotechnology); monoclonal anti-β-tubulin, polyclonal and monoclonal anti-γ-tubulin, and monoclonal anti-Flag (all from Sigma); polyclonal anti-pericentrin (Abcam); and polyclonal anti-PLK1 and vinculin (Sigma).
Substrates used for kinase assays were 5 μg of histone H1 (Roche Diagnostics) and α–casein or 10-20 μg of GST-Cdc25C. Where Roscovitine was used, beads were incubated at final step of the kinase assay in reaction buffer plus 80μM Roscovitine at 37°C for 30 minutes.
Glutathione-S-transferase pull-down assay
In vitro transcription and translation of cyclin E constructs (EL and LMW-E) and Cdc25C proteins were performed using the TNT T7 Quick Coupled Transcription/Translation system (Promega, Madison, WI) as previously described (18).
Phosphatase activity assay
Cdc25C activity was measured using 3-O-methylfluorescein phosphate (3-OMFP) as described previously (19). Values were corrected for background activity by measuring the activity of GST-Cdc25C lacking its catalytic domain or of lysates IPed with IgG antibody. The activity of GST-Cdc25C was used as a positive control.
Statistical analysis
Statistical analyses were performed using GraphPad Prism Software with two-way ANOVA or Stats Direct Software. All statistical tests were two-sided and were considered to be significant at P < 0.05.
Results
LMW-E overexpression induces premature activation of Cdc25C and Cyclin B/CDK1
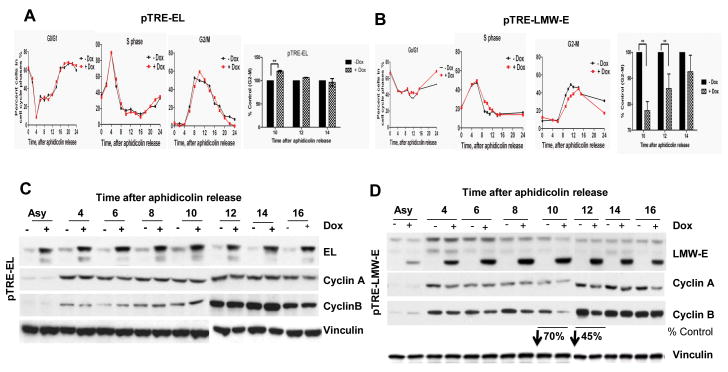
To examine the impact of EL and LMW-E overexpression on the progression of cells through mitosis, we measured the expression of key cell cycle proteins and the activity of key mitotic enzymes, including CDK1 and PLK1 in synchronized population of cells. MCF-7 cells inducibly expressing Flag-tagged EL or LMW-E were synchronized at the G1/S boundary with aphidicolin, induced for 24 h, and harvested at different time points following release from aphidicolin arrest. Flow cytometric analysis revealed that 10 h after release from aphidicolin arrest, 20% more EL-overexpressing cells than uninduced cells had accumulated in the G2/M phase of the cell cycle (n = 3, P < 0.001; Fig. 1A). In contrast, the G2-M population of LMW-E–overexpressing cells was 20-30% less than that of uninduced cells 10-12 h after aphidicolin release (Fig 1B). This occurred concomitantly with an increase in the G0/G1 and S phase populations (Fig. 1B). The EL- overexpressing cells showed no significant change in cyclin B1 expression 10-12 h after aphidicolin release (Fig. 1C), while the LMW-E–overexpressing cells showed a 45-70% reduction in cyclin B1 levels (Fig. 1D).
Figure 1. Overexpression of EL and LMW-E impair G2-M transition differently.
A, pTRE-EL and B, pTRE-LMW-E MCF-7 cells were synchronized at the G1/S transition by aphidicolin block, induced for cyclin E expression with doxycycline (Dox, 1μg/ml) for 24 h, released for the indicated times and analyzed by flow cytometry. The graphs show the percentages of cells in the G0/G1, S, and G2/M phases. Right panel, results are normalized to the percentage of uninduced cells in G2/M. Each bar indicates the percentage of cells in G2/M relative to control cells +/- SD (standard deviation) obtained from seven independent experiments. C, D, Representative western blot analysis of cell lysates described in A and B using specific antibodies against cyclins E, A, and B, and vinculin. Changes in cyclin B expression is as percentage of control after densitometry of uninduced and induced cells (*P < 0.05).
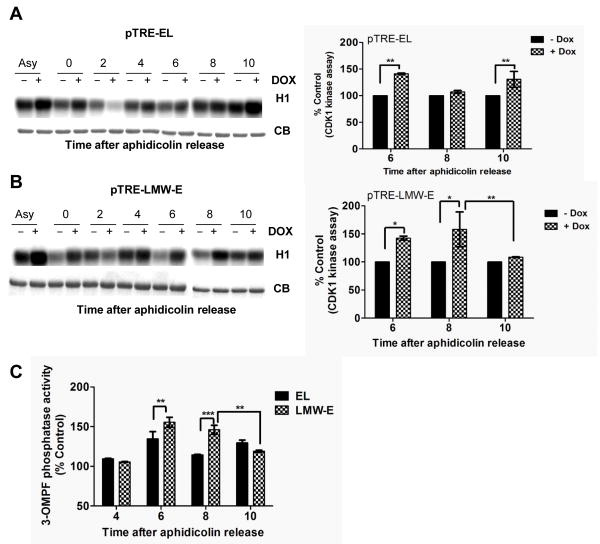
Next, we examined whether the activity of cyclin B/CDK1 is differentially altered by EL versus LMW-E induction using histone H1 as a substrate. The results revealed the premature activation of CDK1 upon induction of both EL (30-40% increase) and LMW-E (40-58% increase) 6-8 h after aphidicolin release compared to uninduced cells (Fig. 2A and B). CDK1 activity is regulated by phosphorylation of residues Thr14 and Tyr15 by the Wee1/Myt kinases and by dephosphorylation of these residues by Cdc25C phosphatases (20-22). Thus, we next examined whether the EL- and LMW-E–mediated premature activation of cyclin B/CDK1 was caused by regulation of Cdc25C phosphatase activity. We measured the activity of Cdc25C in aphidicolin-synchronized populations of EL- and LMW-E–induced cells using 3-OMPF as a substrate (Fig. 2C). These analyses showed that upon induction of EL and LMW-E, the initial burst of Cdc25C activation in the early S phase was larger than that in the uninduced control cells (Fig. 2C). These results coincide with the higher activity of cyclin B/CDK1 in these cells upon induction of EL and LMW-E. Specifically, at 10hr post aphidicolin release in the LMW-E–expressing cells, the Cdc25C activity dropped by 27%, while it remained high in the EL-expressing cells. This LMW-E mediated decreased in Cdc25C activity is consistent with a drop in cyclin B1/CDK1 activity in these cells (Fig. 2B).
Figure 2. Overexpression of EL and LMW-E deregulate cyclinB/CDK1 and Cdc25C activities differentially.
A, pTRE-EL and B, pTRE-LMW-E cells were synchronized at the G1/S transition by aphidicolin block, induced with Dox. (1μg/ml) for 24 h, released for the indicated times, and subjected to immunoprecipitation (IP) with a cyclin B1 antibody. Kinase activity was measured using histone H1 as the substrate. Coomassie-stained gel of the IgG (CB) is used as a loading control. Right panels, quantitation of cyclin B/CDK1 kinase activity normalized to activity in uninduced cells +/- SD obtained from seven independent experiments. C, Cell lysates treated and prepared in A and B were used to measure the 3-OMFP phosphatase activity of Cdc25C. Cells were IPed with Cdc25C and release of phosphate from 3-OMFP was measured by an associated increase in fluorescence. Fluorescent emissions values were corrected for background activity by subtracting the values from IgG IPs. Each bar indicates percent 3-OMPF phosphatase activity relative to control cells +/- SD obtained from seven independent experiments. *P < 0.05, **P < 0.01, ***;p< 0.001.
Cdc25C is a binding partner and substrate of the cyclin E/CDK2 complex
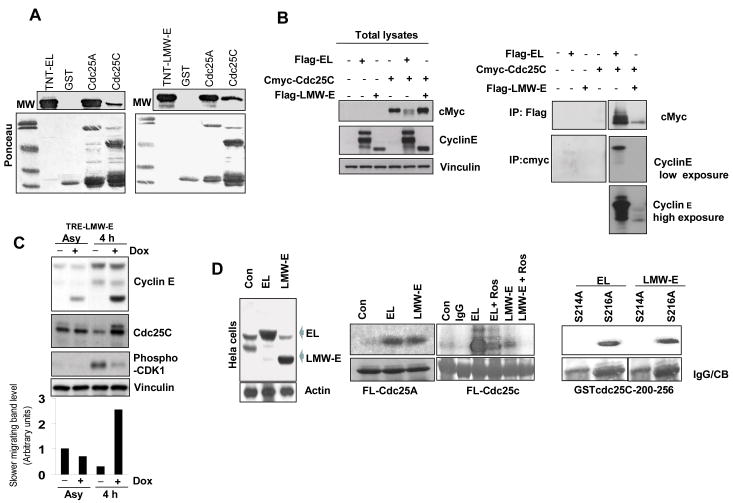
To understand the mechanism by which EL and LMW-E deregulate Cdc25C activity, we first sought to determine whether there is a functional interaction between Cdc25C and cyclin E. GST pull down assays revealed that both EL (Fig. 3A, left panel) and LMW-E (Fig. 3A, right panel) bind to full-length Cdc25C. We also observed an interaction between both EL and LMW-E and Cdc25A (Fig. 3A), as previously described (23). To confirm these in vitro interactions in an ex vivo setting, we transiently transfected human embryonic kidney 293T cells with Flag-tagged EL or LMW-E and c-Myc–tagged Cdc25C expression constructs and examined complex formation in cell lysates (Fig. 3B). Both EL and LMW-E formed immune complexes with Cdc25C, but the EL/Cdc25C complexes were more abundant than LMW-E/Cdc25C complexes (Fig. 3B, right panels). In addition, western blot analysis showed that Cdc25C from LMW-E–overexpressing cells migrated more slowly 4 h after aphidicolin release, suggesting a post-translational event (Fig. 3C). Specifically, upon induction of LMW-E, there was a 3 fold increase in the slower migrating Cdc25C band compared to uninduced LMW-E or induced EL cells (Fig 3C, bar graph). Next, we asked whether the migration shift is caused by Cdc25C phosphorylation, leading to its activation and subsequent cyclin B/CDK1 activation. Using a specific phospho-CDK1 antibody, we observed a lower level of CDK1/pY15 in LMW-E–overexpressing cells, suggesting an increase in CDK1 activity (Fig. 3C). Next, we measured the ability of the EL/CDK2 versus LMW-E/CDK2 complexes to phosphorylate full-length GST-Cdc25C. HeLa cells were used as they (i) do not have the machinery to process full-length cyclin E, (ii) have similar population doubling times to MCF-7 (24 hrs HeLa; 29 hrs MCF-7) and (iii) both have similar cell cycle profiles following release from aphidicolin arrest (24). EL or LMW-E were overexpressed in HeLa cells by adenoviral infection (Fig. 3D, left panel) and assayed for cyclin E–associated kinase activity using full-length Cdc25A and Cdc25C (Fig. 3D, middle panels) as substrates. While both EL and LMW-E–overexpressing HeLa cells could phosphorylate full-length GST-Cdc25A and GST-Cdc25C, the amount of phosphorylated GST-Cdc25C was three fold higher in EL than in LMW-E–overexpressing cells (Fig. 3D, middle panel). This difference was specific to GST-Cdc25C, as the EL and LMW-E complexes phosphorylated GST-Cdc25A to the same extent. In addition, the GST-Cdc25C phosphorylation was specific, as the CDK2 inhibitor Roscovitine attenuated the phosphorylation by about 80% in both EL- and LMW-E–overexpressing cells (Fig. 3D, middle panel). Lastly, we examined the site(s) on Cdc25C which cyclin E/CDK2 can phosphorylate (Fig. 3D, right panel). During interphase, Cdc25C is inhibited by Ser 216 phosphorylation, but during mitosis Cdc25C is phosphorylated on Ser 214, which in turn prevents phosphorylation of S216 (25). HeLa cells were infected with LMW-E adenovirus, synchronized in G1-S with aphidicolin and subjected to a cyclin E–associated kinase–activity assay using S214A or S216A GST-Cdc25C 200-256 as substrates. We observed that only GST-Cdc25C 200-256 containing S216A, and not the construct containing S214A, was phosphorylated by both the EL/CDK2 and LMW-E/CDK2 complexes (Fig. 3D, right panel). Collectively, these experiments suggest that EL and LMW-E directly interact with and phosphorylate Cdc25C on Ser 214, leading to its premature activation during the S phase, followed by dephosphorylation of CDK1 on T14 and Y15 and activation of cyclin B/CDK1, which promotes premature mitotic entry. The difference between the EL and LMW-E complexes is the extent and timing of Cdc25C phosphorylation. Specifically, it is the transient activity of LMW-E/CDK2 in G2/M transition, which leads to a transient activation of cyclin B/CDK1 via phosphorylation of Cdc25C that results in the faster mitotic exit in LMW-E overexpressing cells.
Figure 3. EL and LMW-E interact with and phosphorylate Cdc25C.
A, GST pull-down assay showing the interaction of 35S-labeled EL (left panel) and LMW-E (right panel) with GST-tagged fusion proteins. Ponceau-stained gel is shown below. B, 293T cells were cotransfected with Flag-tagged EL or LMW-E and c-myc-Cdc25C for 24 h. Left panel, total lysates were analyzed by western blot analysis to show expression of transfected proteins. Vinculin used as loading control. Right panel, IP was performed with monoclonal anti-Flag or a polyclonal anti-c-Myc followed by western blotting with the indicated antibodies. C, pTRE-LMW-E MCF-7 cells were synchronized with aphidicolin for 24 h, induced for 24 h, released for 4 h, and analyzed by western blotting for cyclin E, Cdc25C, and CDK1-pY15 (phospho-CDK1). Densitometric values of the Cdc25C slower migrating band is depicted in bar graph, bottom panel. D, Left panel, western blot analysis showing cyclin E expression in HeLa cells infected with control (LacZ), EL- or LMW-E–expressing adenovirus for 24 h. Middle panels, HeLa cells overexpressing cyclin E were IPed with a cyclin E antibody and kinase activity was measured using full-length GST-Cdc25A or GST-Cdc25C as substrates. The CDK2 inhibitor Roscovitine (80 μM) was added to the kinase reaction as indicated. Right panel, HeLa cells were synchronized with aphidicolin for 24 h and infected with EL or LMW-E adenovirus. Cell lysates were prepared from cells released for 6 h and subjected to a kinase assay using GST-Cdc25C 200-256 S214A or S216 A as a substrate (right panel).
LMW-E overexpression abrogates the mitotic arrest caused by nocodazole
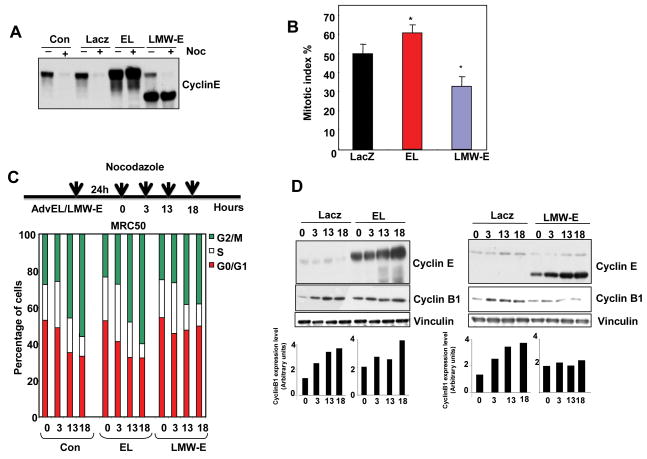
We next assessed whether the LMW-E-mediated alteration of mitotic exit could render these cells resistant to mitotic arrest using a spindle poison. HeLa H2B-GFP cells were infected with adenovirus to EL and LMW-E, treated with nocodazole, followed by counting of the mitotic cells (Fig. 4A and B). More than 50% of cells infected with LacZ- or EL-expressing adenovirus were arrested in mitosis, compared to only 30% of the LMW-E–expressing cells (Fig. 4B) suggesting that LMW-E, but not EL, abrogates the nocodazole-mediated mitotic arrest in HeLa cells. To examine these results in a different system, we overexpressed EL or LMW-E for 24 h in MRC50 normal human fibroblasts, treated the cells with nocodazole for 3-18 h, and then analyzed the cells by flow cytometry. LMW-E–expressing cells accumulated in prometaphase less efficiently than the control or EL-expressing cells, suggesting that overexpression of LMW-E results in a faster exit from mitosis after nocodazole treatment (Fig. 4C). Cyclin B1 protein levels accumulated in both the control and EL-expressing cells after 13 or 18 h of nocodazole treatment, whereas the level of cyclin B1 in LMW-E–expressing cells was not increased (Fig. 4D) In fact, the level of cyclin B1 is increased by about 2 fold after 18 hours incubation with nocodazole, while cyclin B1 remained at its lowest levels in the LMW-E overexpressed cells. Collectively, these data suggest that LMW-E–expressing cells have an increased rate of mitotic slippage and adaptation to the spindle checkpoint, resulting in a faster mitotic exit, as compared with EL-overexpressing cells.
Figure 4. Overexpression of LMW-E overcomes nocodazole-dependent mitotic arrest.
A, HeLa H2B-GFP cells were infected with LacZ-control, EL, or LMW-E expressing adenovirus and then treated with nocodazole (Noc, 100 ng/ml) for 16 h. Cyclin E expression was detected by western blot analysis. B, Mitotic cells treated as in A were counted by microscopy and the mitotic index was calculated +/- S,D. (N=1000 for two independent experiments). C, Human fibroblast MRC50 cells were infected with adenovirus expressing LacZ control, EL, or LMW-E and then exposed to nocodazole for the indicated times and analyzed by flow cytometry. The graphs show the percentages of cells in the G0/G1, S, and G2/M phases and are representative of three independent experiments. D, Western blot analysis of cyclin E, cyclin B1, and vinculin expression in cells treated as described in C. Densitometry was performed using ImageQuant software with the cyclin B1:vinculin ratio.
Downregulation of Cdc25C inhibits LMW-E–mediated chromosome missegregation and centrosome amplification
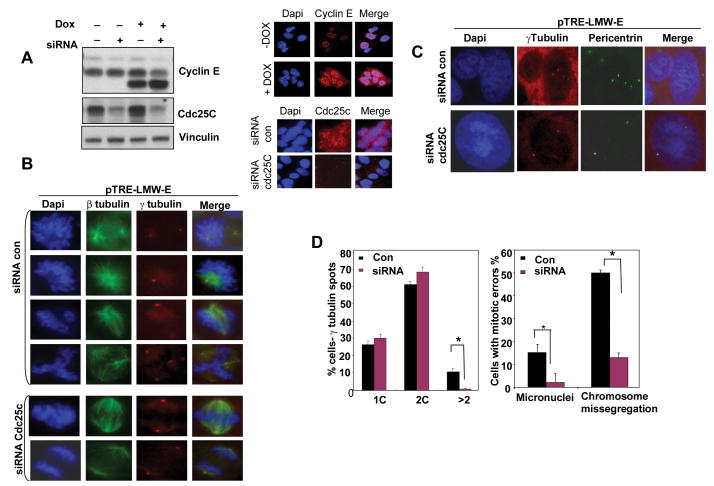
Our results thus far suggest that Cdc25C may mediate LMW-E's effects on deregulation of the G2-M transition, spindle defects, chromosome segregation, centrosome amplification, and polyploidy. To directly address this hypothesis, we transfected pTRE-EL (Supplementary Fig. S1) or pTRE-LMW-E MCF-7 cells with siRNA targeting Cdc25C (Fig. 5A), which was capable of downregulating Cdc25C protein >80% as depicted in western blot analysis and immunostaining (Fig 5A and (S1A). Next, cells were stained for β-tubulin and γ-tubulin to analyze mitotic defects (Fig. 5B, D and Fig S1C, D) or for pericentrin and γ-tubulin to detect centrosomes (Fig. 5C and D). We noted that LMW-E overexpressing cells displayed increases in supernumerary centrosomes (12%), micronuclei (15%) and chromosome missegregation in mitosis (50% of mitotic cells). Depletion of Cdc25C completely inhibited centrosome amplification (Fig. 5C, D), micronuclei formation (Fig. 5D right panel) or chromosome missegregation (Fig. 5D right panel) in these cells and brought the level of abnormalities comparable to that of uninduced LMW-E or EL induced cells. Overexpression of EL did not show a significant increase of centrosome amplification or micronuclei but increased chromosome missegregation during mitosis (supplementary Fig. S1). Depletion of Cdc25C in EL overexpressing cells did not further alter chromosome missegregation during mitosis of these cells (Supplementary Fig. S1). These findings suggest that Cdc25C is a critical target of LMW-E for induction of chromosomal instability in cancer cells.
Figure 5. Depletion of Cdc25C protein prevents LMW-E–mediated centrosome amplification and chromosome missegregation.
A, pTRE-LMW-E MCF-7 cells were transfected with control siRNA or Cdc25C-specific siRNA for 72 h, and Cdc25C and cyclin E expression were analyzed by western blotting (left panels). Cyclin E immunofluorescence (IF) (right panels) was also used to examine cyclin LMW-E levels in the presence or absence of pTRE-LMW-E induction. Cdc25C IF was used to examine Cdc25C levels in the siRNA control or siCdc25C pTRE-LMW-E induced cells. B, pTRE-LMW-E cells were treated with control or Cdc25C-specific siRNA for 72 h, treated with or without LMW- E induction, and costained for β–tubulin (green) and γ–tubulin (red), and DAPI (blue) to analyze the mitotic defects. C, Cells treated as in B were costained with pericentrin (green), γ–tubulin (red), or DAPI (blue) to analyze the number of centrosomes. D, Left panel, centrosomes were counted in cells (n= 500) stained with γ–tubulin and pericentrin for each condition in three independent experiments. *P < 0.01. Right panel, mitotic cells (n= 100) were analyzed for abnormalities such as micronulei and chromosome missegregation for each condition in three independent experiments. *P < 0.01.
EL and LMW-E overexpression differentially modulate PLK1 activity
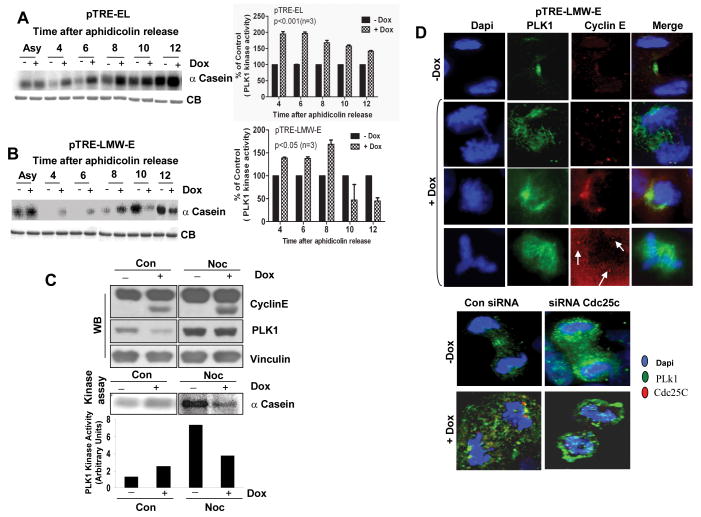
We next assessed the downstream consequences of EL-mediated versus LMW-E–mediated altered Cdc25C activation. One of the proteins that is involved in a feedback loop with Cdc25C and Myt 1 is PLK1, which also functions as a key regulator of CDK1 activity. Hence, we asked whether induction of either EL or LMW-E could alter the activation of PLK1 as they did Cdc25C by measuring PLK1 kinase activity. We used the MCF-7 EL or LMW-E inducible cells, induced with doxycycline and synchronized in the G1/S boundary by aphidicolin. At different time intervals following release, cells were harvested and PLK1 kinase activity was measured using α-casein as a substrate (Fig 6A and 6B). The left panels depict the autoradiogram of the α-casein kinase gels at each time interval in either EL (Fig 6A) or LMW-E (Fig 6B) induced cells. The right panels depict the densitometric measurement of each band. These analyses revealed that induction of EL or LMW-E results in the premature activation of PLK1 activity 4-8 h after aphidicolin release by about 68-97% or 37-68% respectively (Fig. 6A and B). However, the PLK1 activity remained high 12 h after release in the EL-overexpressing cells, while it dropped 3.5 fold 10 h after release in the LMW-E–overexpressing cells when the G2-M population was the largest (Fig. 6A and B). The results from these synchronization experiments show that the changes in Cdc25C activity coincided with the time intervals where there were also changes in cyclin B/CDK1 and PLK1 activities in both EL- and LMW-E–expressing cells (Fig. 2 C). Together these experiments suggest that cyclin B/CDK1 and PLK1 activity are modulated by EL and LMW-E expression differently and through a mechanism involving direct activation of Cdc25C.
Figure 6. Overexpression of EL and LMW-E differentially deregulates PLK1 and leads to its mislocalization.
A, pTRE-EL and B, pTRE-LMW-E cells were synchronized at the G1/S transition by aphidicolin block, induced with Dox for 24 h, released for the indicated times, and subjected to IP with anti-PLK1. Kinase activity was measured using α–casein as a substrate. Coomassie-stained gel of IgG (CB) was used as a loading control. Right panels, PLK1 kinase activity in A and B was measured by densitometry and normalized to the activity in the uninduced condition. +/- SD obtained from at least three independent experiments. C, pTRE-LMW-E cells were treated with Dox for 24 h, including a 16 h nocodazole treatment, and analyzed by western blotting for cyclin E, PLK1, and vinculin. Kinase activity was measured as in A and the bands corresponding to the α-casein were quantitated and depicted as bar graphs. D, pTRE-LMW-E cells were induced to express LMW-E by treatment with Dox for 24 h or depleted of Cdc25C by treating with a Cdc25C-specific siRNA for 72 h. Cells were costained for cyclin E (red), PLK1 (green), and DNA (blue) (top panels) and for PLK1 (green) or Cdc25C (red) (bottom panels).
Normal exit from mitosis requires the inactivation of PLK1, which can occur either by its degradation or dephosphorylation (26). Because we observed a significant reduction in the PLK1 activity in LMW-E –overexpressing cells enriched in the G2-M phase, we asked whether LMW-E induction affects PLK1 activity in cells treated with nocodazole. MCF-7 LMW-E cells were induced by doxycycline in the presence and absence of nocodazole and examined for cyclin E and PLK1 expression and PLK1 kinase activity (Fig 6C). Treatment with nocodazole resulted in a two-fold reduction in PLK1 activity in LMW-E–expressing cells, as compared with uninduced cells (Fig. 6C, bar graphs). These results are consistent with the aphidicolin time-course study, suggesting that the decrease in PLK1 activity in LMW-E–expressing cells during mitosis may force cells to exit mitosis and bypass the spindle assembly checkpoint, and potentially resulting in the mislocalization of PLK1.
Under normal conditions, PLK1 associates with centrosomes from G2 to metaphase, translocates to the kinetochores at metaphase, and localizes to the midbody during anaphase and telophase. It has been shown that the activation state of CDK1 controls the translocation of PLK1 from the centrosomes and kinetochores during metaphase to the central spindles during anaphase (27). We hypothesized that overexpression of LMW-E deregulates the localization of PLK1. We tested this hypothesis in our inducible system and found that upon induction of LMW-E, PLK1 is no longer associated with the central spindles during anaphase and telophase and is now dispersed non-specifically throughout the cellular structure (Fig. 6D, top panels). We also examined PLK1 localization in LMW-E–overexpressing cells in which Cdc25C was depleted by siRNA. We observed that in uninduced cells following Cdc25C siRNA depletion, PLK1 does not localize to the midbody but to the centrosome (Fig. 6D, bottom panels). Cdc25C is not detectable in control cells at the telophase stage, but it is when LMW-E is induced. Additionally, in LMW-E–induced cells depleted of Cdc25C, PLK1 was mislocalized in the same manner as in the Cdc25C-depleted uninduced cells (Fig. 6D, bottom panel). These results suggest that both overexpression of LMW-E and depletion of Cdc25C, lead to mislocalization of PLK1. Since, inhibition of PLK1 activity abolishes the correct localization of PLK1 (28), these results suggest that inhibition of PLK1 activity caused by induction of LMW-E may also lead to its mislocalization and may cause cytokinesis failure by affecting important targets of PLK1.
Discussion
In this report, we describe a novel regulatory pathway involving activation of Cdc25C protein phosphatase, which is differentially phosphorylated by EL and LMW-E to regulate mitotic entry and exit. We also show that deregulation of mitotic progression and chromosomal instability is mediated by the interaction between cyclin E and Cdc25C. EL and LMW-E directly interact with and phosphorylate Cdc25C on residue Ser 214, leading to its premature activation during S phase. Ser 214 is the major phosphorylation site on Cdc25C during mitosis and prevents phosphorylation of Ser 216 (25). This premature activation of Cdc25C causes dephosphorylation of CDK1 on Thr14 and Tyr15, which forms the core of feedback loops that control the activation of cyclin B/CDK1 and PLK1 and promote mitotic entry. A fraction of Cdc25C localizes to the centrosome during the S phase and throughout G2 and mitosis, and cyclin E localizes to the centrosomes (29). Thus, it is likely that cyclin E interacts with Cdc25C at the centrosomes and activates Cdc25C by phosphorylating Ser 214, leading to activation of CDK1-cyclin B1, which in turn activates PLK1 and Cdc25C. However, we cannot exclude the possibility that the cyclin E/CDK complex phosphorylates and directly activates PLK1.
The EL- and LMW-E–expressing cells displayed different mitotic exit phenotypes. LMW-E–expressing cells exited mitosis faster than the control cells and exhibited an accelerated mitotic transition, while EL-overexpressing cells were arrested in mitosis (manuscript #1). Inactivation of Cdc25C is a key upstream event in M-phase promoting factor inactivation during mitotic exit (30). We propose that LMW-E's weak binding to Cdc25C during mitosis may result in a rapid exit from mitosis through the premature inactivation of Cdc25C and further inactivation of CDK1 and PLK1. In contrast, in EL-overexpressing cells, high levels of S214 phosphorylation and hyperactivation of both CDK1 and PLK1 arrest cells in mitosis and delay mitotic exit. These results with EL are consistent with a previous study showing that EL overexpression causes mitotic arrest by inhibiting APC/Ccdh1(6). Normal exit from mitosis has been shown to require the inactivation of PLK1 by either its degradation or dephosphorylation (26). Thus, the drop in PLK1 activity observed during mitosis may be the cause of the premature mitotic exit in LMW-E–expressing cells, whereas the high PLK1 activity observed in EL-expressing cells arrests them in mitosis, resulting in delayed mitotic exit.
We also observed mislocalization of PLK1 in LMW-E–expressing cells in metaphase and anaphase (31, 32), suggesting that such mislocalization may deregulate the activity of PLK's target genes that are necessary for proper cytokinesis. These results may explain the failure of cytokinesis in the LMW-E–expressing cells. We found that expression of LMW-E induced genomic instability by causing the cells to progress faster through mitosis before the chromosomes were properly segregated, leading to an increase in the number of multipolar anaphase spindles, a failure of cytokinesis, and the generation of multinucleated cells with supernumerary centrosomes (manuscript #1). Failure to execute a crucial step in the cell division process, such as late anaphase and cytokinesis, leads to the formation of cells with two nuclei; these 4N cells can then undergo either bipolar or multipolar mitosis with amplified centrosomes (33). Furthermore, we showed that depletion of Cdc25C in LMW-E–overexpressing cells inhibited centrosome amplification, decreased the number of abnormal mitotic spindles, and reduced chromosome missegregation. Our findings suggest that the LMW-E–mediated polyploidy and centrosome amplification require a premature transition through mitosis.
Another key finding from our study is that LMW-E–overexpressing cells showed a reduction in the mitotic index in the presence of nocodazole, suggesting an increased rate of mitotic slippage and adaptation to the spindle checkpoint, a feature that is commonly seen when checkpoint-defective cells are accelerated out of mitosis. In contrast, EL-overexpressing cells accumulated in G2-M and had an increased mitotic index when treated with nocodazole, suggesting delayed entry into and exit from mitosis. These results suggest the spindle assembly checkpoint is defective in LMW-E–overexpressing cells but not in EL-overexpressing cells. The mechanism by which LMW-E overexpression bypasses the spindle assembly checkpoint needs further investigation. Collectively, these results suggest that the generation of LMW-E isoforms only in tumor cells primes these cells to accrue chromosomal instability by affecting the transition of cells through mitosis, leading to the generation of supernumerary centrosomes and contributing to transformation in cancer cells.
Supplementary Material
Acknowledgments
We thank Said Akli and Yan Liu for generation of the cyclin E–expressing adenovirus, Wendy D. Schober and Nalini Patel for assistance with the flow cytometry analysis. We thank Helen Piwnica-Worms, Stephen J. Elledge, Yolanda Sanchez, and Jian Kuang for providing the Cdc25C constructs. This work was supported by NIH grants CA87458 and P50CA116199 and a grant from the Clayton Foundation to K.K. and Susan G. Komen grant PDF0707621 to A.B. and K.K.
References
- 1.Lacey KR, Jackson PK, Stearns T. Cyclin-dependent kinase control of centrosome duplication. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2817–22. doi: 10.1073/pnas.96.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Resnitzky D, Gossen M, Bujard H, Reed SI. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Molecular and cellular biology. 1994;14:1669–79. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohtsubo M, Roberts JM. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science (New York, NY) 1993;259:1908–12. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- 4.Ekholm SV, Reed SI. Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol. 2000;12:676–84. doi: 10.1016/s0955-0674(00)00151-4. [DOI] [PubMed] [Google Scholar]
- 5.Minella AC, Loeb KR, Knecht A, et al. Cyclin E phosphorylation regulates cell proliferation in hematopoietic and epithelial lineages in vivo. Genes Dev. 2008;22:1677–89. doi: 10.1101/gad.1650208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keck JM, Summers MK, Tedesco D, et al. Cyclin E overexpression impairs progression through mitosis by inhibiting APC(Cdh1) J Cell Biol. 2007;178:371–85. doi: 10.1083/jcb.200703202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- 8.Lindqvist A, Rodriguez-Bravo V, Medema RH. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol. 2009;185:193–202. doi: 10.1083/jcb.200812045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cimini D, Moree B, Canman JC, Salmon ED. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. Journal of cell science. 2003;116:4213–25. doi: 10.1242/jcs.00716. [DOI] [PubMed] [Google Scholar]
- 10.Keyomarsi K, Tucker SL, Buchholz TA, et al. Cyclin E and survival in patients with breast cancer. N Engl J Med. 2002;347:1566–75. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- 11.Porter D, Zhang N, Danes C, et al. Tumor Specific Proteolytic Processing of Cyclin E Generates Hyper-Active Lower Molecular Weight Forms. Mol Cell Bio. 2001;21:6254–69. doi: 10.1128/MCB.21.18.6254-6269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delk NA, Hunt KK, Keyomarsi K. Altered subcellular localization of tumor-specific cyclin E isoforms affects cyclin-dependent kinase 2 complex formation and proteasomal regulation. Cancer Res. 2009;69:2817–25. doi: 10.1158/0008-5472.CAN-08-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akli S, Zheng PJ, Multani AS, et al. Tumor-specific low molecular weight forms of cyclin E induce genomic instability and resistance to p21, p27, and antiestrogens in breast cancer. Cancer research. 2004;64:3198–208. doi: 10.1158/0008-5472.can-03-3672. [DOI] [PubMed] [Google Scholar]
- 14.Wingate H, Zhang N, McGarhen MJ, Bedrosian I, Harper JW, Keyomarsi K. The tumor-specific hyperactive forms of cyclin E are resistant to inhibition by p21 and p27. The Journal of biological chemistry. 2005;280:15148–57. doi: 10.1074/jbc.M409789200. [DOI] [PubMed] [Google Scholar]
- 15.Ogg S, Gabrielli B, Piwnica-Worms H. Purification of a serine kinase that associates with and phosphorylates human Cdc25C on serine 216. The Journal of biological chemistry. 1994;269:30461–9. [PubMed] [Google Scholar]
- 16.Sanchez Y, Wong C, Thoma RS, et al. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science (New York, NY) 1997;277:1497–501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 17.Kanda T, Sullivan KF, Wahl GM. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr Biol. 1998;8:377–85. doi: 10.1016/s0960-9822(98)70156-3. [DOI] [PubMed] [Google Scholar]
- 18.Bagheri-Yarmand R, Mandal M, Taludker AH, et al. Etk/Bmx tyrosine kinase activates Pak1 and regulates tumorigenicity of breast cancer cells. The Journal of biological chemistry. 2001;276:29403–9. doi: 10.1074/jbc.M103129200. [DOI] [PubMed] [Google Scholar]
- 19.Gottlin EB, Xu X, Epstein DM, et al. Kinetic analysis of the catalytic domain of human cdc25B. The Journal of biological chemistry. 1996;271:27445–9. doi: 10.1074/jbc.271.44.27445. [DOI] [PubMed] [Google Scholar]
- 20.Liu F, Stanton JJ, Wu Z, Piwnica-Worms H. The human Myt1 kinase preferentially phosphorylates Cdc2 on threonine 14 and localizes to the endoplasmic reticulum and Golgi complex. Molecular and cellular biology. 1997;17:571–83. doi: 10.1128/mcb.17.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGowan CH, Russell P. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. The EMBO journal. 1993;12:75–85. doi: 10.1002/j.1460-2075.1993.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sebastian B, Kakizuka A, Hunter T. Cdc25M2 activation of cyclin-dependent kinases by dephosphorylation of threonine-14 and tyrosine-15. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:3521–4. doi: 10.1073/pnas.90.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann I, Draetta G, Karsenti E. Activation of the phosphatase activity of human cdc25A by a cdk2-cyclin E dependent phosphorylation at the G1/S transition. The EMBO journal. 1994;13:4302–10. doi: 10.1002/j.1460-2075.1994.tb06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goswami PC, He W, Higashikubo R, Roti Roti JL. Accelerated G1-transit following transient inhibition of DNA replication is dependent on two processes. Exp Cell Res. 1994;214:198–208. doi: 10.1006/excr.1994.1249. [DOI] [PubMed] [Google Scholar]
- 25.Bulavin DV, Higashimoto Y, Demidenko ZN, et al. Dual phosphorylation controls Cdc25 phosphatases and mitotic entry. Nature cell biology. 2003;5:545–51. doi: 10.1038/ncb994. [DOI] [PubMed] [Google Scholar]
- 26.Lindon C, Pines J. Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. The Journal of cell biology. 2004;164:233–41. doi: 10.1083/jcb.200309035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neef R, Gruneberg U, Kopajtich R, et al. Choice of Plk1 docking partners during mitosis and cytokinesis is controlled by the activation state of Cdk1. Nat Cell Biol. 2007;9:436–44. doi: 10.1038/ncb1557. [DOI] [PubMed] [Google Scholar]
- 28.Dai W, Wang X. Grabbing Plk1 by the PBD. Mol Cell. 2006;24:489–90. doi: 10.1016/j.molcel.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto Y, Maller JL. A centrosomal localization signal in cyclin E required for Cdk2-independent S phase entry. Science (New York, NY) 2004;306:885–8. doi: 10.1126/science.1103544. [DOI] [PubMed] [Google Scholar]
- 30.Forester CM, Maddox J, Louis JV, Goris J, Virshup DM. Control of mitotic exit by PP2A regulation of Cdc25C and Cdk1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19867–72. doi: 10.1073/pnas.0709879104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golsteyn RM, Mundt KE, Fry AM, Nigg EA. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. The Journal of cell biology. 1995;129:1617–28. doi: 10.1083/jcb.129.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee KS, Yuan YL, Kuriyama R, Erikson RL. Plk is an M-phase-specific protein kinase and interacts with a kinesin-like protein, CHO1/MKLP-1. Molecular and cellular biology. 1995;15:7143–51. doi: 10.1128/mcb.15.12.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nigg EA. Centrosome aberrations: cause or consequence of cancer progression? Nature reviews. 2002;2:815–25. doi: 10.1038/nrc924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.