Abstract
Stearic acid (stearate) is an 18-carbon saturated fatty acid that has been shown to inhibit invasion and proliferation and induce apoptosis in various human cell types. The specificity of stearate-induced apoptosis for cancerous versus non-cancerous breast cells has not been examined and the mechanism underlying stearate-induced apoptosis is unknown. Morphological analysis, cell viability and caspase-3 activity assays demonstrated that stearate activated apoptosis preferentially in cancerous breast cells in a time and dose dependent manner. Inhibition of de novo diacylgycerol synthesis or protein kinase C (PKC) blocked stearate-induced caspase-3 activity, indicating the involvement of a novel or classical PKC isozyme. To our knowledge this is the first study showing that stearate induces apoptosis preferentially in breast cancer cells and implicates protein kinase C in the signaling cascade. These results raise the possibility of dietary stearate having a beneficial role in the prevention or treatment of breast cancer.
Keywords: stearate, apoptosis, protein kinase c, diacylglycerol
Introduction
Stearic acid (stearate) is an 18-carbon saturated fatty acid found in relatively high concentrations in several foods in the western diet, including beef and chocolate. In vitro and in vivo studies suggest stearate may have unique properties, especially in terms of breast cancer development and neoplastic progression. For example, stearate has been shown to inhibit epidermal growth factor (EGF) receptor-mediated proliferation in Hs578t breast cancer cells (1), inhibit invasion of HT-1080 fibrosarcoma cells (2), and induce apoptosis of MDA-MB-231 breast cancer cells (3). In vivo, dietary stearate has been associated with a decrease in mammary tumor development and incidence in spontaneous carcinogenesis models (4, 5). A recent meta-analysis of 13 studies compared human erythrocyte membrane, serum and adipocyte fatty acid composition relative to breast cancer risk (6). The study found that stearate was associated with a decreased risk of breast cancer in post-menopausal women but showed no association with breast cancer risk in the remainder of cohort studies (6). A more recent case-control study from Shanghai, China, also reported that stearate had no association with breast cancer risk (7). These results indicate that dietary stearate either reduces or has no effect on breast cancer risk in humans. Taken together, in vitro, in vivo and epidemiological studies point to stearate as having potential for breast cancer prevention and treatment; however, little is known concerning the mechanism of stearate's action(s), including its induction of apoptosis.
Apoptosis is a form of programmed cell death that is generally executed by a family of cysteine proteases known as caspases. Activation of the extrinsic cascade through the cleavage of caspase-8, or the intrinisic cascade through the cleavage of caspase-9 leads to the cleavage and activation of executioner caspases such as caspase-3, resulting in cell death (Reviewed in (8)).
When stearate enters into a cell, it is converted to stearoyl-Coenzyme A (CoA) by one of the acyl-CoA synthetases (ACS). Based on the subcellular location of the synthetase, the stearoyl-CoA can lead to the de novo generation of acyl-glycerols, phospholipids, ceramide, mitochondrial β-oxidation, or protein acylation. In this manuscript, we investigated these pathways and found that de novo diacylglycerol (DAG) synthesis and protein kinase C (PKC) activation are necessary for stearate-induced caspase-3 activity. We also compared the ability of stearate to induce apoptosis in several breast cancer and non-cancer cell lines and found that stearate has a significant preferential effect on breast cancer cells.
Materials and Methods
Materials
Protease inhibitor cocktail, insulin, EGF, actin antibody, stearic acid, Etomoxir, Fumonisin B1, Triacsin C and diatomaceous earth were all obtained from Sigma-Aldrich (St. Louis, MO). GF-109203X was obtained from Biomol (Plymouth Meeting, PA). Hs578t and Hs578Bst were obtained from the ATCC (Manassas, VA), MDA-MB-435 cells were a gift from Dr. Danny Welch (UAB, Birmingham, AL), MDA-MB-231 cells were a gift from Dr. Selvarangan Ponnazhagan (UAB, Birmingham AL), and MCF-10A cells were a gift from Dr. Andra Frost (UAB, Birmingham, AL). DMEM, fetal bovine serum (FBS), heat-inactivated FBS, DMEM:Ham F12, sodium pyruvate, glutamine, non-essential amino acids, PBS, and penicillin/streptomycin were obtained from Cellgro (Mediatech, Inc. Herdon, VA). NEFA C was purchased from Wako Chemicals (Neuss, Germany). The BCA Protein Assay was obtained from Pierce Biotechnology (Rockford, IL). Cleaved caspase-3, cleaved PARP, and phospho-PKC (pan) (βII Ser 660) antibodies were obtained from Cell Signaling Technologies (Danvers, MA). Anti-mouse and anti-rabbit conjugated to horseradish peroxidase and ECL reagents were from Amersham Biosciences (Piscataway, NJ) and the caspase-3 activity assay was obtained from Invitrogen (Carlsbad, CA). Finally, the Matrigel coated Boyden chambers were from BD Biosciences (Franklin Lakes, NJ) and the lactate dehydrogenase assay was from Roche (Nutley, NJ).
Cell Culture
Human Hs578t breast cancer cells, MDA-MB-231 breast cancer cells, Hs578Bst myoepithelial breast cells, and MCF-10A epithelial cells were grown and maintained as recommended by the ATCC. MDA-MB-435 cells breast cancer cells were cultured in 5% FBS in DMEM:Ham F12 media supplemented with 2 mM glutamine, 1 mM sodium pyruvate and 0.2X non-essential amino acids (5% CO2). Hs578t cells were serum starved with DMEM without phenol red supplemented with 0.5% heat inactivated FBS, 1 mM sodium pyruvate, 1% penicillian/streptomycin. MDA-MB-231 breast cancer cells were serum starved with L-15 without phenol red supplemented with 0.5% heat inactivated FBS. MCF-10A cells were serum starved with DMEM:F12 without phenol red supplemented with 0.5% heat inactivated FBS, 20 ng/mL EGF, 100ng/mL cholera toxin, 500ng/mL hydrocortisone, and .01mg/mL bovine insulin. MDA-MB-435 breast cancer cells were serum starved with DMEM:F12 without phenol red supplemented with 0.5% heat inactivated FBS, 1mM sodium pyruvate, and 0.2% non-essential amino acids. All media were supplemented with 1% penicillian/streptomycin.
Stearic Acid Preparation
Stearate was bound to fatty acid free-BSA (FAF-BSA) by the method of Spector and Hoak (9). Briefly, stearate was added to hexane, mixed with diatomaceous earth, and dried under a stream of nitrogen gas to form a powder. The powder was stirred with starvation media containing 1% FAF-BSA and then the diatomaceous earth was filtered out. The pH of the BSA-stearate mixture was adjusted to 7.4 with 1M HCl. The addition of the powder, filtration, and pH adjustment were repeated 3 more times. The BSA-fatty acid mixture was sterilized by filtration and the concentration of fatty acids was measured using the NEFA C enzymatic-colorimetric analysis. FAF-BSA was prepared the same way without the addition of stearate to the diatomaceous earth.
Scratch Assay for Cell Migration
Hs578t cells were plated on a 24-well plate in cell media containing 10% FBS. When the cells attained 90% confluence a 200 μl pipette tip was used to make one mid-horizontal and one mid-vertical scratch across the cell layer in each well. The cells were gently washed 3 times with PBS and complete media was added with either FAF-BSA (control) or stearate (50 μM). The cells within a 1cm2 area within the scratch region were counted under a microscope in 4 fields of view before stearate treatment (time 0) and at 6, 12, and 24 hours after stearate treatment.
Cell Invasion Assay
Matrigel coated chambers were rehydrated using complete media for 2 hours. Hs578t cells were trypsinized from the plate, and diluted to 2.5 × 104 cells/mL in starvation media. Complete media was added to the lower well and 500 μL of cells in starvation media were added to each invasion chamber. After two hours, 50 μM stearate or FAF-BSA was added to the upper and lower chambers. After 24 hours of incubation, media was removed and cells were fixed with 3.7% paraformaldehyde, stained with 0.5% crystal violet, rinsed with distilled water and allowed to dry overnight. Five random areas on each insert were then counted at 100X magnification.
Cytotoxicity Assays
40,000 cells/ well were plated on a 24 well plate. Wells were treated with stearate at the concentrations mentioned. 12 hours after stearate treatment, conditioned media was collect and cells and debris were removed by centrifugation. Lactate dehydrogenase (LDH) activity was measured in the conditioned media according to the manufacturer's instructions. Triton X was used as a positive control as it will lyse all cells in the well. Cells were treated with 2% Triton X 10 minutes and conditioned media was collected as stated above. The values obtained with Triton X were taken as 100% cytoxicity and stearate's values were adjusted accordingly. Each experiment was performed in triplicate.
Immunoblots
Hs578t cells were starved for 24 hours in starvation medium, and then treated with 50 μM stearate for the times indicated. Cells were lysed using lysis buffer (25 mM HEPES; 150 mM NaCl; 1% NP-40; 10 mM MgCl2; 1 mM EDTA; 2% Glycerol; 1 mM NaF; 1 mM Na3V04; 25 mM β-glycerophosphate; 1 mM tetrasodium pyrophosphate; 1:1000 PMSF [100 mM]; 1:1000 Protease Inhibitor Cocktail), equal amounts of protein were prepared by boiling the lysate with 6X sample buffer (300 mM Tris-HCl, pH 6.8; 12% SDS; 0.05% Bromophenol blue; 60% Glycerol; 12% β-mercaptoethanol), and were separated using 10% SDS-PAGE gels. Proteins were then transferred to PVDF membrane and were probed with primary antibodies and incubated with secondary antibody conjugated to horseradish peroxidase.
Caspase-3 Activity Assay
Cells were plated to 60-70% confluence and were then serum starved for 24 hours. One hour before treatment with 50 μM stearate of FAF-BSA, cells were treated with either 5μM Triascin C, 25 μM Etomoxir, 50 μM Fumonisin B1, 10 nM GF-109203X or DMSO (vehicle). After an additional 12 hours, cells were lysed and caspase-3 activity was measured according to the manufacturer's protocol using the peptide Z-DEVD-AMC assay (Invitrogen). Cell lysates were transferred to a 96-well black plate and incubated with the peptide for 30 minutes. Fluorescence was measured using a fluorescent microplate reader.
Statistical Analysis
Statistics were analyzed using either ANOVA (SigmaStat3.0) or student's t-test (Excel). Data presented are mean +/− standard error of the mean.
Results
Stearate Inhibits Breast Cancer Cell Invasion and Migration
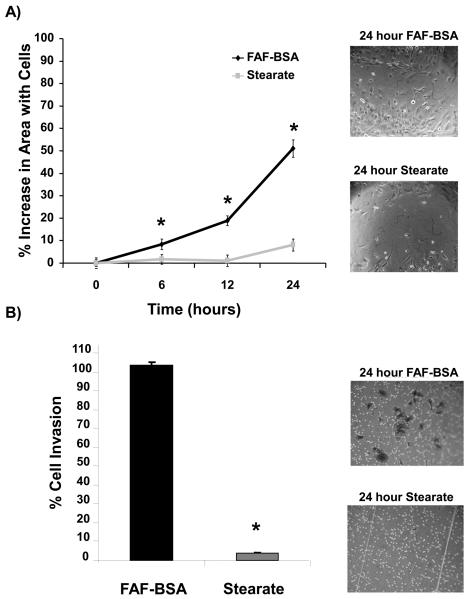
Stearate has previously been demonstrated to markedly inhibit invasion of HT-1080 fibrosarcoma cells and induce morphological changes, such as retraction of cell processes and cell rounding in vitro, although the molecular mechanisms of these effects are currently not known (2). To determine whether or not stearate had a similar effect on breast cancer cells, we analyzed the effect of stearate on migration and invasion. Because albumin is the physiological carrier of free fatty acids in vivo, cells were treated either with FAF-BSA or 50 μM stearate conjugated to BSA. Using an in vitro wound-healing assay, stearate significantly inhibited cell migration at 6, 12 and 24 hours compared to FAF-BSA (Figure 1A). To determine whether inhibition of cell motility by stearate would result in decreased cell invasion in vitro, we performed invasion assays using Matrigel coated chambers. We found that stearate completely blocked breast cancer cell invasion (Figure 1B).
Figure 1. Stearate inhibited cell migration and invasion.
A) Hs578t cells were treated with FAF-BSA or 50 μM stearate for 12 hours and then a scratch assay was performed. Representative images of the wound 24 hours after treatment with stearate or FAF-BSA are shown. Stearate significantly decreased the migratory ability of the cells 12 and 24 hours post treatment. (n=3; *p<0.001 by ANOVA). B) Hs578t cells were plated on Matrigel coated invasion chambers and treated with 50 μM stearate. After 24 hours the number of cells that invaded through the Matrigel was counted. Stearate treatment abolished invasion of the Hs578t cells (n=3; *p<0.0001 by ANOVA). Representative images of the crystal violet stained cells 24 hours post-treatment are shown.
Stearate Preferentially Induces Apoptosis of Human Breast Cancer Cells
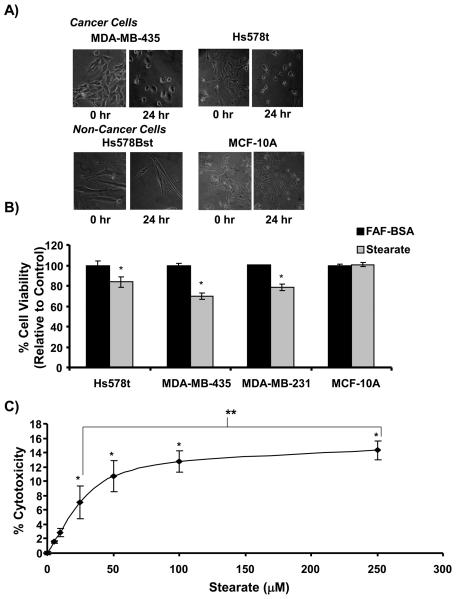
Following treatment of the Hs578t breast cancer cells with stearate, we observed that stearate induced cell rounding 24 hours post-treatment. To determine if this effect was specific to this cell line, we analyzed the MDA-MB-435 and MDA-MB-231 breast cancer cell lines 24 hours after stearate treatment and found that both cell lines rounded in the presence of stearate (Figure 2A and data not shown). When we analyzed the Hs578Bst myoepithelial breast cells and the MCF-10A breast epithelial cells, we found that even after 24 hours of stearate treatment, the morphology of the cells did not significantly change. These results indicated that the effects of stearate are preferential for the cancer versus the non-cancer cells.
Figure 2. Stearate decreased the viability of the human breast cancer cells.
A) Stearate induced morphological changes of the breast cancer cells but not the non-cancerous cells 24 hours post treatment. B) Twelve hours post-treatment, stearate decreased the viability of the breast cancer cells but not the non-cancerous MCF-10A cells using the trypan blue exclusion assay (n=2 performed in triplicate; *p<0.005 by the Student's t-test). C) To confirm the decrease in cell viability, cytotoxicity induced by stearate 12 hours post-treatment was estimated by measuring lactate de-hydrogenase activity in the cell culture media. Stearate decreased the viability of the cancer cells in a dose dependent manner. (n=3 performed in triplicate; *p<0.04 compared to FAF-BSA, **p<0.02 by ANOVA).
Because morphological changes such as cell rounding and cell shrinkage are hallmark characteristics of apoptosis, we next tested whether or not stearate was affecting the viability of the cancer cells. As stearate has been shown to induce death of the MDA-MB-231 breast cancer cells previously, they were used as a positive control (3). As shown in Figure 2B, stearate significantly decreased the viability of the three cancer cell lines tested. Twelve hours post-treatment, stearate decreased the viability of the Hs578t, MDA-MB-435, and MDA-MB-231 cells by 16.4%, 30.5%, and 21.5%, respectively. Stearate did not affect the viability of the MCF-10A cells, a finding that was consistent with the lack of stearate induced morphological changes in this cell line. To confirm the cells were undergoing cell death, cell media from the Hs578t cells was tested for lactate dehydrogenase activity (LDH). LDH is secreted into the media as cells die and activity was measured using colormetric assay. As shown in Figure 2C, stearate induced cytotoxicity in a dose dependent manner.
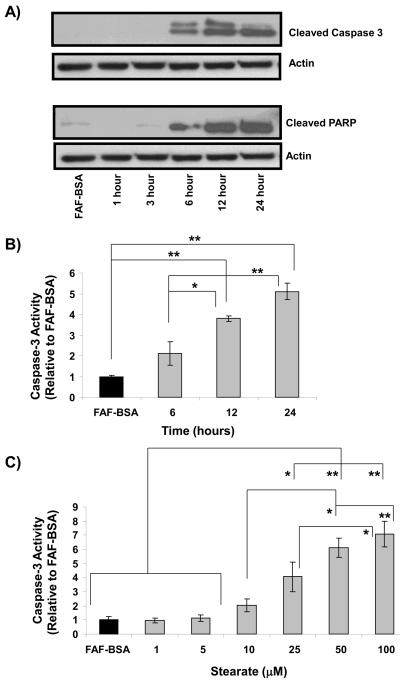
Analysis of lysate from the Hs578t breast cancer cells confirmed that stearate is inducing caspase-dependent apoptosis as shown by the presence of cleaved caspase-3 (Figure 3A). Furthermore, the well-characterized caspase-3 target poly-ADP-ribosomal polymerase (PARP) is cleaved at the same time, indicating that caspase-3 is active (10). To confirm the immunoblot data, caspase-3 activity was measured using a fluorescent based assay. As shown in Figure 3B, stearate induced caspase-3 activity in a time dependent manner. Furthermore, we wanted to determine if stearate induced caspase-3 activity correlated with the cytotoxicity data. Cells were treated with varying concentrations of stearate for 12 hours and activity was increased in a dose dependent manner (Figure 3C).
Figure 3. Stearate induces cleavage and activation of caspase-3 in the Hs578t cells.
A) Beginning approximately 6 hours after stearate treatment, cleaved caspase-3 appeared and was present 24 hours post-treatment. Concurrent with cleaved caspase-3 was the presence of cleaved PARP, a well-characterized downstream target of caspase-3. The immunoblots are representative of 5 independent experiments. B) To confirm that stearate is activating caspase-3 in a time dependent manner, caspase-3 activity was measured 6, 12 and 24 hours after treatment. Stearate significantly increased caspase-3 activity approximately 12 hours post-treatment. (n=3-4; **p<0.03, *p<0.05 by ANOVA). C) Stearate induces caspase-3 activity in dose dependent manner (n=3-4; *p<0.04, **p<0.005 by ANOVA).
Stearate Induces Apoptosis in Human Breast Cancer Cells through the de novo Synthesis of DAG and the Activation of PKC
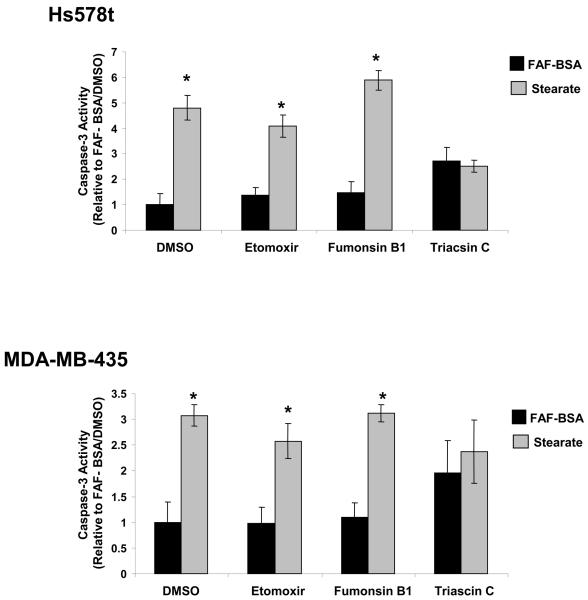
To determine if stearate was being metabolized through a particular pathway to induce apoptosis, cells were treated with metabolic pathway inhibitors and stearate or FAF-BSA. When carnitine palmitoyltransferase-1 (CPT-1) was inhibited with Etomoxir, a partial reversal of caspase-3 activity was seen in the Hs578t cells, but not in the MDA-MB-435 cells (Figure 4). When cells were treated with Fumonisin B1 to inhibit ceramide synthesis, no effect was seen on either cell line. In contrast, when the ACS was inhibited with Triacsin C, stearate-induced caspase-3 activity was reversed completely in both cell lines. It is worth noting, however, that in the MDA-MB-435 cell lines, Triacsin C induced caspase-3 activity without stearate. The difference between the FAF-BSA/ Triacsin C group was not different from the stearate/ Triacsin C group. All other FAF-BSA and stearate combinations tested were significant.
Figure 4. Inhibition of acyl-CoA synthetase inhibited stearate-induced caspase-3 activity at 12 hours.
In the presence of the ACS inhibitor Triacsin C, stearate-induced caspase-3 activity was inhibited in the Hs578t and MDA-MB-435 cell lines (n=3; FAF-BSA vs. stearate for each inhibitor, *p<0.03 by the Student t-test).
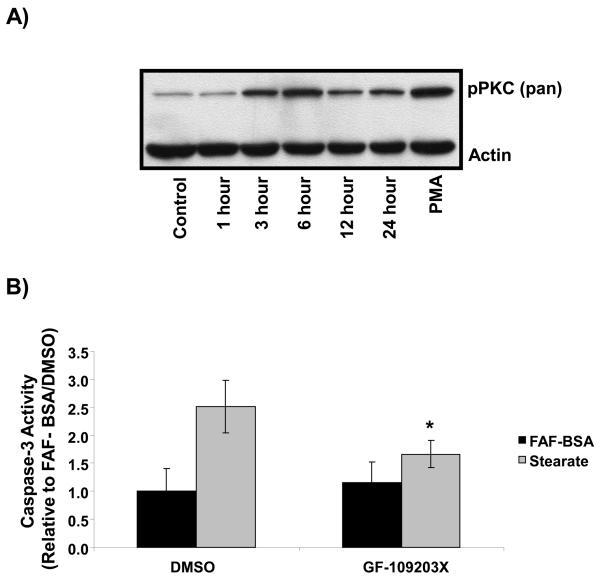
Finally, as DAG is a well-characterized activator of PKC, we tested whether or not stearate activated PKC and if PKC was involved in stearate-induced capase-3 activity. Cells were treated with 50 μM stearate and PKC activation was assessed using a pan-phosphorylation antibody. Stearate induced phosphorylation of PKC beginning at 1-3 hours after treatment (Figure 5A). Furthermore, when PKC was inhibited with the pan-PKC inhibitor GF-109203X, stearate-induced caspase-3 activity was completely reversed indicating a direct role for PKC in stearate-induced apoptosis (Figure 5B).
Figure 5. Inhibition of PKC inhibited stearate-induced caspase-3 activity at 12 hours.
A) Hs578t cells were treated with 50 μM stearate for the times indicated, lysates were collected and immunoblotted with a pan phospho-PKC antibody. Stearate induced phosphorylation of PKC 1-3 hours post treatment (n=4). B) Hs578t cells were treated with the pan PKC inhibitor GF-109203X for 1 hour prior to treatment with 50 μM stearate. Inhibition of PKC blocked stearate-induced caspase-3 activity (n=5; * different compared to stearate treated controls p<0.02 but not different from non-stearate treated controls p>0.07 by the paired t-test).
Discussion
Numerous studies have indicated that long chain saturated fatty acids including stearate and palmitate are capable of causing apoptosis in various cell types (3, 11-20). However, these studies generally use elevated, pathophysiological concentrations of stearate. When non-esterified plasma fatty acids where investigated in 8 men and 7 women, stearate concentrations ranged between 35 μM at the start of the study to 66 μM after 12 hours of fasting (21). Few studies have investigated concentrations of stearate less than 100 μM. In one study, human granulosa cells were incubated for 3 days with 50 μM stearate and there was no difference in cell viability between the stearate treated cells and controls while 100μM stearate significantly inhibited cell viability at 3 days (12). In another study, HT-29 human colon cancer cells were cultured with 30μM stearate for 15 days and cell growth did not differ from 4 other groups (22). Wicha et al. demonstrated a preferential ability of stearate to inhibit the growth of DMBA-induced rat mammary tumor cells compared to normal rat epithelial cells in vitro (23). It seems clear that stearate can induce apoptosis, although this effect is cell type and concentration dependent. Our results concur with these general conclusions but are the first to directly show a preferential pro-apoptotic effect of stearate at physiological concentrations (50 μM) for human breast cancer cell lines compared to non-cancerous breast cell lines. Interestingly, stearate completely inhibited invasion through Matrigel after 24 hours of treatment. Trypan blue staining showed that approximately 50% of the cells are dead at 24 hours (unpublished data). This argues another potential mechanism underlying the inhibition of invasion.
Little work has been done on the mechanism of stearate-induced apoptosis in breast cancer cells, although, some studies have been performed on palmitate-induced apoptosis. These studies using the MDA-MB-231 cell line concluded that fatty acid metabolism unrelated to β-oxidation, ceramide synthesis, ROS production or changes in PI3-kinase activity affected palmitate-mediated apoptosis (3). Similar with these studies, we found inhibition of ceramide synthesis with Fumonisin B1 had no effect on stearate-induced caspase-3 activity in the Hs578t and MDA-MB-435 breast cancer cells. Stearate induced caspase-3 activity was partially blocked in the Hs578t cells by Etomoxir, an inhibitor of CPT-1, indicating a possible role of β-oxidation in the induction of apoptosis.
It was suggested that palmitate incorporation into diacylglycerol rather than triacylglycerol was related to fatty acid-induced apoptosis of the MDA-MB-231 breast cancer cells (3). In our studies, inhibition of DAG synthesis with Triacsin C completely reversed stearate-induced caspase 3 activity in both the Hs578t and MDA-MB-435 cells. Triacsin C has been shown previously to inhibit fatty acid-induced de novo synthesis of diacylglycerol (DAG), and stearate has been shown to induce DAG synthesis in cultured bovine aortic smooth muscle cells (24). Furthermore, Triacsin C has been reported previously to inhibit de novo DAG synthesis without effecting phospholipid synthesis (25). Studies with rat homologues of ACS found that only certain isoforms were inhibited by Triacsin C. It has been hypothesized that there are different ACS isforms located at distinct subcellular regions and that Triacsin C inhibits the ACS that feeds acyl-CoAs into the synthesis of DAG de novo. (Reviewed in (26)). Because the PKC isozymes are well characterized downstream targets of DAG, we hypothesized that stearate was activating PKC through the generation of DAG to induce apoptosis.
The PKC isozymes are a family of serine/threonine kinases that are subdivided into three classes. The classical isozymes (α, βI, βII, γ) are dependent on DAG and Ca2+ for activation. The novel isozymes (δ, ε, η, θ, μ) lack the Ca2+ dependency and are dependent on DAG. Finally, the atypical isozymes (ζ and λ) lack both the Ca2+ and DAG requirements. The PKC isozymes as a class are already known to play a role in numerous cellular processes including apoptosis, proliferation, invasion and migration. In general, PKCα and PKCε have been portrayed as being anti-apoptotic whereas PKCδ is generally regarded as pro-apoptotic (Reviewed in (27)). However, the exact role of PKC isoenzymes in apoptosis is known to depend on the specific isozymes present and the cell type being analyzed. For example PKCδ has been demonstrated to play an anti-apoptotic role in human colon cancer cells, being mediated by NFκB and cIAP-2 (28).
We found that stearate activated PKC in the Hs578t cells and that inhibition of PKC with GF-109203X was able to reverse caspase-3 activity. Identifying the specific PKC isoenzyme that mediates stearate-induced apoptosis in breast cancer cells is an ongoing area of research for our laboratory.
To date, very few studies have attempted to address the role of individual fatty acids in human breast cancer prevention studies. Saturates, as a whole class, are generally associated with an increase risk of breast cancer (29), (30). However, using erythrocyte and adipose tissue compositions as a correlation to total fat intake, the individual saturates have varying effects on breast cancer. For example, palmitate positively correlates with breast cancer risk whereas stearate has a negative association (6). Although we found no such studies that found a positive correlation between stearate and breast cancer some studies found stearate to be neutral (no positive or negative effect). This may be related to the proportion of stearate in the diet compared to other dietary fatty acids. It is possible that by lowering the background of other dietary fatty acids stearate may become effective in vivo. Of course we cannot rule out other factors such as the environment and gene pools of the neutral studies. A number of human studies are necessary to determine the efficacy of stearate in breast cancer prevention and treatment. To date, no studies have been performed to determine if a diet rich in stearic acid could be an effective treatment. Along the same lines, minimal concentrations, optimal doses and mechanism of administration have not been monitored.
Long chain staturated fatty acids have been linked to high cholesterol concentrations. Dietary stearate, unlike palmitate (C16:0), has not been shown to increase cholesterol and may even decrease low density lipoprotein cholesterol (LDLc) without affecting high density lipoprotein cholesterol (31). Several studies have claimed that a high stearic acid diet promotes coagulation (32). However, several more recent studies dispute this fact, finding stearate is not prothrombotic and may actually decrease thrombotic and athrogenic factors in vivo such as mean platelet volume and plasma fatty acid concentrations (33) (34). Interestingly, the opposite was observed with a diet high in palmitate (34). It is also worth noting a diet high in stearate does not appear to effect insulin sensitivity indicating that unlike other saturated fatty acids, it may not be involved in the development of type 2 diabetes (35).
Thus, the growing body of literature indicating stearate may be a potential cancer preventative and therapeutic, suggest that clinical testing of stearate should be seriously considered.
In summary, stearate inhibited breast cancer cell migration and invasion in vitro as well as preferentially induced apoptosis of human breast cancer cells. Inhibition of de novo DAG synthesis reversed stearate induced caspase-3 activation. Stearate activated PKC and inhibition of PKC also reversed caspase-3 activation. This is the first study we are aware of that has implicated PKC isozymes in stearate-induced apoptosis and supports in vivo studies indicating that stearate may inhibit breast cancer development.
Acknowledgements
The authors would like to thank A. Ibrahim-Hashim and E. Toline for help with the preparation of the manuscript. These studies were supported by the NIH/National Center for Complementary and Alternative Medicine (NCCAM), R21 AT 001636 and R21 AT 002922 and (RWH). Its contents are solely the responsibility of the authors and do not necessarily represents of the official views of the NCCAM, or the National Institutes of Health. This study was also made possible by a pre-doctoral fellowship from the National Cattlemen's Beef Association (LME/RWH).
References
- 1.Wickramasinghe NS, Jo H, McDonald JM, Hardy RW. Stearate inhibition of breast cancer cell proliferation. A mechanism involving epidermal growth factor receptor and G-proteins. Am J Pathol. 1996;148:987–995. [PMC free article] [PubMed] [Google Scholar]
- 2.Singh RK, Hardy RW, Wang MH, Williford J, Gladson CL, et al. Stearate inhibits human tumor cell invasion. Invasion Metastasis. 1995;15:144–155. [PubMed] [Google Scholar]
- 3.Hardy S, El-Assaad W, Przybytkowski E, Joly E, Prentki M, et al. Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. A role for cardiolipin. J Biol Chem. 2003;278:31861–31870. doi: 10.1074/jbc.M300190200. [DOI] [PubMed] [Google Scholar]
- 4.Tinsley IJ, Schmitz JA, Pierce DA. Influence of dietary fatty acids on the incidence of mammary tumors in the C3H mouse. Cancer Res. 1981;41:1460–1465. [PubMed] [Google Scholar]
- 5.Bennett AS. Effect of dietary stearic acid on the genesis of spontaneous mammary adenocarcinomas in strain A/ST mice. Int J Cancer. 1984;34:529–533. doi: 10.1002/ijc.2910340416. [DOI] [PubMed] [Google Scholar]
- 6.Saadatian-Elahi M, Norat T, Goudable J, Riboli E. Biomarkers of dietary fatty acid intake and the risk of breast cancer: a meta-analysis. Int J Cancer. 2004;111:584–591. doi: 10.1002/ijc.20284. [DOI] [PubMed] [Google Scholar]
- 7.Shannon J, King IB, Moshofsky R, Lampe JW, Gao DL, et al. Erythrocyte fatty acids and breast cancer risk: a case-control study in Shanghai, China. Am J Clin Nutr. 2007;85:1090–1097. doi: 10.1093/ajcn/85.4.1090. [DOI] [PubMed] [Google Scholar]
- 8.Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4:139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 9.Spector AA, Hoak JC. An improved method for the addition of long-chain free fatty acid to protein solutions. Anal Biochem. 1969;32:297–302. doi: 10.1016/0003-2697(69)90089-x. [DOI] [PubMed] [Google Scholar]
- 10.Rosen A, Casciola-Rosen L. Macromolecular substrates for the ICE-like proteases during apoptosis. J Cell Biochem. 1997;64:50–54. doi: 10.1002/(sici)1097-4644(199701)64:1<50::aid-jcb8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Lu ZH, Mu YM, Wang BA, Li XL, Lu JM, et al. Saturated free fatty acids, palmitic acid and stearic acid, induce apoptosis by stimulation of ceramide generation in rat testicular Leydig cell. Biochem Biophys Res Commun. 2003;303:1002–1007. doi: 10.1016/s0006-291x(03)00449-2. [DOI] [PubMed] [Google Scholar]
- 12.Mu YM, Yanase T, Nishi Y, Tanaka A, Saito M, et al. Saturated FFAs, palmitic acid and stearic acid, induce apoptosis in human granulosa cells. Endocrinology. 2001;142:3590–3597. doi: 10.1210/endo.142.8.8293. [DOI] [PubMed] [Google Scholar]
- 13.Staiger K, Staiger H, Weigert C, Haas C, Haring HU, et al. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation. Diabetes. 2006;55:3121–3126. doi: 10.2337/db06-0188. [DOI] [PubMed] [Google Scholar]
- 14.Ulloth JE, Casiano CA, De Leon M. Palmitic and stearic fatty acids induce caspase-dependent and -independent cell death in nerve growth factor differentiated PC12 cells. J Neurochem. 2003;84:655–668. doi: 10.1046/j.1471-4159.2003.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artwohl M, Roden M, Waldhausl W, Freudenthaler A, Baumgartner-Parzer SM. Free fatty acids trigger apoptosis and inhibit cell cycle progression in human vascular endothelial cells. Faseb J. 2004;18:146–148. doi: 10.1096/fj.03-0301fje. [DOI] [PubMed] [Google Scholar]
- 16.Beeharry N, Chambers JA, Green IC. Fatty acid protection from palmitic acid-induced apoptosis is lost following PI3-kinase inhibition. Apoptosis. 2004;9:599–607. doi: 10.1023/B:APPT.0000038039.82506.0c. [DOI] [PubMed] [Google Scholar]
- 17.Paumen MB, Ishida Y, Muramatsu M, Yamamoto M, Honjo T. Inhibition of carnitine palmitoyltransferase I augments sphingolipid synthesis and palmitate-induced apoptosis. J Biol Chem. 1997;272:3324–3329. doi: 10.1074/jbc.272.6.3324. [DOI] [PubMed] [Google Scholar]
- 18.Mishra R, Simonson MS. Saturated free fatty acids and apoptosis in microvascular mesangial cells: palmitate activates pro-apoptotic signaling involving caspase 9 and mitochondrial release of endonuclease G. Cardiovasc Diabetol. 2005;4:2. doi: 10.1186/1475-2840-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, et al. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144:4154–4163. doi: 10.1210/en.2003-0410. [DOI] [PubMed] [Google Scholar]
- 20.Eitel K, Staiger H, Rieger J, Mischak H, Brandhorst H, et al. Protein kinase C delta activation and translocation to the nucleus are required for fatty acid-induced apoptosis of insulin-secreting cells. Diabetes. 2003;52:991–997. doi: 10.2337/diabetes.52.4.991. [DOI] [PubMed] [Google Scholar]
- 21.Mougios V, Ring S, Petridou A, Nikolaidis MG. Duration of coffee- and exercise-induced changes in the fatty acid profile of human serum. J Appl Physiol. 2003;94:476–484. doi: 10.1152/japplphysiol.00624.2002. [DOI] [PubMed] [Google Scholar]
- 22.Hunter DJ, Spiegelman D, Adami HO, Beeson L, van den Brandt PA, et al. Cohort studies of fat intake and the risk of breast cancer--a pooled analysis. N Engl J Med. 1996;334:356–361. doi: 10.1056/NEJM199602083340603. [DOI] [PubMed] [Google Scholar]
- 23.Wicha MS, Liotta LA, Kidwell WR. Effects of free fatty acids on the growth of normal and neoplastic rat mammary epithelial cells. Cancer Res. 1979;39:426–435. [PubMed] [Google Scholar]
- 24.Yu HY, Inoguchi T, Kakimoto M, Nakashima N, Imamura M, et al. Saturated non-esterified fatty acids stimulate de novo diacylglycerol synthesis and protein kinase c activity in cultured aortic smooth muscle cells. Diabetologia. 2001;44:614–620. doi: 10.1007/s001250051668. [DOI] [PubMed] [Google Scholar]
- 25.Igal RA, Wang P, Coleman RA. Triacsin C blocks de novo synthesis of glycerolipids and cholesterol esters but not recycling of fatty acid into phospholipid: evidence for functionally separate pools of acyl-CoA. Biochem J. 1997;324 (Pt 2):529–534. doi: 10.1042/bj3240529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleman RA, Lewin TM, Van Horn CG, Gonzalez-Baro MR. Do long-chain acyl-CoA synthetases regulate fatty acid entry into synthetic versus degradative pathways? J Nutr. 2002;132:2123–2126. doi: 10.1093/jn/132.8.2123. [DOI] [PubMed] [Google Scholar]
- 27.Musashi M, Ota S, Shiroshita N. The role of protein kinase C isoforms in cell proliferation and apoptosis. Int J Hematol. 2000;72:12–19. [PubMed] [Google Scholar]
- 28.Wang Q, Wang X, Evers BM. Induction of cIAP-2 in human colon cancer cells through PKC delta/NF-kappa B. J Biol Chem. 2003;278:51091–51099. doi: 10.1074/jbc.M306541200. [DOI] [PubMed] [Google Scholar]
- 29.Howe GR, Hirohata T, Hislop TG, Iscovich JM, Yuan JM, et al. Dietary factors and risk of breast cancer: combined analysis of 12 case-control studies. J Natl Cancer Inst. 1990;82:561–569. doi: 10.1093/jnci/82.7.561. [DOI] [PubMed] [Google Scholar]
- 30.Boyd NF, Stone J, Vogt KN, Connelly BS, Martin LJ, et al. Dietary fat and breast cancer risk revisited: a meta-analysis of the published literature. Br J Cancer. 2003;89:1672–1685. doi: 10.1038/sj.bjc.6601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grundy SM. Influence of stearic acid on cholesterol metabolism relative to other long-chain fatty acids. Am J Clin Nutr. 1994;60:986S–990S. doi: 10.1093/ajcn/60.6.986S. [DOI] [PubMed] [Google Scholar]
- 32.Mitropoulos KA, Miller GJ, Martin JC, Reeves BE, Cooper J. Dietary fat induces changes in factor VII coagulant activity through effects on plasma free stearic acid concentration. Arterioscler Thromb. 1994;14:214–222. doi: 10.1161/01.atv.14.2.214. [DOI] [PubMed] [Google Scholar]
- 33.Tholstrup T. Influence of stearic acid on hemostatic risk factors in humans. Lipids. 2005;40:1229–1235. doi: 10.1007/s11745-005-1490-1. [DOI] [PubMed] [Google Scholar]
- 34.Kelly FD, Sinclair AJ, Mann NJ, Turner AH, Abedin L, et al. A stearic acid-rich diet improves thrombogenic and atherogenic risk factor profiles in healthy males. Eur J Clin Nutr. 2001;55:88–96. doi: 10.1038/sj.ejcn.1601122. [DOI] [PubMed] [Google Scholar]
- 35.Louheranta AM, Turpeinen AK, Schwab US, Vidgren HM, Parviainen MT, et al. A high-stearic acid diet does not impair glucose tolerance and insulin sensitivity in healthy women. Metabolism. 1998;47:529–534. doi: 10.1016/s0026-0495(98)90235-9. [DOI] [PubMed] [Google Scholar]