Abstract
Stearate is an 18-carbon saturated fatty acid found in many foods in the western diet, including beef and chocolate. Stearate has been shown to have anti-cancer properties during early stages of neoplastic progression. However, previous studies have not investigated the effect of dietary stearate on breast cancer metastasis. In this study, we present evidence that exogenously supplied dietary stearate dramatically reduces the size of tumors that formed from injected human breast cancer cells within the mammary fat pads of athymic nude mice by approximately 50% and partially inhibits breast cancer cell metastasis burden in the lungs in this mouse model system. This metastatic inhibition appears to be independent of primary tumor size, as stearate fed animals that had primary tumors comparable in size to littermates fed either a safflower oil enriched diet or a low fat diet had reduced lung metastasis. Also stearate fed mice sub-groups had different primary tumor sizes but no difference in metastasis. This anti-metastasis effect may be due, at least in part, to the ability of stearate to induce apoptosis in these human breast cancer cells. Overall, this study suggests the possibility of dietary manipulation with selected long-chain saturated fatty acids such as stearate as a potential adjuvant therapeutic strategy for breast cancer patients wishing to maximize the suppression of metastatic disease.
Keywords: Breast cancer, dietary fat, metastasis, linoleic acid, stearic acid, stearate
Introduction
The role that dietary fat plays in breast cancer development and progression has remained a controversial area for over 60 years. While epidemiological studies have produced conflicting results, in vivo studies consistently support a role for dietary fat in breast cancer, suggesting it is not only the amount of fat, but also the type, present in the diet that affects tumorigenesis, cancer growth and metastasis (Reviewed in (1, 2). To date, studies suggest the omega-6 unsaturated fatty acid linoleic acid (C18:2) promotes breast cancer growth and metastasis (3-5). In contrast, the omega-3 unsaturated fatty acids linolenic acid (C18:3), eicosapentaenoic acid (20:5; EPA) and docosahexaenoic acid (22:6; DHA) inhibit tumor development and metastasis (6, 7). The saturated fatty acid stearic acid (C18:0) also inhibits tumorigenesis although the effect on metastasis has not been tested.
Stearic acid, or stearate, is an 18-carbon saturated fatty acid found in high concentrations in many foods in the western diet including beef, chocolate, and milk fats. Unlike other saturated fatty acids, such as palmitate (C16:0), stearate does not increase plasma cholesterol concentrations and has been shown to decrease plasma low density lipoprotein concentrations in humans (8). Interestingly, stearate has been found to have ‘anti-cancer properties’ both in vitro and in vivo, targeting proliferation, migration, and tumor invasion.
In vitro, stearate has been shown to inhibit breast cancer cell proliferation, induce breast cancer cell apoptosis, and inhibit fibrosarcoma cell invasion (9-11). To date, the molecular mechanisms underlying these effects have yet to be determined. In vivo, dietary stearate has been shown to decrease tumor incidence and delay tumor development in spontaneous and chemically-induced mammary tumor carcinogenesis models. Tinsley et al. fed CH3 mice diets composed of different concentrations of oils and, using linear regression, found that increased levels of dietary stearate correlated with decreased incidence of spontaneous mammary tumors (12). Similarly, when A/St mice were fed a 14% stearate/1% safflower oil diet, they developed fewer spontaneous tumors than the low fat diet, indicating that a diet composed of pure stearate, as opposed to an enriched oil, could effect tumorigenesis (13, 14). Habib et al. found that injecting stearate into rats treated with NMU decreased the average number of tumors/rat (14). So while stearate clearly effects the transformation and early neoplastic progression steps of cells, its role in an orthotopic cancer model of metastases using human cells derived from carcinomas has not previously been investigated.
Metastasis is defined as tumor growth at a secondary site distant and discontinuous from the primary site (15). This growth is a multi-step process and for tumors which spread by lymphohematogenous routes such as breast cancer begin with growth and invasion if extracellular matrix at the primary site, intravasion into the blood or lymphatic system, survival in this system during circulation, extravasion from the vessel, and colonization and growth at the secondary site. Inhibition of any of these steps results in an inhibition of secondary sites of tumor growth, i.e. metastatic foci. (Reviewed in 16).
Based on the known in vivo and in vitro data, we hypothesized that dietary stearate would inhibit breast cancer metastasis. Using the athymic nude mouse mammary fat pad injection model, we found that animals fed a high fat diet containing principally stearate demonstrate significantly decrease growth of primary tumors by approximately 50% and demonstrated a statistically significant decrease in macroscopic lung metastases. Further, we present evidence that this decrease in metastatic lung tumors is dependent on factors other than primary tumor size. Finally, we demonstrate that stearate in vitro induces cleavage of caspase-3 and PARP raising the possibility that stearate inhibits tumor growth and metastasis via apoptosis of the breast cancer cells.
Materials and Methods
Animals and Diets
3-4 week old female athymic mice were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN) and were maintained in microisolater cages in pathogen-free facilities. Animals were placed on one of three diets – a low fat diet comparable to normal rodent chow, a 20% safflower oil diet, or a 17% stearic acid/3% safflower oil diet for 3 weeks prior to the injection of human breast cancer cells. The diets were prepared by Harlan-Teklad (Madison, WI) and dietary compositions are shown in Table 1. The animals were fed ad libitum and the amount of food consumed was recorded. Mice were anesthetized with 3% Isoflurane in 2.5% O2 and weighed weekly.
Table 1.
| Control | Safflower | Stearate | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ingredients (g/kg) | Protein | CHO | Fat | Fiber | Protein | CHO | Fat | Fiber | Protein | CHO | Fat | Fiber | ||
| Casein | 174 | 0.4 | 2 | 0 | 203.58 | 0.47 | 2.34 | 0 | 203.58 | 0.47 | 2.34 | 0 | ||
| L-Cystine | 3 | 0 | 0 | 0 | 3.51 | 0 | 0 | 0 | 3.51 | 0 | 0 | 0 | ||
| Corn Starch | 1.34 | 375.74 | 0.83 | 0 | 0.72 | 202.1 | 0.45 | 0 | 0.72 | 202.1 | 0.45 | 0 | ||
| Maltodextrin (Lo-Dex) | 0 | 126.46 | 0 | 0.13 | 0 | 126.5 | 0 | 0.13 | 0 | 126.46 | 0 | 0.13 | ||
| Sucrose | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | ||
| Corn Oil | 0 | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Safflower Oil | 0 | 0 | 0 | 0 | 0 | 0 | 200 | 0 | 0 | 0 | 30 | 0 | ||
| Stearate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 170 | 0 | ||
| Cellulose (Fiber) | 0 | 0 | 0 | 48.13 | 0 | 0 | 0 | 48.13 | 0 | 0 | 0 | 0 | ||
| Mineral Mix, AIN-93G-MX | 0 | 7.73 | 0 | 0 | 0 | 9.04 | 0 | 0 | 0 | 0 | 0 | 48.13 | ||
| Vitamin Mix, AIN-93-VX | 0 | 9.75 | 0 | 0 | 0 | 11.4 | 0 | 0 | 0 | 9.04 | 0 | 0 | ||
| Choline Bitartrate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11.4 | 0 | 0 | ||
| TBHQ (Antioxidant) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 0 | 0 | 0 | 0 | |||||||||||
| Diet % | 17.83 | 62.01 | 5.28 | 4.83 | 20.78 | 44.95 | 20.3 | 4.83 | 20.78 | 44.95 | 20.28 | 4.83 | ||
| kcal/g | 0.71 | 2.48 | 0.48 | 0.83 | 1.8 | 1.83 | 0.83 | 1.8 | 1.83 |
Cancer Cells
MDA-MB-435 human breast cancer cells (obtained from Dr. Dan Welch; UAB) were grown and maintained in DMEM:F12 supplemented with 5% FBS, 2 mM glutamine, 1 mM sodium pyruvate, 0.2X non-essential amino acids and 1% penicillin/streptomycin (5% CO2). Cells were grown to 80-90% confluence prior to preparation for injection. To detach cells from the plates, cells were washed with 1× PBS and then treated with 3mM versene, pH 7.2. Cells were pelleted by centrifugation and resuspended in Hank's Buffered Saline Solution (HBSS). Cells were diluted to 1×107 cells/ml and were kept on ice until the time of injection to prevent clumping.
Experimental Design
The experimental timetable is shown in Figure 1. Briefly, animals were divided randomly into one of four groups – a low fat diet group, a safflower oil diet group, and two stearate diet groups. All animals were placed on the diets three weeks prior to injection of cancer cells. The low fat and safflower oil diet exposed tumors were allowed to reach an approximate mean tumor diameter of 10mm2-12mm2 (~253.6mm3-904.8mm3) at which time the primary tumors were removed (~9 weeks post-injection). One stearate group followed the same pattern although the tumors were not as large at 9 weeks post-injection as the tumors from the two control groups. The second stearate group's tumors were allowed to grow to the size of the tumors in the safflower and low fat groups (10mm2-12mm2) or for a maximum of 13 weeks after injection. After removal of the primary tumors, the animals were allowed to develop metastases for four weeks, sacrificed and the lungs were collected. All in vivo procedures were approved by the Institutional Animal Care and Use Committee.
Figure 1. Experimental Timetable.
Nude mice were placed on either a low fat diet, a high linoleic diet (Safflower) or a high stearic acid diet (Stearate (A)) for 3 weeks prior to injection with cancer cells. Tumors were allowed to develop for 9 weeks and were removed. Four weeks post-tumor removal, animals were sacrificed and their lungs were analyzed for metastases. A fourth group of animals were placed on the high stearic acid diet (Stearate (B)) and were injected with cancer cells 3 weeks later. The tumors were allowed to grow to the size of the tumors in the low fat and safflower groups before removal. Due to variation within this stearate group, it was broken down into 3 smaller groups – B-i, B-ii, and B-iii – based on when the tumors were removed. After 4 weeks, animals were sacrificed and their lungs were analyzed for metastases.
Mammary Fat Pad Injections
Animals were anesthetized with 3% Isoflurane in 2.5% O2. A small incision was made between the right 2nd and 3rd mammary fat pads and 1×106 MDA-MB-435 cells suspended in HBSS were injected into the 2nd mammary fat pad using a 27mm gauge needle (final volume of 100 μl). A single wound clip was used to close the incision and removed the following week.
Tumor Measurement
After the injection of the cells, mice were monitored weekly for the development of primary tumor masses. Once the tumors became visible (1-2 weeks post-injection), they were measured using a digital caliper. The tumor volume was estimated using the equation for a prolate ellipsoid where volume=(4/3)(length/2)(width/2)((length + width)/4).
Tumor Excision
The animals were anesthetized with isoflurane. The skin overlying the mammary tumor area was cleaned with a betadine solution and an incision was made circumferentially around the tumor down to its base. The tumor was excised and weighed. The wound was closed using wound clips which were removed one week later.
Necropsy
Four weeks after the removal of the primary tumor, mice were anesthetized with a combination of ketamine and xylazine and then decapitated. A visual examination of the lymph nodes and liver found no metastasis. The lungs were dissected from the mice and stored in Bouin's solution prior to the counting of visible tumors on all surfaces of the lungs. The counter was blinded to the identity of the samples prior to counting.
Cell Treatment with Stearate
Cells were grown to 60-70% confluence and serum starved for 24 hours prior to treatment with 50 μM stearate conjugated to fatty acid free BSA (FAF-BSA) in a manner previously described (17). Fatty acid concentrations were measured using the NEFAC kit from Wako Chemicals (Neuss, Germany).
Western Blotting
Following treatment, cells were washed with 1x PBS and lysed in lysis buffer composed of 25 mM HEPES; 150 mM NaCl; 1% NP-40; 10 mM MgCl2; 1 mM EDTA; 2% Glycerol; 1 mM NaF; 1 mM Na3V04; 25 mM β-glycerophosphate; 1 mM tetrasodium pyrophosphate; 1:1000 PMSF (100 mM) and 1:1000 Protease Inhibitor Cocktail. Proteins were separated by SDS-PAGE and transferred to PVDF membranes. Standard western blotting protocols were followed to detect cleaved caspase-3 (Cell Signaling; Danvers, MA), cleaved PARP (Cell Signaling), or β-actin (Sigma Aldrich; St. Louis, MO).
Caspase-3 Activity
Cells were grown to 60-70% confluence prior to serum starvation for 24 hours. After treatment with FAF-BSA or stearate for 12 hours, cells were washed with 1X PBS and caspase-3 activity was measured using the EnzCheck Capase-3 Activity Kit #1 (Invitrogen; Carlsbad, CA) according to the manufacturer's instructions.
Stastics
All data represent means +/- the S.E.M. ANOVA calculations were carried out using the SigmaStat 3.1® software program and statistical significance was confirmed using Tukey tests. Tumor metastasis was examined by the Cochran-Armitage Test for trend. Differences in tumor weight were tested by the generalized linear models with Tukey adjustment for multiple comparisons. The analyses of subcutaneous tumor volume and body weight were performed using a repeated measures model with PROC MIXED (SAS®Ver. 9.1). The effects of treatment group, time in days and the interaction of treatment group and time were evaluated by F tests. Curvature in the models was tested for by a quadratic term for time. The a priori planned comparisons of specific differences in predicted treatment means averaged over time and at the last timepoint were computed by t-statistics. For all analyses a two-sided p value of < 0.05 was deemed statistically significant.
Results
Based on the known in vitro and in vivo data, we hypothesized that dietary stearate would inhibit breast cancer cell metastasis. To test this hypothesis, we injected MDA-MB-435 breast cancer cells into the mammary fat pad of athymic mice and monitored the animals for metastases to the lungs.
Diets, Food Intake and Weight Gain
With the help of Harlan Teklad, three experimental diets were developed - one low fat and two high fat. The low fat diet was 5% fat derived from corn oil and comparable to normal rodent chow. The stearate diet was 17% pure stearic acid and 3% safflower oil to ensure the animals received adequate amounts of the essential fatty acids. Finally, a 20% safflower oil diet was used as a positive low fat, as linoleic acid, the predominant fatty acid in safflower oil, has been shown to promote metastasis in this model (3-5).
Because the safflower oil and stearate diets were isocaloric, but not the low fat diet, we monitored food consumption and weight gain to ensure the animals did not have significant discrepancies in energy intake. As shown in Figure 2A, the low fat diet animals consumed the most kilocalories/day, followed by the stearate diet animals, and then the safflower oil diet injesting mice. Despite difference in food intake, there was no overt difference in weight gain between the diets (Figure 2B). The slight decrease in weight at week 10 was mostly likely due to recovery from the tumor removal at week 9. The relatively large increase in mouse weight observed in the first week is consistent with the normal nude mouse growth pattern (Harland, data not shown). The drop off in mouse weight seen with all three diets from week 12-13 is likely due to an overall decrease in health due to metastases however we cannot rule out other causes. To determine if there were differences in overall weight gain, the total weight gain at week 10, minus the weight of the tumor, was determined. No significant difference was observed between the three diets (Figure 2C). The stearate (B) group was nearly identical to the stearate (A) group and, therefore, was not shown.
Figure 2. Food Intake and Weight Gain.
A) The average kcal consumed per animal per day was calculated. Of the three diets, the animals on the low fat diet ate the most, followed by the high stearate diet and then the safflower oil diet. Each group's intake was significantly different from the other two diets (§p value of stearate (A) vs. low fat <0.001; ¥p value of low fat vs. safflower <0.001; *p value of stearate (A) vs. safflower <0.001 by ANOVA). B) The weight of the animals was measured once a week for the duration of the experiment. Although differences were observed at individual weeks in the experiment, overall no significant change was seen. (n=14-21 per diet per week; §p value of stearate (A) vs. low fat <0.05; *p value of stearate vs. safflower <0.05 by ANOVA). The second stearate group was not different from the first and was therefore not represented on the graphs. C) The total weight gain of the animals at week 10 was determined. The weight does not include the weight of the tumors removed from the animals. No significant difference was observed between the three groups.
Mammary Tumor Analysis and Metastatic Burden of Low fat, Safflower, Stearate (A), and Stearate (B) Diets
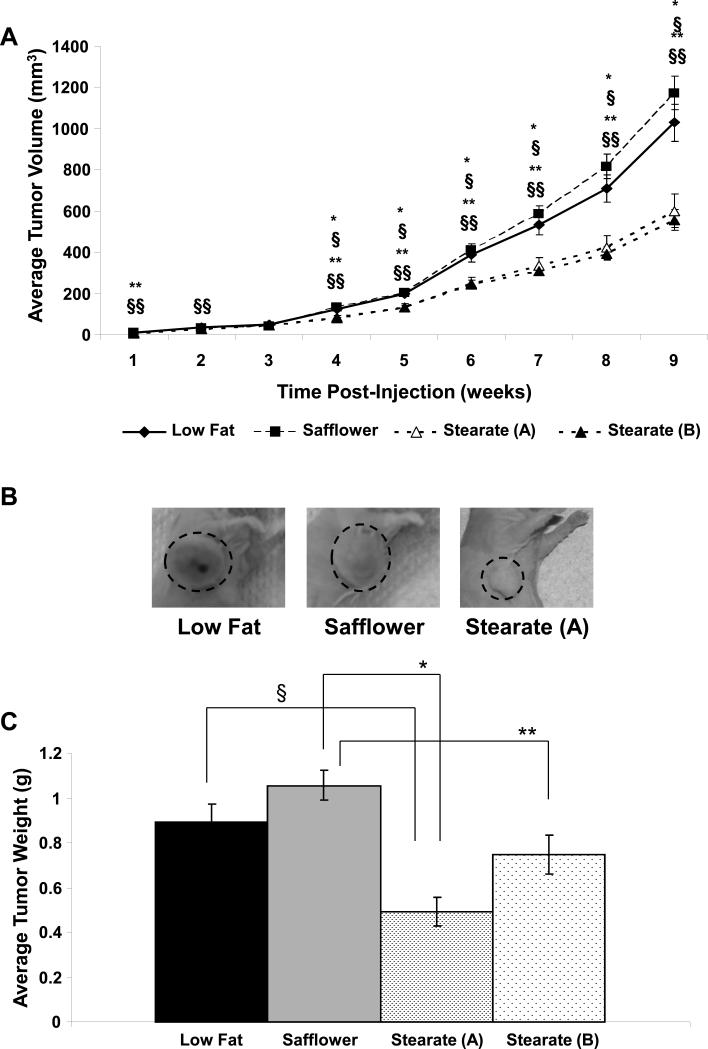
Following injection of the MDA-MB-435 cancer cells, the length and width of the tumors was measured weekly and used to calculate the volume (Figure 3a). Between 1 and 3 weeks post-injection, no significant difference was seen in the mean tumor volumes of the four groups. Starting four weeks post-injection, the stearate groups began to become significantly smaller than the other two diets. This trend continued until the 9th week post-injection. At that point, the tumors in the low fat, safflower and stearate (A) groups were excised. As described in the materials and methods and shown in Figure 1, the stearate (B) tumors were allowed to grow longer until the size reached that of the low fat and safflower tumors. Figure 3B depicts representative images of the tumors present in the three different diets.
Figure 3. Tumor Weights and Volumes for the Low fat, Safflower, and Stearate (A) Groups.
A) Mammary fat pad tumors of the animals were measured weekly after injection. Based on the estimated volumes, stearate began to decrease the average volume of the tumors approximately 4 weeks post-injection. The stearate (A) and stearate (B) primary tumors were not different. (n=20-21 animals per diet; §p value of stearate (A) vs. low fat <0.009; §§p value of stearate (B) vs. low fat<0.021; *p value of stearate (A) vs. safflower <0.003; **p value of stearate (B) vs. safflower<0.015 by ANOVA). When the data were analyzed using a repeated measures model and the curvature of the data points were estimated, all diets were different at week 9 except low fat vs. safflower (p<0.05). B) Immediately prior to removal of the mammary fat pad tumors at week 9, photographs were taken of mice in the low fat, safflower, and stearate (A) groups. All three of these tumors were removed on the same day. Representative images are shown. Animals on the stearate (A) diet tended to have noticeably smaller tumors compared to the other two diets. C) Following tumor removal, tumors were weighed and averages were calculated for each dietary group. Note: safflower oil, low fat and stearate A primary tumors were removed at 9 weeks post injection while the stearate B group tumors were removed and weighed at 11, 12 and 13 weeks post injection of cells. No difference was seen between the low fat and safflower oil treated animals, the low fat and stearate (B) animals, or the stearate (A) and stearate (B) animals. Differences were observed between all other diets (n=20-21; §p value of low fat vs. stearate (A) <0.002; *p value of stearate (A) vs. safflower <0.001; **p value of stearate (B) vs. safflower <0.003 by ANOVA).
Primary tumor weights are shown in Figure 3C. The tumors from the low fat and safflower fed groups (black and grey solid bars) were significantly larger than the stearate (A) fed animals. The stearate (B) fed mice tumors were significantly different in weight than the safflower tumors, but not the stearate (A) or low fat fed mice tumors.
As shown in Figure 4A, the stearate diet significantly decreased the number of lung metastases in the mice compared to the pulmonary metastases in the mice fed either the low fat or safflower diets. When the number of metastases was quantitated by diet, a larger number of stearate fed animals had lower lung tumor burdens as compared to the low fat and safflower diets, indicating stearate successfully inhibited metastases (Figure 4B). When the tumor volume to metastatic burden was calculated, no difference was observed indicating this effect may be independent of the primary tumor size (Figure 4C).
Figure 4. Analysis of Lung Metastases from the Low fat, Safflower and Stearate (A) Groups.
A) The number of macroscopic lung metastases was counted following necropsy. Stearate significantly decreased the number of macroscopic lung metastases in both the stearate (A) and stearate (B) groups. (n=18-20 animals per diet; §p value of stearate (A) vs. low fat <0.021; *p value of stearate (A) vs. safflower<0.024; §§p value of stearate (B) vs. low fat<0.022; **p value of stearate (B) vs. safflower<0.024 by Cochran-Armitage trend test). B) The animals on the low fat and safflower diets tended to have more metastatic tumors than those on the stearate diet. Interestingly, a large difference was not seen between the two groups of animals fed the stearate diet. C) When the tumor volume to metastases was calculated, no difference was observed.
Mammary Tumor Analysis and Metastatic Burden of the Stearate (B) Subgroups
When using the mammary fat pad model of metastasis, the size of the primary tumor appears to correlate to the number of metastases on the lung surface (18). Therefore, we hypothesized, if stearate decreases the size of the primary mammary fat pad tumor, any decrease in metastasis would be due to this decrease in primary tumor size rather than an effect on another part of the metastatic cascade. To test this hypothesis, a second group of athymic mice carrying human tumor loads was fed the stearate diet, but rather than removing the primary tumors at the time the tumors were removed from the low fat and safflower animals, the tumors were allowed to grow larger.
One of the most striking results of this experiment was that stearate (B) animals displayed a much larger difference in the range of tumor volumes. Tumors in subgroups B-i and B-ii were removed as they reached approximately 10mm2-12mm2 in size (diagrammed in Figure 1). Since it appeared the tumors in the B-iii group reached a plateau 6 weeks post-injection, it was decided to remove the primary tumors 13 weeks post-injection. Tumor volumes for these groups are shown in Figure 5A. Interestingly, the tumor volumes were not statistically significant until 8 weeks post-injection. Week 11 post-injection was the only point in which all three groups were statistically significant. The tumors in B-i and B-ii appeared to continue growing, even in the presence of the stearate diet, whereas the B-iii reached a maximum volume at six weeks. Comparing the volume of the tumors at the times of removal for each sub group, B-iii tumors were significantly smaller than both B-i and B-ii tumors. No difference was observed between B-i and B-ii (Fig. 5B) and the tumor weight and volume of B-i and B-ii groups were not different than tumors of the low fat and safflower groups. However, as with the tumor volume, the average weight of the B-iii tumors was significantly less than the other two groups.
Figure 5. Tumor Analysis of Animals on the Stearate (B) Diet.
A) Tumor volumes were calculated weekly for each of the stearate (B) sub groups. Approximately week 8 post injection, 2c became statistically smaller than B-ii and around week 10 than 2b. At week 11 post injection, all groups were different. (n=7 animals per group; £p value of B-i vs. B-iii <0.001; &p value of B-ii vs. B-iii <0.002; #p value of B-i vs. 2bii <0.02). B) After the tumors were removed, they were weighed. No difference was observed between B-i and B-ii. B-iii tumors were statistically smaller than both B-i and B-ii. C) There was no difference in the metastatic tumor number per animal between the three stearate (B) subgroups.
Finally, each stearate (B) subgroup was sacrificed four weeks after primary tumor removal (Figure 1) and the number of metastases was counted. There was no significant difference in the number of macroscopic metastases present between the subgroups; B-i, B-ii, and B-iii had 15 +/-7, 4 +/- 2, and 6 +/-3 average metastatic lesions per animal, respectively. The slightly larger average for the B-i group was most likely due to one mouse that had 52 macroscopic lung metastases. The number of metastatic tumors per animal was similar for the three subgroups irrespective of the fact that primary tumor weight was significantly lower in the B-iii subgroup compared to B-i and B-ii (Figure 5C). Thus, the stearate diet appears to decrease the number of metastases independent of primary tumor size. In addition, the primary tumor sizes for B-i and B-ii were similar to low fat and safflower groups but with significantly fewer metastases per animal, also indicating that stearate decreases metastasis independent of primary tumor size.
Stearate Induces Apoptosis of MDA-MB-435 Breast Cancer Cells In Vitro
As stearate has been shown to induce apoptosis of human breast cancer cells in vitro, we tested MDA-MB-435 cells for cleaved caspase-3 and cleaved PARP following treatment with stearate. As shown in Figure 6A, stearate induces cleavage of caspase-3 and its downstream target PARP. To determine what concentration of stearate induces caspase-3 activity, cells were treated with concentrations ranging from 1-100 μM. Stearate begins to induce apoptosis of the MDA-MB-435 breast cancer cells at a concentration of approximately 25 μM (Figure 6B).
Figure 6. Stearate Induced Apoptosis of MDA-MB-435 Breast Cancer Cells In Vitro.
A) Cells were treated with 50 μM stearate for the times indicated and the presence of cleaved caspase-3 and cleaved PARP was determined by immunblot. As shown, stearate induces cleavage of the two proteins 12-24 hours post-treatment indicating stearate is inducing apoptosis of the breast cancer cells (n=3). B) Cells were treated with 1-100 μM stearate for 12 hours and caspase-3 activity was measured using a fluorescence based assay. Stearate activated caspase-3 in a dose dependent manner (n=4; *p<0.023 ANOVA).
Discussion
Stearate has been found to inhibit invasion, inhibit proliferation and induce apoptosis of breast cancer and other cells, as well as induce cytotoxicity in a variety of normal and malignant cell lines (9-11, 19). This body of work indicates the possibility for stearate to function as an anti-metastasic agent in vivo. This is the first study to demonstrate that stearate does inhibit breast cancer metastasis in vivo and does so by approximately 50%. Interestingly stearate also inhibits the primary tumor size and weight by approximately 50% although stearate's inhibition of metastasis was independent of primary tumor size. While this does not necessarily rule out a relationship between the effects of stearate on the primary tumor and decreased metastasis it certainly increases the possibility that stearate effects another part of the metastatic cascade. In vitro, stearate induced apoptosis of the MDA-MB-435 breast cancer cells in a dose dependent manner (Figure 6). As 50 μM can be considered a high-normal stearate concentration in the plasma of humans, this suggests that physiological concentrations of stearate are sufficient to induce apoptosis of breast cancer cells. This may partially explain the decrease of tumor size and metastases seen in the stearate-fed mice compared to the low fat and high safflower fed mice. However, because the primary tumors have similar growth patterns up to 3 weeks post-injection, if stearate is affecting the viability of the cancer cells, it is most likely not acting through an initial selection of sub-populations of cells that are sensitive to the apoptotic effects of stearate.
The three week delay in dietary stearate decreasing primary tumor volumes may suggest changes in the tumor microenvironment such as an inhibition of angiogenesis. Compounds derived from arachidonic acid and those derived from EPA appear to have opposing roles in the cell. Studies have found that linoleic acid enhanced the development of tumors in animals that were injected in the mammary fat pad with MDA-MB-435 or MDA-MB-231 breast cancer cells. To date, the mechanism underlying this increase is unknown. However, it has been hypothesized that the increase in linoleic acid causes an increase in arachidonic acid, thereby increasing prostaglandin synthesis (Reviewed in 20, 21). Consistent with this hypothesis, treatment of animals on a high linoleic acid diet with indomethacin, a cyclooxygenase inhibitor, inhibited prostaglandin synthesis and linoleic acid induced metastasis (22). Omega-3 fatty acids such as EPA, and DHA have been shown to inhibit tumor angiogensis and breast cancer cell metastasis, perhaps due to a difference in lipid metabolism or an inhibition of arachidonic-derived eicosanoids (7, 23-25). To date, the role of saturated fatty acids on prostaglandin synthesis has not been investigated in breast cancer tumors. However, in vitro, 20 μM stearate is sufficient to almost completely inhibit cyclooxgenase-1 activity and partially inhibit cyclooxygenase-2 activity (26). Furthermore, stearate has been shown to induce apoptosis of human endothelial cells, although the 200-300 μM concentrations tested are within the pathophysiological range (27, 28). These results indicate stearate may inhibit angiogenesis by inhibiting prostaglandin synthesis or affecting vessel viability in the mammary fat pad tumors, and, therefore, may cause a decrease in primary tumor size and inhibit metastasis.
Stearate could also be modulating the immune response of nude mice to the tumors, thereby accounting for the variation observed in the stearate (B) subgroups. These animals have been shown previous to display a large amount of heterogeneity concerning specific T-cell subpopulations (29). This suggests that although the mice are an inbred population, the immune response to the tumor may vary between mice. Interestingly, macrophages, cells known to be modulated by fatty acids, have been extensively studied for their cancer promoting or cancer inhibiting effects. Two lineages of macrophages have been uncovered that effect tumors in opposing manners. The M1 lineage is generally associated with tumor suppression and resistance whereas the M2 linage is associated with tumor promotion and angiogenesis (Reviewed in 30). Although much work has been done analyzing the effect of n-3 and n-6 fatty acids in regard to immune response, little is known concerning the saturated fatty acids. Stearate has been demonstrated previously to induce apoptosis of the murine macrophage cell line J774 (31). Dietary stearate has been associated with a decrease in natural killer T-cells in the spleen of mice, whereas palmititate, a 16-carbon saturated fatty acid has been associated with an increase in activity (32). NK cells are known to stimulate M2 macrophages through the release of certain cytokines (30). These results suggest that dietary stearate may inhibit the differentiation of monocytes into M2 macrophages, thereby inhibiting tumor progression. These data also suggest that the saturated fatty acids have differing effects in vivo. Future studies are necessary to the cause of the decrease in tumor size.
Interestingly, the animals in the stearate (B) group displayed a range in terms of tumor growth, but not macroscopic metastases. The tumors in the B-i and B-ii subgroups reached a size comparable to the low fat and safflower groups although an additional 2-3 weeks of tumor growth were necessary for this to occur (9 weeks post-injection for the low fat and safflower animals versus 11 and 12 weeks post injection for B-i and B-ii, respectively). The tumors in the B-iii group reached a maximum volume approximately 6 weeks after cancer cell injection. The underlying cause of this plateau is unknown although stearate could be inhibiting proliferation or inducing apoptosis of the cancer cells. Stearate has been shown previously to inhibit epidermal growth factor dependent proliferation in the Hs578t human breast cancer cells and we present evidence here that the MDA-MB-435 breast cancer cells are sensitive to stearate induced apoptosis ((9); Figure 6). Furthermore, as cancer cell masses are generally accepted as a heterogeneous population of neoplastic, there could be a selection occurring for stearate-insensitive cells. Further studies are necessary to test these hypotheses.
Given the complexity of dietary studies, the effect seen with the high stearate diet may be due to a decrease in linoleate, the predominate fatty acid in safflower oil. Safflower oil is composed of 77% linoleic acid. The stearate diet was 3% safflower oil, meaning the diet was 2.3% linoleic acid as compared to the 15.5% in the safflower diet. Using the same mammary fat pad injection model with the MDA-MB-435 breast cancer cells, Rose et al. reported that significantly more macroscopic lung metastases in animals fed a diet composed of 12% linoleic acid than animals fed a diet with 2% linoleic acid, although no difference was observed in the growth rate of the primary tumor. (4, 5). These results would argue our effect may, at least in part, be due to a decrease in linoleic acid. However, corn oil is 56% linoleic acid, meaning that our low fat diet was composed of 2.8% linoleic acid. Given no significant difference was observed between the low fat (2.8% linoleic acid) and safflower (15.5% linoleic acid) diets in terms of tumor volume or lung metastases, the effects seen with the stearate diet are most likely not due to a decrease in linoleic acid. Also, there was a significant reduction in primary tumor and metastasis seen with the stearate diet compared to the low fat diet that contained comparable amounts of linoleic acid.
In summary, dietary stearate inhibited the growth of MDA-MB-435 human breast cancer cells in the mammary fat pad model system and partially reduced metastatic burden in the lung. The inhibition of metastasis was independent of the size of the primary tumor as animals that developed larger tumors also had an inhibition of metastasis. Physiological concentrations of stearate where also sufficient to induce apoptosis in vitro, providing a potential mechanism observed in vivo. There is also evidence in the literature to support other potential in vivo mechanisms, including inhibition of angiogenesis and modulation of the immune system, and these are on going areas of investigation in our laboratory. The degree of inhibition of metastasis by dietary stearate indicates that it may be a potential adjuvant therapeutic strategy for breast cancer patients to increase the suppression of metastatic disease.
Acknowledgements
This project was funded in part, by beef and veal producers and importers through their $1-per-head checkoff and was produced for the Cattlemen's Beef Board and state beef councils by the National Cattlemen's Beef Association. This work was also funded by Grant Number R21 AT 001636 (RWH) from the National Center for Complementary and Alternative Medicine (NCCAM). Its contents are solely the responsibility of the authors and do not necessarily represents of the official views of the NCCAM, or the National Institutes of Health.
Abbreviations
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- NK
natural kill T cells
References
- 1.Welsch CW. Relationship between dietary fat and experimental mammary tumorigenesis: a review and critique. Cancer Res. 1992;52:2040s–2048s. [PubMed] [Google Scholar]
- 2.Lee MM, Lin SS. Dietary fat and breast cancer. Annu Rev Nutr. 2000;20:221–48. doi: 10.1146/annurev.nutr.20.1.221. [DOI] [PubMed] [Google Scholar]
- 3.Rose DP, Connolly JM, Liu XH. Effects of linoleic acid and gamma-linolenic acid on the growth and metastasis of a human breast cancer cell line in nude mice and on its growth and invasive capacity in vitro. Nutr Cancer. 1995;24:33–45. doi: 10.1080/01635589509514391. [DOI] [PubMed] [Google Scholar]
- 4.Rose DP, Connolly JM, Liu XH. Effects of linoleic acid on the growth and metastasis of two human breast cancer cell lines in nude mice and the invasive capacity of these cell lines in vitro. Cancer Res. 1994;54:6557–62. [PubMed] [Google Scholar]
- 5.Rose DP, Hatala MA, Connolly JM, Rayburn J. Effect of diets containing different levels of linoleic acid on human breast cancer growth and lung metastasis in nude mice. Cancer Res. 1993;53:4686–90. [PubMed] [Google Scholar]
- 6.Rose DP, Connolly JM, Rayburn J, Coleman M. Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. J Natl Cancer Inst. 1995;87:587–92. doi: 10.1093/jnci/87.8.587. [DOI] [PubMed] [Google Scholar]
- 7.Rose DP, Connolly JM. Effects of dietary omega-3 fatty acids on human breast cancer growth and metastases in nude mice. J Natl Cancer Inst. 1993;85:1743–7. doi: 10.1093/jnci/85.21.1743. [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM. Influence of stearic acid on cholesterol metabolism relative to other long-chain fatty acids. Am J Clin Nutr. 1994;60:986S–990S. doi: 10.1093/ajcn/60.6.986S. [DOI] [PubMed] [Google Scholar]
- 9.Wickramasinghe NS, Jo H, McDonald JM, Hardy RW. Stearate inhibition of breast cancer cell proliferation. A mechanism involving epidermal growth factor receptor and G-proteins. Am J Pathol. 1996;148:987–95. [PMC free article] [PubMed] [Google Scholar]
- 10.Hardy S, El-Assaad W, Przybytkowski E, Joly E, Prentki M, Langelier Y. Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. A role for cardiolipin. J Biol Chem. 2003;278:31861–70. doi: 10.1074/jbc.M300190200. [DOI] [PubMed] [Google Scholar]
- 11.Singh RK, Hardy RW, Wang MH, Williford J, Gladson CL, McDonald JM, Siegal GP. Stearate inhibits human tumor cell invasion. Invasion Metastasis. 1995;15:144–55. [PubMed] [Google Scholar]
- 12.Tinsley IJ, Schmitz JA, Pierce DA. Influence of dietary fatty acids on the incidence of mammary tumors in the C3H mouse. Cancer Res. 1981;41:1460–5. [PubMed] [Google Scholar]
- 13.Bennett AS. Effect of dietary stearic acid on the genesis of spontaneous mammary adenocarcinomas in strain A/ST mice. Int J Cancer. 1984;34:529–33. doi: 10.1002/ijc.2910340416. [DOI] [PubMed] [Google Scholar]
- 14.Habib NA, Wood CB, Apostolov K, Barker W, Hershman MJ, Aslam M, Heinemann D, Fermor B, Williamson RC, Jenkins WE, et al. Stearic acid and carcinogenesis. Br J Cancer. 1987;56:455–8. doi: 10.1038/bjc.1987.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welch DR. Do we need to redefine a cancer metastasis and staging definitions? Breast Dis. 2006;26:3–12. doi: 10.3233/bd-2007-26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 17.Spector AA, Hoak JC. An improved method for the addition of long-chain free fatty acid to protein solutions. Anal Biochem. 1969;32:297–302. doi: 10.1016/0003-2697(69)90089-x. [DOI] [PubMed] [Google Scholar]
- 18.Price JE, Polyzos A, Zhang RD, Daniels LM. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990;50:717–21. [PubMed] [Google Scholar]
- 19.Fermor BF, Masters JR, Wood CB, Miller J, Apostolov K, Habib NA. Fatty acid composition of normal and malignant cells and cytotoxicity of stearic, oleic and sterculic acids in vitro. Eur J Cancer. 1992;28A:1143–7. doi: 10.1016/0959-8049(92)90475-h. [DOI] [PubMed] [Google Scholar]
- 20.Rose DP, Connolly JM. Regulation of tumor angiogenesis by dietary fatty acids and eicosanoids. Nutr Cancer. 2000;37:119–27. doi: 10.1207/S15327914NC372_1. [DOI] [PubMed] [Google Scholar]
- 21.Harris RE, Robertson FM, Abou-Issa HM, Farrar WB, Brueggemeier R. Genetic induction and upregulation of cyclooxygenase (COX) and aromatase (CYP19): an extension of the dietary fat hypothesis of breast cancer. Med Hypotheses. 1999;52:291–2. doi: 10.1054/mehy.1998.0009. [DOI] [PubMed] [Google Scholar]
- 22.Connolly JM, Liu XH, Rose DP. Dietary linoleic acid-stimulated human breast cancer cell growth and metastasis in nude mice and their suppression by indomethacin, a cyclooxygenase inhibitor. Nutr Cancer. 1996;25:231–40. doi: 10.1080/01635589609514447. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Stavro PM, Thompson LU. Dietary flaxseed inhibits human breast cancer growth and metastasis and downregulates expression of insulin-like growth factor and epidermal growth factor receptor. Nutr Cancer. 2002;43:187–92. doi: 10.1207/S15327914NC432_9. [DOI] [PubMed] [Google Scholar]
- 24.Hardman WE, Sun L, Short N, Cameron IL. Dietary omega-3 fatty acids and ionizing irradiation on human breast cancer xenograft growth and angiogenesis. Cancer Cell Int. 2005;5:12. doi: 10.1186/1475-2867-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardman WE. Dietary canola oil suppressed growth of implanted MDA-MB 231 human breast tumors in nude mice. Nutr Cancer. 2007;57:177–83. doi: 10.1080/01635580701277445. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto Y, Yonemura T, Sakuma S. Stearic acid potently modulates the activity of cyclooxygenase-1, but not cyclooxygenase-2, in the form of its CoA ester. Prostaglandins Leukot Essent Fatty Acids. 2008;78:81–4. doi: 10.1016/j.plefa.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 27.Artwohl M, Lindenmair A, Sexl V, Maier C, Rainer G, Freudenthaler A, Huttary N, Wolzt M, Nowotny P, Luger A, Baumgartner-Parzer SM. Different mechanisms of saturated versus polyunsaturated free fatty acid-induced apoptosis in human endothelial cells. J Lipid Res. 2008 doi: 10.1194/jlr.M800393-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Artwohl M, Roden M, Waldhausl W, Freudenthaler A, Baumgartner-Parzer SM. Free fatty acids trigger apoptosis and inhibit cell cycle progression in human vascular endothelial cells. Faseb J. 2004;18:146–8. doi: 10.1096/fj.03-0301fje. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald HR, Lees RK, Bron C, Sordat B, Miescher G. T cell antigen receptor expression in athymic (nu/nu) mice. Evidence for an oligoclonal beta chain repertoire. J Exp Med. 1987;166:195–209. doi: 10.1084/jem.166.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 31.Lima TM, Kanunfre CC, Pompeia C, Verlengia R, Curi R. Ranking the toxicity of fatty acids on Jurkat and Raji cells by flow cytometric analysis. Toxicol In Vitro. 2002;16:741–7. doi: 10.1016/s0887-2333(02)00095-4. [DOI] [PubMed] [Google Scholar]
- 32.Jeffery NM, Sanderson P, Newsholme EA, Calder PC. Effects of varying the type of saturated fatty acid in the rat diet upon serum lipid levels and spleen lymphocyte functions. Biochim Biophys Acta. 1997;1345:223–36. doi: 10.1016/s0005-2760(96)00174-9. [DOI] [PubMed] [Google Scholar]