Abstract
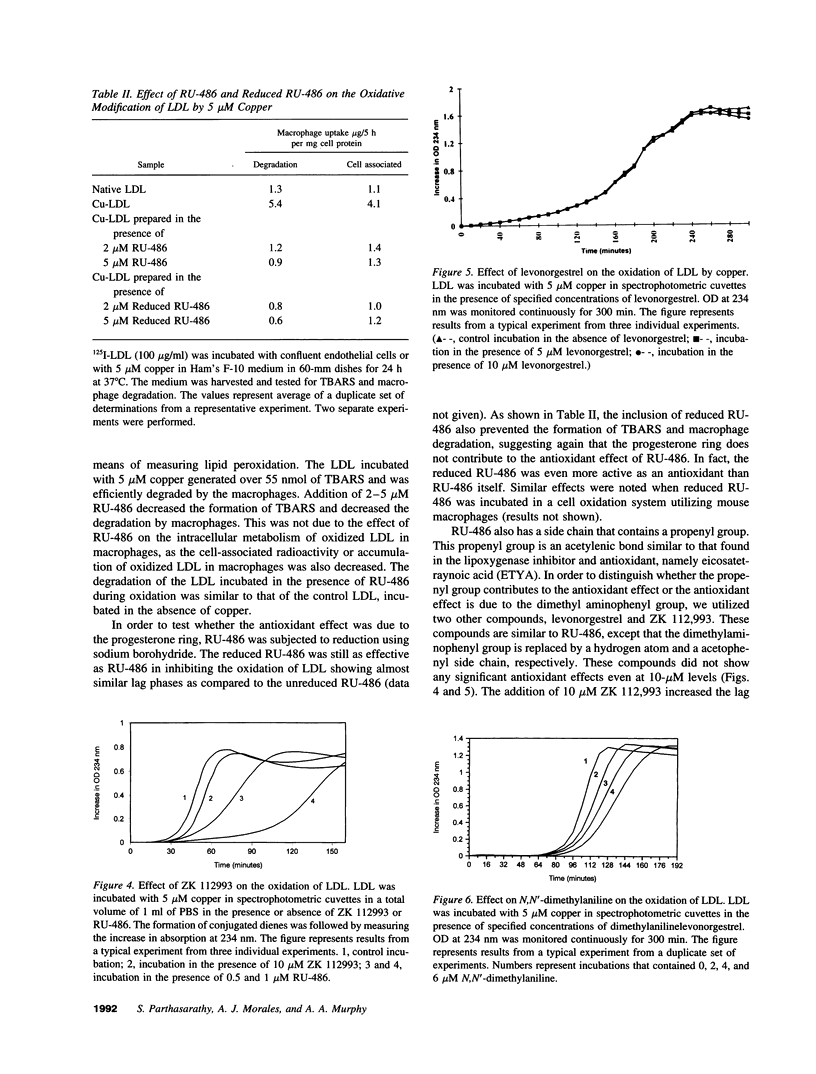
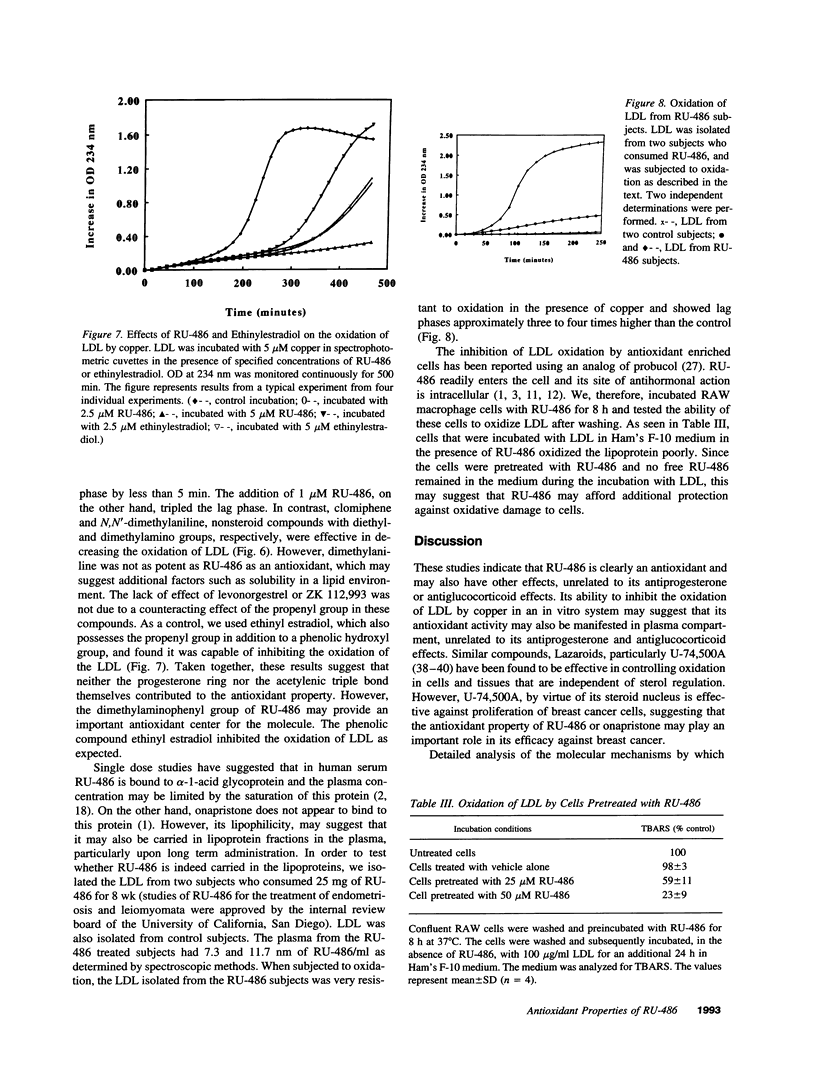
RU-486 (17 beta-hydroxy-4-dimethylaminophenyl-17-alpha-propenyl estrone 4,9 diene-3-one; mifepristone) is suggested to act by binding to progesterone and glucocorticoid receptors. Based on its chemical nature, we anticipated that RU-486 may have potent antioxidant properties. We used the oxidation of LDL as our model system. RU-486 and a similar compound, onapristone, at 1-5-microM concentrations, decreased the formation of oxidized LDL. LDL isolated from plasma of subjects who were orally supplemented with RU-486 was resistant to oxidation, as compared to LDL isolated from control plasma. The antioxidant effect of RU-486 appears to reside in the dimethylaminophenyl side chain moiety. Reduction of the A-ring of the steroid molecule had no effect on its antioxidant property. Analogs of RU-486 which lack the dimethylaminophenyl group, were without antioxidant activity. Levonorgestrel, which lacks the dimethylaminophenyl group failed to inhibit the oxidation of LDL even at 100-microM levels. In contrast, ethinylestradiol and estradiol which do not possess the dimethylamino group, were able to inhibit the oxidation of LDL by virtue of their phenolic steroid "A" ring. Thus RU-486, with its long half life, high plasma concentrations, association with lipoproteins, and ability to readily enter the cell may have additional intra- and extra-cellular antioxidant effects.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aeba R., Killinger W. A., Keenan R. J., Yousem S. A., Hamamoto I., Hardesty R. L., Griffith B. P. Lazaroid U74500A as an additive to University of Wisconsin solution for pulmonary grafts in the rat transplant model. J Thorac Cardiovasc Surg. 1992 Nov;104(5):1333–1339. [PubMed] [Google Scholar]
- Bakker G. H., Setyono-Han B., Portengen H., De Jong F. H., Foekens J. A., Klijn J. G. Treatment of breast cancer with different antiprogestins: preclinical and clinical studies. J Steroid Biochem Mol Biol. 1990 Dec 20;37(6):789–794. doi: 10.1016/0960-0760(90)90421-g. [DOI] [PubMed] [Google Scholar]
- Bardon S., Vignon F., Chalbos D., Rochefort H. RU486, a progestin and glucocorticoid antagonist, inhibits the growth of breast cancer cells via the progesterone receptor. J Clin Endocrinol Metab. 1985 Apr;60(4):692–697. doi: 10.1210/jcem-60-4-692. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Henriksson-Freyschuss A., Breuer O., Diczfalusy U., Berglund L., Henriksson P. The antioxidant butylated hydroxytoluene protects against atherosclerosis. Arterioscler Thromb. 1991 Jan-Feb;11(1):15–22. doi: 10.1161/01.atv.11.1.15. [DOI] [PubMed] [Google Scholar]
- Bowden R. T., Hissom J. R., Moore M. R. Growth stimulation of T47D human breast cancer cells by the anti-progestin RU486. Endocrinology. 1989 May;124(5):2642–2644. doi: 10.1210/endo-124-5-2642. [DOI] [PubMed] [Google Scholar]
- Bygdeman M., Swahn M. L. Progesterone receptor blockage. Effect on uterine contractility and early pregnancy. Contraception. 1985 Jul;32(1):45–51. doi: 10.1016/0010-7824(85)90115-5. [DOI] [PubMed] [Google Scholar]
- Carew T. E., Schwenke D. C., Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasserot-Golaz S., Beck G. How the potency of the steroid RU486 is related to P450 activities induced by dexamethasone and phenobarbital in rat hepatoma cells. J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):653–657. doi: 10.1016/0960-0760(92)90399-4. [DOI] [PubMed] [Google Scholar]
- Cohen-Solal K., Bailly A., Rauch C., Quesne M., Milgrom E. Specific binding of progesterone receptor to progesterone-responsive elements does not require prior dimerization. Eur J Biochem. 1993 May 15;214(1):189–195. doi: 10.1111/j.1432-1033.1993.tb17912.x. [DOI] [PubMed] [Google Scholar]
- Couzinet B., Le Strat N., Ulmann A., Baulieu E. E., Schaison G. Termination of early pregnancy by the progesterone antagonist RU 486 (Mifepristone). N Engl J Med. 1986 Dec 18;315(25):1565–1570. doi: 10.1056/NEJM198612183152501. [DOI] [PubMed] [Google Scholar]
- Cushing S. D., Berliner J. A., Valente A. J., Territo M. C., Navab M., Parhami F., Gerrity R., Schwartz C. J., Fogelman A. M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Zweifel B. S., Schonfeld G. Probucol attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits. Br J Pharmacol. 1989 Oct;98(2):612–618. doi: 10.1111/j.1476-5381.1989.tb12635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieber-Rotheneder M., Puhl H., Waeg G., Striegl G., Esterbauer H. Effect of oral supplementation with D-alpha-tocopherol on the vitamin E content of human low density lipoproteins and resistance to oxidation. J Lipid Res. 1991 Aug;32(8):1325–1332. [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Gravanis A., Schaison G., George M., de Brux J., Satyaswaroop P. G., Baulieu E. E., Robel P. Endometrial and pituitary responses to the steroidal antiprogestin RU 486 in postmenopausal women. J Clin Endocrinol Metab. 1985 Jan;60(1):156–163. doi: 10.1210/jcem-60-1-156. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H., Benhamou B., Berry M., Bocquel M. T., Gofflo D., Garcia T., Lerouge T., Metzger D., Meyer M. E., Tora L. Mechanisms of antihormone action. J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):217–221. doi: 10.1016/0960-0760(92)90347-l. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H. Transcription activation by estrogen and progesterone receptors. Annu Rev Genet. 1991;25:89–123. doi: 10.1146/annurev.ge.25.120191.000513. [DOI] [PubMed] [Google Scholar]
- Grunberg S. M., Weiss M. H., Spitz I. M., Ahmadi J., Sadun A., Russell C. A., Lucci L., Stevenson L. L. Treatment of unresectable meningiomas with the antiprogesterone agent mifepristone. J Neurosurg. 1991 Jun;74(6):861–866. doi: 10.3171/jns.1991.74.6.0861. [DOI] [PubMed] [Google Scholar]
- Heikinheimo O., Haukkamaa M., Lähteenmäki P. Distribution of RU 486 and its demethylated metabolites in humans. J Clin Endocrinol Metab. 1989 Feb;68(2):270–275. doi: 10.1210/jcem-68-2-270. [DOI] [PubMed] [Google Scholar]
- Heikinheimo O., Kekkonen R. Dose-response relationships of RU 486. Ann Med. 1993 Feb;25(1):71–76. doi: 10.3109/07853899309147861. [DOI] [PubMed] [Google Scholar]
- Heikinheimo O., Lähteenmäki P. L., Koivunen E., Shoupe D., Croxatto H., Luukkainen T., Lähteenmäki P. Metabolism and serum binding of RU 486 in women after various single doses. Hum Reprod. 1987 Jul;2(5):379–385. doi: 10.1093/oxfordjournals.humrep.a136554. [DOI] [PubMed] [Google Scholar]
- Hirsch L. J., Mazzone T. Dexamethasone modulates lipoprotein metabolism in cultured human monocyte-derived macrophages. Stimulation of scavenger receptor activity. J Clin Invest. 1986 Feb;77(2):485–490. doi: 10.1172/JCI112327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacolot F., Simon I., Dreano Y., Beaune P., Riche C., Berthou F. Identification of the cytochrome P450 IIIA family as the enzymes involved in the N-demethylation of tamoxifen in human liver microsomes. Biochem Pharmacol. 1991 Jun 15;41(12):1911–1919. doi: 10.1016/0006-2952(91)90131-n. [DOI] [PubMed] [Google Scholar]
- Kettel L. M., Murphy A. A., Mortola J. F., Liu J. H., Ulmann A., Yen S. S. Endocrine responses to long-term administration of the antiprogesterone RU486 in patients with pelvic endometriosis. Fertil Steril. 1991 Sep;56(3):402–407. doi: 10.1016/s0015-0282(16)54531-2. [DOI] [PubMed] [Google Scholar]
- Kim R. S., Zaborniak C. L., Begleiter A., LaBella F. S. Antiproliferative properties of aminosteroid antioxidants on cultured cancer cells. Cancer Lett. 1992 May 30;64(1):61–66. doi: 10.1016/0304-3835(92)90023-o. [DOI] [PubMed] [Google Scholar]
- Kita T., Nagano Y., Yokode M., Ishii K., Kume N., Ooshima A., Yoshida H., Kawai C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lähteenmäki P., Heikinheimo O., Croxatto H., Spitz I., Shoupe D., Birgerson L., Luukkainen T. Pharmacokinetics and metabolism of RU 486. J Steroid Biochem. 1987;27(4-6):859–863. doi: 10.1016/0022-4731(87)90160-9. [DOI] [PubMed] [Google Scholar]
- Meyer M., Schreck R., Baeuerle P. A. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993 May;12(5):2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton K. S., Wu H., Barnett J., Parthasarathy S., Glass C. K. Regulated expression of the human acetylated low density lipoprotein receptor gene and isolation of promoter sequences. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8102–8106. doi: 10.1073/pnas.89.17.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A. A., Kettel L. M., Morales A. J., Roberts V. J., Yen S. S. Regression of uterine leiomyomata in response to the antiprogesterone RU 486. J Clin Endocrinol Metab. 1993 Feb;76(2):513–517. doi: 10.1210/jcem.76.2.8432797. [DOI] [PubMed] [Google Scholar]
- Nieman L. K., Chrousos G. P., Kellner C., Spitz I. M., Nisula B. C., Cutler G. B., Merriam G. R., Bardin C. W., Loriaux D. L. Successful treatment of Cushing's syndrome with the glucocorticoid antagonist RU 486. J Clin Endocrinol Metab. 1985 Sep;61(3):536–540. doi: 10.1210/jcem-61-3-536. [DOI] [PubMed] [Google Scholar]
- Parhami F., Fang Z. T., Fogelman A. M., Andalibi A., Territo M. C., Berliner J. A. Minimally modified low density lipoprotein-induced inflammatory responses in endothelial cells are mediated by cyclic adenosine monophosphate. J Clin Invest. 1993 Jul;92(1):471–478. doi: 10.1172/JCI116590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Khoo J. C., Miller E., Barnett J., Witztum J. L., Steinberg D. Low density lipoprotein rich in oleic acid is protected against oxidative modification: implications for dietary prevention of atherosclerosis. Proc Natl Acad Sci U S A. 1990 May;87(10):3894–3898. doi: 10.1073/pnas.87.10.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Rankin S. M. Role of oxidized low density lipoprotein in atherogenesis. Prog Lipid Res. 1992;31(2):127–143. doi: 10.1016/0163-7827(92)90006-5. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Steinberg D., Witztum J. L. The role of oxidized low-density lipoproteins in the pathogenesis of atherosclerosis. Annu Rev Med. 1992;43:219–225. doi: 10.1146/annurev.me.43.020192.001251. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Young S. G., Witztum J. L., Pittman R. C., Steinberg D. Probucol inhibits oxidative modification of low density lipoprotein. J Clin Invest. 1986 Feb;77(2):641–644. doi: 10.1172/JCI112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifici V. A., Khachadurian A. K. The inhibition of low-density lipoprotein oxidation by 17-beta estradiol. Metabolism. 1992 Oct;41(10):1110–1114. doi: 10.1016/0026-0495(92)90295-l. [DOI] [PubMed] [Google Scholar]
- Sartorius C. A., Tung L., Takimoto G. S., Horwitz K. B. Antagonist-occupied human progesterone receptors bound to DNA are functionally switched to transcriptional agonists by cAMP. J Biol Chem. 1993 May 5;268(13):9262–9266. [PubMed] [Google Scholar]
- Schreck R., Albermann K., Baeuerle P. A. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic Res Commun. 1992;17(4):221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- Schreck R., Grassmann R., Fleckenstein B., Baeuerle P. A. Antioxidants selectively suppress activation of NF-kappa B by human T-cell leukemia virus type I Tax protein. J Virol. 1992 Nov;66(11):6288–6293. doi: 10.1128/jvi.66.11.6288-6293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F., Mizel S. B. In vitro activation and nuclear translocation of NF-kappa B catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol Cell Biol. 1989 Jun;9(6):2424–2430. doi: 10.1128/mcb.9.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J. P., Bonin P. D. Inhibition of proliferation of fibroblasts by lazaroids (21-aminosteroids). Life Sci. 1991;49(26):2053–2058. doi: 10.1016/0024-3205(91)90648-u. [DOI] [PubMed] [Google Scholar]
- Skafar D. F. Differences in the binding mechanism of RU486 and progesterone to the progesterone receptor. Biochemistry. 1991 Nov 12;30(45):10829–10832. doi: 10.1021/bi00109a003. [DOI] [PubMed] [Google Scholar]
- Sparrow C. P., Doebber T. W., Olszewski J., Wu M. S., Ventre J., Stevens K. A., Chao Y. S. Low density lipoprotein is protected from oxidation and the progression of atherosclerosis is slowed in cholesterol-fed rabbits by the antioxidant N,N'-diphenyl-phenylenediamine. J Clin Invest. 1992 Jun;89(6):1885–1891. doi: 10.1172/JCI115793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Tora L., White J., Brou C., Tasset D., Webster N., Scheer E., Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989 Nov 3;59(3):477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Verlangieri A. J., Bush M. J. Effects of d-alpha-tocopherol supplementation on experimentally induced primate atherosclerosis. J Am Coll Nutr. 1992 Apr;11(2):131–138. [PubMed] [Google Scholar]
- Wiseman H., Paganga G., Rice-Evans C., Halliwell B. Protective actions of tamoxifen and 4-hydroxytamoxifen against oxidative damage to human low-density lipoproteins: a mechanism accounting for the cardioprotective action of tamoxifen? Biochem J. 1993 Jun 15;292(Pt 3):635–638. doi: 10.1042/bj2920635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtczak A. B., Famulski K. S., Buszkowska M., Latos P. Effect of glucagon and phorbol myristate acetate on oxidative demethylation and lipid peroxidation in isolated hepatocytes. Acta Biochim Pol. 1991;38(2):251–263. [PubMed] [Google Scholar]
- Zurth C., Kagels F. Determination of onapristone and its N-desmethyl metabolite in human plasma or serum by high-performance liquid chromatography. J Chromatogr. 1990 Oct 26;532(1):115–123. doi: 10.1016/s0378-4347(00)83757-3. [DOI] [PubMed] [Google Scholar]
- el-Ashry D., Oñate S. A., Nordeen S. K., Edwards D. P. Human progesterone receptor complexed with the antagonist RU 486 binds to hormone response elements in a structurally altered form. Mol Endocrinol. 1989 Oct;3(10):1545–1558. doi: 10.1210/mend-3-10-1545. [DOI] [PubMed] [Google Scholar]



