Abstract
Importance of the field
Biomarkers are essential for the identification of high risk children as well as monitoring of prevention outcomes for type 1 diabetes (T1D).
Areas covered in this review
This review discusses progress, opportunities and challenges in biomarker discovery and validation using high throughput genomic, transcriptomic and proteomic technologies. The authors also suggest potential solutions to deal with the current challenges.
What the reader will gain
Readers will gain an overview of the current status on T1D biomarkers, an integrated review of three omic technologies, their applications and limitations for biomarker discovery and validation, and a critical discussion of the major issues encountered in biomarker development.
Take home message
Better biomarkers are still urgently needed for T1D prediction and prevention. The high throughput omic technologies offer great opportunities but also face significant challenges that have to be solved before their potential for biomarker development is fully realized.
Keywords: Biomarkers, Prediction, Prevention, Type 1 Diabetes
1. Introduction
Type 1 diabetes (T1D) is an autoimmune disease resulting from the poorly understood interactions between susceptibility genes, the environment and the immune system. It has been projected that the incidence of T1D will continue to increase with an average rate of 3% per year (1-4) in underdeveloped and developing countries. In attempts to curtail this rising incidence, novel prediction and prevention strategies are urgently needed. The long silent prodomal period before the onset of clinical disease offers many opportunities for the prevention of T1D. The disease can be prevented in numerous ways in NOD mouse models. Although various prevention trials have been conducted in humans, no breakthroughs have yet been made in preventing the disease. Possible reasons for this failure include the difficulties of accurately identifying a sufficiently large high risk population at the early stages of the disease, the inability to conduct a large numbers of clinical trials, poorly understood etiology of the disease, and heterogeneity of the disease pathogenesis. Therefore, prevention tailored for the whole at-risk population may not be effective and personalized prevention strategies based on one’s own risk and etiology may prove to be more efficient.
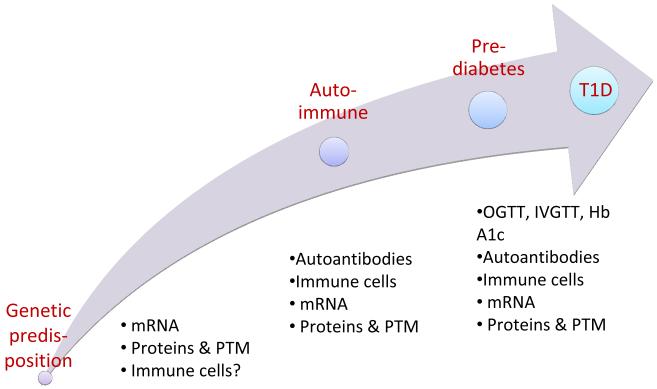
Biomarkers play essential roles for both the identification of the high risk population and more importantly for tailoring and monitoring of therapies for the disease. T1D results from a cascade of molecular, cellular and metabolomic changes starting at a very early stage in life, probably in utero. Therefore, biomarkers for T1D may be derived from a variety of sources that can be grouped into six major categories: metabolomic, autoantibodies, immune cells, proteomic, transcriptomic, and genetic (Fig. 1). Before the clinical onset, diabetes can be accurately diagnosed with several glucose tolerance tests and hemoglobin A1c levels. Although these metabolomic changes remain the golden standard for pre-diabetes diagnosis, these changes occur at late stages of the disease process and have relatively little value for disease prevention. Earlier prediction of T1D became possible three decades ago through the use of islet cell autoantibodies (ICA). The discovery of autoantibodies against specific islet autoantigens such as insulin and GAD (Glutamate dehydrogenase) and the continuously improving assays for these autoantibodies has allowed significant increase in the predictive value of these autoantibodies (5-7). However, islet autoantibodies also have major limitations. Most importantly, the appearance of islet autoantibodies marks a relatively late stage in disease development. Because T1D prevention may be more effective before active autoimmune response, identification of the high risk population before the appearance of islet autoantibodies would be of great value. Furthermore, islet autoantibodies are not implicated in the disease pathogenesis and are not useful for assessing therapeutic outcomes.
Figure 1.
Natural history of Type 1 Diabetes with molecular, cellular and metabolic events during disease progression. All T1D patients probably have a genetic predisposition that does not change over time. The appearance of islet autoantibodies mark two important transitions, one being from AbN to AbP, and the other being from AbP to T1D. Molecular and cellular events associated with these transitions may serve as T1D biomarkers.
A number of studies have also suggested that T1D patients are defective in the number and/or function of immune cells including CD4+ regulatory T cells (8,9), antigen presenting cells (10,11) and NKT cells (12). Furthermore, autoreactive T cells against islet-cell antigens are increased in T1D patients (13). These and other cellular changes have great potential to become excellent biomarkers for T1D prediction and prevention as they are likely implicated in the disease pathogenesis and may occur before the onset of islet autoantibodies. The development of such biomarkers also faces great challenges that have been reviewed elsewhere (13) and will not be discussed here.
It is well known that the interaction between susceptibility genes and environmental factors result in a cascade of changes in gene and protein expression levels. Therefore, susceptibility genes, gene expression, protein expression and post-translational modifications (PTM) are rich sources of biomarkers for T1D. Such biomarkers have great potential as they may occur early in the disease process and can be easily and cheaply assayed. The developments in high throughput technologies in the last two decades have opened the door to a variety of exciting advances with regards to biomarker discovery. The extensive set of omic methodologies can be used to capture the molecular changes occurring at various levels of an organism and during the entire disease progression process. The knowledge gained through these technologies should provide new biomarkers for T1D prediction and prevention. In this review, we will discuss progress, opportunities and challenges in the discovery and validation of T1D biomarkers using genetic, transcriptomic and proteomic technologies.
2. T1D susceptibility genes as risk factors for T1D
A genetic predisposition to T1D has been suggested by the familial clustering of the disease and demonstrated by a variety of genetic studies. Earlier studies indicated that the strongest genetic susceptibility for T1D is encoded within the HLA class II region and accounts for approximately 50% of the familial clustering (14). Genome-wide linkage analysis suggested that a large number of susceptibility genes may be implicated in T1D. Recently, genome-wide association studies (GWAS) have become a method of choice for mapping genes involved in many complex diseases including inflammatory bowel disease (15,16), multiple sclerosis (17,18), type 2 diabetes (19-22), and type 1 diabetes (23-30). To date GWAS have identified over 750 regions associated with more than 148 traits (GWAS Catalog www.genome.gov/gwastudies). The number of genomic regions associated with each disease phenotype is quite large. Often, the regions showing association harbor many genes that potentially could contribute to the observed disease phenotypes. It has also become apparent that some of the associated regions are shared by multiple diseases with similar etiology (31).
Table 1 summarizes the genomic intervals identified for T1D in multiple GWAS studies. The main finding of these GWAS studies is the large number of associated intervals with variable degrees of association evidence. An intrinsic problem with GWAS is the potentially high false-positive rate due to the large number of markers analyzed in the studies (32). The intervals with evidence in multiple studies are likely to contain susceptibility genes while others may be spurious associations. Among the large numbers of associated intervals, the overall evidence for the intervals in Table 1 is generally convincing. Other intervals remained to be confirmed in additional studies. Although GWAS are typically conducted on a relatively large sample sizes, the earlier GWAS were designed to detect variants of relatively larger effect size. Various studies have shown that as sample size in GWAS increases, the number of loci that are able to be identified and validated will also increase. It is also likely that studies of different populations will identify more associated intervals. The early GWAS also employed simpler statistical methods and assume independence between variants. These approaches do not account for the interactions that are occurring between multiple loci contributing to disease risk. Future GWAS should take the limitations of the earlier GWAS into consideration and may allow for the discovery of additional associations.
Table 1.
Summary of T1D genomic intervals identified by genetic mapping studies
| WTCCC | Todd et al. 2007 |
Barrett et al. 2009 | Other Studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Chr. | Gene of interest |
P-value | OR | P-value | OR | P-value | OR | P-value | OR | Ref |
| rs2476601 | 1p13.2 | PTPN22 | 1.7E-32 | 2.04 | 8.5E-85 | 8.7E-4 | 1.32 | (26) | |||
| rs2269241 | 1p31.3 | PGM1 | 5.9E-6 | 1.10 | |||||||
| rs3024505 | 1q32.1 | IL10 | 2.2E-6 | 0.84 | |||||||
| rs2816316 | 1q31.2 | RGS1 | 3.1E-5 | ||||||||
| rs1990760 | 2q24.2 | IFIH1 | 1.4E-10 | 0.86 | 3.8E-3 | 0.89 | 6.6E-9 | 0.027 | 0.849 | (26) | |
| rs1534422 | 2p25.1 | NA | 6.7E-6 | 1.08 | |||||||
| rs3087243 | 2q33.2 | CTLA4 | 1.2E-15 | 0.02 | 1.191 | (26) | |||||
| rs11711054 | 3p21.31 | CCR5 | 1.7E-5 | ||||||||
| rs1051708 | 4p15.2 | NA | 2.8E-7 | ||||||||
| rs4505848 | 4q27 | IL2 | 6.3E-7 | 1.27 | 4.7E-13 | ||||||
| rs6897932 | 5p13.2 | IL7R | 0.095 | 0.93 | 0.026 | 6.9E-7 | 1.13 | (91) | |||
| rs9268645 | 6p21.32 | MHC | <10−100 | ||||||||
| rs11755527 | 6q15 | BACH2 | 5.4E-8 | 0.0075 | 1.216 | (26) | |||||
| rs9388489 | 6q22.32 | C6orf173 | 5.1E-8 | 1.17 | |||||||
| rs4948088 | 7p12.1 | COBL | 2.7E-6 | 0.77 | |||||||
| rs78043256 | 7p15.2 | NA | 3.3E-8 | 0.00051 | |||||||
| rs4729562 | 7q22 | LOC646282 | 8.8E-5 | 0.81 | (91) | ||||||
| rs7020673 | 9p24.2 | GLIS3 | 1.9E-9 | 0.88 | 2.9E-4 | 1.30 | (26) | ||||
| rs947474 | 10p15 | PRKCQ | 1.2E-7 | ||||||||
| rs1225130 | 10p15.1 | IL2RA | 1.3E-13 | 4.6E-4 | 1.30 | (26) | |||||
| rs10509540 | 10q23.31 | C10orf59 | 6.9E-9 | 0.75 | |||||||
| rs7111341 | 11p15.5 | INS | 4.4E-48 | 4.3E-9 | 0.622 | (26) | |||||
| rs3764021 | 12p13 | CLEC2D | 7.1E-5 | 0.64 | 0.0267 | 0.93 | |||||
| rs4763879 | 12p13.31 | CD69 | 2.8E-7 | 1.09 | |||||||
| rs773107 | 12q13 | SUOX, | 2.8E-5 | 1.25 | (27) | ||||||
| rs10876864 | 12q13 | ERBB3 | 8.3E-5 | 1.33 | (26) | ||||||
| rs1701704 | 12q13 | CDK2 | 9.8E-6 | 1.25 | (27) | ||||||
| rs2292239 | 12q13 | ERBB3 | 1.4E-9 | 0.77 | 1.8E-14 | 1.28 | 2.2E-25 | ||||
| rs17696736 | 12q24 | C12orf30 | 7.2E-14 | 1.37 | 1.8E-6 | 1.16 | 8.7E-8 | 0.86 | (91) | ||
| rs3184504 | 12q24.12 | SH2B3 | 2.8E-27 | ||||||||
| rs1465788 | 14q24.1 | NA | 1.4E-8 | 0.86 | |||||||
| rs941578 | 14q32.2 | DLK1 | 0.049 | 0.9 | 0.00042 | 1.09 | 9.8E-7 | 0.9 | (92) | ||
| rs3825932 | 15q24 | CTSH | 7.7E-8 | ||||||||
| rs4788084 | 16p11.2 | IL27 | 5.2E-8 | 0.86 | (91) | ||||||
| rs1244426 | 16p12.3 | NA | 2.0E-6 | 1.1 | |||||||
| rs12708716 | 16p13 | KIAA0350 | 1.28 × 10−8 | 0.77 | 7.07 × 10−9 |
0.83 | 2.2E-16 | 1.0E-6 | 0.63 | (26) | |
| rs7202877 | 16q23.1 | NA | 5.7E-11 | 1.28 | |||||||
| rs1695693 | 17p13.1 | NA | 3.2E-6 | 0.92 | |||||||
| rs2290400 | 17q12 | ORMDL3 | 1.3E-7 | 0.87 | |||||||
| rs7221109 | 17q21.2 | NA | 9.9E-8 | 0.95 | |||||||
| rs2542151 | 18p11 | PTPN2 | 8.4E-8 | 1.33 | 3.3E-10 | 1.29 | |||||
| rs1893217 | 18p11.21 | PTPN2 | 3.6E-15 | ||||||||
| rs763361 | 18q22 | CD226 | 1.5E-5 | 1.18 | 1.2E-5 | ||||||
| rs2304256 | 19p13.2 | TYK2 | 2.6E-3 | 0.84 | 1.4E-10 | 0.86 | (92) | ||||
| rs425105 | 19q13.32 | NA | 1.5E-7 | 0.86 | |||||||
| rs2281808 | 20p13 | NA | 5.0E-7 | 0.9 | |||||||
| rs2232613 | 20q11 | NA | 0.738 | 0.98 | 8.1E-5 | 1.11 | (91) | ||||
| rs5753037 | 22q12.2 | NA | 1.8E-14 | 1.1 | |||||||
| rs229541 | 22q13 | C1QTNF6 | 2.1E-7 | ||||||||
| rs2664170 | Xq28 | NA | 3.0E-5 | 1.16 | |||||||
NA-Not Available
GWAS also confirmed that the strongest genetic susceptibility genes for T1D were encoded in the human leukocyte antigen (HLA) region. All non-HLA genes appear to have very weak contribution to T1D susceptibility. The previously confirmed disease genes such as INS and PTPN22 are among the stronger non-HLA T1D susceptibility genes (33-36). The newly discovered T1D association intervals generally have weaker contribution. The weak effect of these susceptibility genes poses a serious challenge for the identification of the specific genes implicated in the disease. This task will require extensive sequencing and genotyping of large number of subjects for all genes in the associated intervals as well as functional characterization of the associated genes and variants. Identification of the specific disease genes and elucidation of the underlying functional mechanism will undoubtedly contribute valuable information for understanding the pathogenesis of T1D.
T1D susceptibility genes, particularly the HLA class II genes, have been widely used in population-based studies for the identification of high risk individuals. Although HLA genes allow the identification of a population at increased risk for the development of T1D, testing HLA genes alone lacks specificity, sensitivity and positive predictive value. It has been hoped that the identification of the non-HLA genes would increase the predictive value of genetic testing for T1D. It is believed by some investigators that the use of computer based modeling approaches with GWA data may be able to improve the assessment of disease risk (37). This may be possible only if significant gene-gene and/or gene-environment interactions occur. Although such interactions are certainly expected to occur in T1D, there is no available evidence to suggest that they will significantly improve our ability to accurately identify the high risk subjects with high specificity and sensitivity. We believe that one remaining hope to gain predictive value with T1D susceptibility genes is the possibility that rare variants with much larger effect on disease that are difficult to detect by GWAS will be identified by large scale sequencing studies. This hypothesis remains to be tested in future studies.
3. Gene expression as potential biomarkers for T1D
The microarray technologies for gene expression profiling have provided unparalleled opportunities for the discovery of gene expression changes associated with disease. The approach allows genome-wide characterization of genes implicated in disease pathogenesis as well as biomarkers for risk assessment, molecular classification of disease subtypes and therapeutic monitoring. The approach has also been applied to the studies of T1D in both human patients and animal models. A major focus of the microarray studies has been on the elucidation of the immunological mechanism of the disease using the NOD mouse as a model. For example, our gene expression profiling in NOD mouse spleens identified two distinct groups corresponding to an immature (1-4 weeks) and mature (6-10 weeks) state. The rapid switch of gene expression occurring around 5 weeks of age defines a key immunological checkpoint for the development of disease (38). Analysis of three different tissues (pancreatic lymph nodes, spleen and peripheral blood cells) at six different stages defined a “road map” of gene expression profiles in NOD mice (39). Microarray technology was also successfully used to reveal the important roles of chemokines in T1D pathogenesis (40). Analysis of the CD4+ T cells from NOD congenic mice identified Cd55 (Daf1) and Acadl as candidate genes for T1D (41). Microarray technology was also used to elucidate the role of regulatory T cells (42), to define a defect in central tolerance in NOD (43) and the molecular events associated with cyclophosphamide-induced diabetes (44). A series of studies in Dr. Eizirik’s laboratory has extensively characterized the gene expression profiles of pancreatic islet cells and cytokine-induced apoptosis (45-50).
Several studies attempted to characterize the gene expression profiles in T1D patients and/or prediabetic subjects (51,52). Our group conducted the first microarray study on peripheral blood cells (PBC) from human T1D patients and autoantibody-positive (AbP) subjects (51). The analysis resulted in the identification of over 100 genes found to be up-regulated in PBC of T1D and AbP subjects. Consistent with the studies in the NOD mice, many of the differentially expressed genes are involved in important immunological functions including antigen processing and presentation (e.g. HLA-b,c, CD74), cytotoxicity and apoptosis (e.g. GZMB, GNLY), and immune regulation (e.g. MNDA, SELL). Gene expression profiles in PBC of T1D patients were also characterized in two subsequent studies (53,54), each identifying a different set of genes as differentially expressed in T1D patients. Although the studies suggested a proinflammatory response in T1D patients, the specific genes identified in each study are non-overlapping. Microarray was also used to characterize gene expression in PBC from a small number of prediabetic subjects (52). This study suggested a down-regulation of genes involved antigen presentation, which contradicts the findings in our previous study with a slightly large sample size (51). Furthermore, microarray has been used as a readout system to assess serum protein differences in T1D and at-risk subjects (55) and this clever approach also indicates a proinflammatory state in T1D patients.
Review of the published literature on gene expression studies clearly indicates that the results are largely inconclusive and sometimes contradictory. Although the approach holds great promise that has yet to be realized, major improvement in the experimental design and conduct is necessary to obtain reliable and reproducible results. The first challenge for genomic studies on human T1D is the availability of study materials. It is evident that the most relevant materials to study T1D are cells from the pancreatic lymphnodes and pancreatic islets where the pathological events mainly take place. However, such samples are severely limited and have only become accessible with the establishment of the nPOD program (http://www.jdrfnpod.org/index.php). Therefore, all previous studies focused on PBC that only contain low frequencies of cells implicated in the disease process. PBC contain a very heterogeneous pool of immune cells and it is difficult and expensive to obtain sufficient number of the most relevant cells (regulatory T cells, dendritic cells and autoreactive cells, for example). Some attempts have been made to study cells purified from human PBC, e.g. monocytes (56,57). These studies confirmed an inflammatory profile in T1D patients, consistent with the results obtained from studying PBC. However, whether these genes can be used as biomarkers for disease prediction or therapeutic monitoring remains an open question.
A second major issue with the previous studies is the small sample sizes that range from a few subjects to a few dozens of subjects. Gene expression can be altered by a large number of factors including sex, age, genetic factors, diet, health status and others. Although investigators can and have attempted to match these covariates to their best ability, it is necessary to use large sample sizes to assess the impact of these variables. It is likely that the inconsistent results from different studies are mainly due to the small sample sizes used in all the previous studies. If the lessons from the genetic association studies can serve as guidelines, we believe that sample sizes in the thousands may also be required for gene expression studies.
A third major issue relates to the disease stages that are most relevant for gene expression studies. While most previous studies investigated T1D patients due to sample availability, the most relevant stages for studying both disease pathogenesis and biomarkers are the transitions from autoantibody-negative (AbN) to AbP and from AbP to clinical onset. Such studies require longitudinal samples from a very early time point, probably at birth, to disease onset. Ideally, RNA samples from different immune cell subsets should be banked for all subjects in large prospective cohorts such as the environmental determinants of diabetes in the young (TEDDY) (58). Joint analysis on gene expression, susceptibility genes and environmental factors using samples banked in TEDDY will undoubtedly offer the greatest power in defining the molecular events during T1D progression.
4. Serum proteins have great potential as T1D biomarkers
While proteomic studies are also limited by the availability of pathological tissues, the studies on serum proteins may reflect, at least partially, the events occurring in the pathological sites. Recent technological innovations have increased our potential to evaluate global changes in proteins in a comprehensive manner. This is especially true in mass spectrometry (MS)-based research where improvements, including ease-of-use, in high performance liquid chromatography (HPLC), column chemistries, instruments, software, and molecular databases have advanced the field of proteomics considerably. These technologies have been used in biomedical research including biomarker discovery, elucidation of molecular mechanisms and identification of drug targets. The major proteomic technologies in current research are summarized in Table 2 and Figure 2. These different techniques have been comprehensively reviewed elsewhere (59).
Table 2.
Current technologies in proteomics for identification of marker proteins
| Proteomics Approach |
Sample Prep | Technology | Detection | Number of Molecules |
Multiplex | Number of Samples |
Detection limit | Pro’s/Cons |
|---|---|---|---|---|---|---|---|---|
| ELISA | None to Minimal | Immunoassay | Absorbance | 1 | No | Unlimited | mid pg/ml to high mg/ml | High through put Only 96 wells |
| Multiplex | None to Minimal | Luminex-200 | Fluorescence | upto 100 molecules/well |
Yes | Unlimited | mid pg/ml to high mg/ml | High through put 96 wells |
| FlexMAP 3D | Fluorescence | upto 500 molecules/well |
Yes | Unlimited | mid pg/ml to high mg/ml | High through put, 96 and 384 well, Assay availability |
||
| Flow cytometry |
Fluorescence | upto 20 molecules/well |
Yes | Unlimited | mid pg/ml to high mg/ml | High through put 96 wells |
||
| Antibody arrays |
Fluroescence | 1-200 spots/slide |
Yes | 2 | low pg/ml to ng/ml | Integrated view of a single pathway |
||
| 2D | Low Salt buffers Protein labelling |
IEF SDS-PAGE |
Fluroescence MS |
abundant proteins |
Duplex | Limited | 1000-1200 spots | Protein map Time and labor intensive Interference by abundance proteins |
| 2D-HPLC MS |
Extensive prep: Digestion of proteins Removal of High Abundance proteins |
HPLC MS |
MS | Unlimited | Available | Limited | Low abundant | deeper scan of the proteome Sohpisticated Instrumentation Steep learning curve |
| 3D | Protein labeling 1st Separation by IEF 2nd Separation by RPHPLC 3rd Separation by SDS-PAGE |
IEF HPLC SDS-PAGE Fluorescence MS |
Fluroescence MS |
3000-5000 | Single Plex Duplex |
Limited | Low abundant | Deep scan Extremely time and labor intensitve |
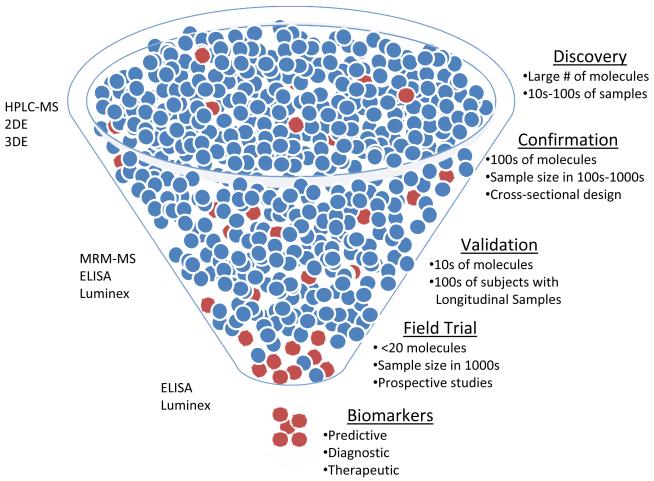
Figure 2.
Phases of Biomarker Discovery Pipeline. Each phase requires different technologies and experimental design. HPLC-MS (High precision liquid chromatography-mass spectrometry), 2DE (2-Dimension gel-electrophoresis), 3DE (3-Dimension gel electrophoresis), MRM-MS (Multiple-reaction monitoring-mass spectrometry).
From the perspective of biomarker discovery, serum and plasma proteins are great sources for biomarkers but also pose a significant and well documented analytical challenge (60). The main challenge to overcome in proteomic research is that candidate biomarkers are present in trace amounts among a large background of non-relevant and abundant proteins. Diversity in the human proteome often gives rise to pluralities of structurally similar but functionally distinct proteins (61). Such micro-heterogeneity generally escapes proteomics discovery technologies and perhaps conventional immunoassays (61).
Several MS-based techniques have been developed and used to discover new biomarker candidates in a variety of human diseases including T1D. Two-dimensional gel electrophoresis (2DE) used to be the platform of proteomic discoveries for a long time. Separation in 2DE is based on isoelectric point (pI) as a first dimension and SDS-PAGE as second dimension. Despite widespread use, 2DE has been plagued by low resolution in that only those high abundant proteins are visible on the gels. This issue has been resolved by the addition of a third dimension (3DE) based on the hydrophobicity of the proteins (59,62). Introducing hydrophobic properties in 3DE resolves the issue of low resolution of 2DE; however, it remains to be a very labor intensive platform. A new proteomic tool, called Mud-PIT based 2D-HPLC, coupled with MS/MS, has resolved some of the issues related to proteomic studies (63-67).
Since proteins are involved in all cellular processes, their cumulative expression profile reflects the specific activity of cells. Early proteomic studies in relation to T1D were conducted in the 1980s using 2D-based techniques and described the changes in the protein expression patterns in islets from mice (68,69). A large effort was dedicated to insulin production and secretion as a part of normal islet physiology. Today no proteomic data is available based on human materials directly studying beta-cell destruction and development of T1D. Most proteomic data are derived from animal models and cell lines (70-72). One study has used SELDI-TOF MS to identify autoantibodies to glial fibrillary acidic protein in both NOD mice and human patients (73). Another study has described the global protein expression in the whole human pancreas allowing for the creation of a reference 2D electrophoresis map of 302 proteins. Although useful for understanding components involved, due to the fact that the proteins identified were from both endocrine and exocrine tissue, it is difficult to use the data for T1D pathogenesis research (74). This gap in our understanding has begun to be filled by a recent study, which identified 6873 proteins from pooled islets, with a role in development of diabetes (69). A number of islet-derived β-cell lines (75) and animal models (76) have contributed to our understanding of the pathogenesis of T1D. Proteomics has been applied in studies of differentiating β-cells, cytokine exposed islets, dietary manipulated islets, and in transplanted islets. A detailed account of proteomic studies on pancreas and pancreatic islets is described in previous reports (77,78). Although various studies have revealed a complex and detailed picture of the protein expression profiles many functional implications remain to be answered. What has been ascertained to this point is a rather detailed picture of protein expression in β-cell lines, islets, and transplanted islets both in vitro and in vivo (71,72,77). Some of the identified proteins in these proteomic discoveries are listed in Table 3. The available data indicate that the β-cell is an active participant in its own destruction during diabetes development. Furthermore, as is the case seen in genomics, no single protein alone seems to be responsible for the development of diabetes. Rather, the cumulative pattern of changes seems to be what favors a transition from dynamic stability in the unperturbed β-cell to dynamic instability and eventually to β-cell destruction (69,75,76).
Table 3.
Proteomic studies identifying unique proteins associated with pancreatic islets or beta-cells
| Tissue/Cell | Model | Species | Platform | Up | Down | Identified Protein | # of Proteins | Ref | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pancreas | Rough endoplasmic reticulum in normal and acute pancreatitis |
Rat | ITRAQ, MS | 3 | 3 | pancreatic triacylglycerol lipase precursor, Erp27, and prolyl 4- hydroxylase beta polypeptide, fibrinogen alpha, beta and gamma chains |
NA/469 | (93) |
| 2 | INS-1 | 11.6 and 30mM Glucose | Cell Line | SILIAC, MS | fatty acid synthase, E-FABP, CRABP-1, Glucose-6 -phosphate isomerase, Cytosolic acetyl-CoA acetyltransferease |
NA/816 | (94) | ||
| 3 | Pancreas | Development of Pancreas |
Porcine | 2DE | Pdx1, Ptf1a, Pax4 | (95) | |||
| 4 | Islets | Islet | murine | LC-MS | 7014/6873 | (69) | |||
| 5 | Islets | Glucose stimulation | murine | LC-MS | 77 | 65 | Prdx3, DJ-1 (Park7), Sod2, VAMP-2, Sytl4, Rab3b |
1487/NA | (69) |
| 6 | beta-TC-6 | Endoplasmic reticulum, Glucose toxicity |
Cell Line | 2D, MS | 4 | 12 | ERp46 | 46/23 | (96) |
| 7 | INS-1 | Identify the potential islet molecules related to pancreatic cancer- associated diabetes |
Cell Line | 2D,MS | 10 | 5 | Prp19, HMOX1 and GRP78 | (97) | |
| 8 | Islets | Type-2 diabets | Human | FTIR-MS, SELDI | Multiple pathways | (98) | |||
| 9 | Panc-1 | MHC peptidome | Cell Line | LC-MS | 131/ | (99) | |||
| 10 | NIT-1 | Secretory Vesicles | Cell Line | LC-MS | Secretory Proteins | 270/ | (100) | ||
| 11 | RNAKT-15 | Islet differentiation | Cell Line | Microarray, LC-MS | (101) | ||||
| 12 | Human Islets, MIN-6 |
ER Stress and beta-cell apopotosis |
2DE | Carboxypeptidase E | (102) |
Beta-cell destruction is proposed to be the result of a dominant Th-1 like cytokine profile at the time of disease onset (79-81), others have countered by proposing a loss of immune response at the time of T1D onset (82,83) in response to cytokine and autoantigen stimulation. Ryden et. al. (84) obtained PBMC from T1D patients and high-risk children, and stimulated them with the autoantigen GAD 65 and mitogen phytohaemagglutinin (PHA). Cytokines and chemokines were detected in cell-culture supernatants by protein microarray in relation to clinical outcome (C-peptide). Increased secretion of IFN-γ, IL-2, TNF-α (Th-1 response) was observed in high-risk children, when PBMC were stimulated, either spontaneously or with GAD-65. This Th-1 dominance was associated with a high risk (40%) of developing T1D within 5 years. PHA stimulation lead to increased secretion of cytokine IL-5 (Th-2 response) in PBMC from the high-risk children, indicating that still healthy high-risk individuals have the ability to switch a Th1-like profile into a more protective Th2-like profile in the presence of the autoantigens GAD65 and insulin (81). PBMC from newly diagnosed T1D children has increased TGF-β and IL-10 secretion (a Th-3 response), upon stimulation, irrespective of the mode. TGF-β is directly involved in generation of FOXP3+ Treg cells (85). Interleukin-10 (IL-10) is a regulatory cytokine that plays a central role in controlling inflammatory processes, and IL-10-secreting T cells may constitute an additional mechanism that are responsible for peripheral tolerance (86,87). Although the protective Th-3 response is high at the onset of T1D, the reduced function of the Treg cells reported by us and others (9), is insufficient to counter the dominant Th-1 response leading to the destruction of β-cells.
Identification of surrogate biomarkers predictive of those at high risk for developing T1D would be beneficial, particularly if such surrogate biomarkers result in higher sensitivity and specificity, better positive predictive value, or earlier detection of at-risk subjects. Biomarker development for T1D using proteomic tools has been slow but is increasing rapidly. A pilot proteomic analysis of human plasma and serum from a subset of controls and T1D patients enrolled in the Diabetes Autoantibody Standardization Program identified Alpha-2-glycoprotein 1 (zinc), Clusterin, Corticosteroid-binding globulin, Lumican, and Serotransferrin as putative biomarkers (88). Our own global peptide finger print approach using SELDI-TOF MS identified 146 protein/peptide peaks. Validation on the test dataset showed 82.8% specificity and 76.2% sensitivity in prediction of T1D from AbN controls (89). However, the identity of these proteins from SELDI study is difficult to determine and validate. A recent study identified transthyretin, apolipoprotein A1, apolipoprotein C1 and cystatin C as markers for diabetic nephropathy (90).
Despite some progress in discovering proteomic biomarker candidates for T1D, no new biomarkers approaching the predictability of islet autoantibodies have been published to date. This sad truth is due, in addition to the difficulties in biomarker discovery, to a series of technical and biological issues related to biomarker validation. The proteomic discovery platforms discussed earlier are usually not suitable for the validation studies that require large numbers of samples. In this regard, the sandwich immunoassays (ELISA and bead-based Luminex assays) are excellent platforms and tools of choice for validation of protein biomarker candidates due to their robustness and high-throughput capabilities in terms of measuring large numbers of samples. These techniques have already been widely used to study candidate serum proteins including cytokines, chemokines, soluble forms of various receptors and inflammatory mediators. The literature has suggested that all studies only analyzed one or a few serum proteins and generally suffered from small sample size and therefore the results were difficult to replicate (89). Our extensive unpublished results indicate that serum is an excellent source for T1D biomarkers but their validation requires thousands of cross-sectional and prospective samples.
5. Expert Opinion
T1D is a disease for which there are fortunately excellent biomarkers for disease prediction. Impending disease can be accurately identified before the appearance of clinical symptoms using metabolic tests (OGTT and IVGTT) and/or hemoglobin A1c. Furthermore, children at high risk for the development of T1D can be identified using a combination of multiple autoantibodies against pancreatic islet cell antigens. These tests are routinely used in clinical studies and patient care. Improvement on the assays, addition of new islet autoantibodies and delineation of the antibody subclasses may further enhance the utility of the islet autoantibody tests. However, these existing biomarkers do not fully meet the need for T1D prediction and prevention due to the imperfect positive predictive value and more importantly the relative late appearance of autoantibodies as well as the lack of causal relationship with disease pathogenesis.
There is still an urgent need for better and earlier biomarkers for T1D prediction and prevention. Successful prevention of the disease requires the identification of high risk populations at the earliest time possible and before the appearance of islet autoantibodies. Furthermore, surrogate biomarkers are needed to access the outcomes of prevention therapies in early stages. Due to the long asymptomatic period for diabetes, it is too expensive and time consuming for clinical trials to wait for the final clinical outcome. The lack of suitable surrogate biomarkers for T1D has severely hampered progress in clinical trials. As discussed in this review, the high throughput omic technologies have offered new opportunities to develop such biomarkers. However, new biomarkers with the potential to fundamentally change diabetes research and care are yet to come due to the many challenges that are being, or will be, resolved.
The first major challenge for all biomarker development programs is the availability of biological samples. It has become very clear that biomarker validation will require thousands of samples irrespective of the technologies and the type of molecules. Furthermore, samples from large cohorts prospectively monitored at high intensity must be used to validate the biomarker candidates. This most difficult challenge is being addressed by several international consortia such as TEDDY and TrialNet. Retrospective analysis of the banked samples should significantly improve the experimental design and outcomes. The second challenge for T1D biomarker development concerns further improvement of technologies used for discovery and validation. In this regard, technologies for genetic and transcriptomic studies are quite mature and the costs are rapidly reducing. However, proteomic analysis still faces severe challenges in both the discovery and validation platforms. Finally, biomarker development programs have to solve the computational challenge. Cumulative evidence suggests that no single biomarker can provide adequate power for T1D or other complex diseases due to the multifactorial nature of the diseases. Therefore, biomarkers for T1D have to rely on the combination of multiple markers. As a result, the simultaneous consideration of genetic, transcriptomic, proteomic, other omic and cellular changes occurring during disease progression will be required for accurate assessment of disease risks and monitoring of therapeutic outcomes. Advanced computational and statistical analyses are needed to develop and validate multivariate models as biomarkers. The development of multivariate models requires the solving of two statistical issues: first, selecting an optimal subset of markers (a single multivariate model) from all available sets of variables with which to make predictions; and second, predicting the phenotypic statuses based on the selected subset of markers. As progress is being made in all three challenge areas, we anticipate that new and improved biomarkers will be become available for T1D in the near future.
Article Highlights.
Currently available biomarkers allow the identification of at-risk subjects but are not useful for therapeutic monitoring.
New biomarkers are urgently needed for early disease prediction and therapeutic monitoring.
High throughput genetic, transcriptomic and proteomic technologies offer great opportunities for T1D biomarker discovery.
Better biomarkers are yet to be discovered and validated due to technical and biological challenges.
Significant effort needs to be devoted to the collection of samples from large prospective cohorts, improvement on technological platforms for both biomarker discovery and validation, and development of computational technologies that integrate multiple types of biomarkers.
Acknowledgments
Declaration of Interest: JS, CC, MW and YJ are supported by grants from the National Institutes of Health (4R33HD050196, 4R33DK069878 and 2RO1HD37800) and JDRF (1-2004-661) given to JS. SP is supported by Fellowships from JDRF, New York (10-2006-792). WZ is supported also by Fellowships from JDRF, New York (3-2009-275)
References
- 1.Borchers AT, Uibo R, Gershwin ME. The geoepidemiology of type 1 diabetes. Autoimmun.Rev. 2009 doi: 10.1016/j.autrev.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Bruno G, Novelli G, Panero F, et al. The incidence of type 1 diabetes is increasing in both children and young adults in Northern Italy: 1984-2004 temporal trends. Diabetologia. 2009;52(12):2531–2535. doi: 10.1007/s00125-009-1538-x. [DOI] [PubMed] [Google Scholar]
- 3.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 4.Levitt NS. Diabetes in Africa: epidemiology, management and healthcare challenges. Heart. 2008;94(11):1376–1382. doi: 10.1136/hrt.2008.147306. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson MA. ADA Outstanding Scientific Achievement Lecture 2004. Thirty years of investigating the autoimmune basis for type 1 diabetes: why can’t we prevent or reverse this disease? Diabetes. 2005;54(5):1253–1263. doi: 10.2337/diabetes.54.5.1253. [DOI] [PubMed] [Google Scholar]
- 6.Bingley PJ, Bonifacio E, Williams AJ, et al. Prediction of IDDM in the general population: strategies based on combinations of autoantibody markers. Diabetes. 1997;46(11):1701–1710. doi: 10.2337/diab.46.11.1701. [DOI] [PubMed] [Google Scholar]
- 7.Bonifacio E, Genovese S, Braghi S, et al. Islet autoantibody markers in IDDM: risk assessment strategies yielding high sensitivity. Diabetologia. 1995;38(7):816–822. doi: 10.1007/s001250050358. [DOI] [PubMed] [Google Scholar]
- 8.Jin Y, Chen X, Podolsky R, et al. APC dysfunction is correlated with defective suppression of T cell proliferation in human type 1 diabetes. Clin.Immunol. 2009;130(3):272–279. doi: 10.1016/j.clim.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedman M, Faresjo M, Axelsson S, et al. Impaired CD4 and CD8 T cell phenotype and reduced chemokine secretion in recent-onset type 1 diabetic children. Clin.Exp.Immunol. 2008;153(3):360–368. doi: 10.1111/j.1365-2249.2008.03720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Makala LH, Jin Y, et al. Type 1 diabetes patients have significantly lower frequency of plasmacytoid dendritic cells in the peripheral blood. Clin.Immunol. 2008;129(3):413–418. doi: 10.1016/j.clim.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinkmann C, Knerr I, Hahn EG, et al. Reduced frequency of peripheral plasmacytoid dendritic cells in type 1 diabetes. Horm.Metab Res. 2008;40(11):767–771. doi: 10.1055/s-2008-1080896. [DOI] [PubMed] [Google Scholar]
- 12.Driver JP, Scheuplein F, Chen YG, et al. Invariant natural killer T-cell control of type 1 diabetes: a dendritic cell genetic decision of a silver bullet or Russian roulette. Diabetes. 2010;59(2):423–432. doi: 10.2337/db09-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenbarth GS, Kotzin BL. Enumerating autoreactive T cells in peripheral blood: a big step in diabetes prediction. J.Clin.Invest. 2003;111(2):179–181. doi: 10.1172/JCI17621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noble JA, Valdes AM, Cook M, et al. The role of HLA class II genes in insulin-dependent diabetes mellitus: molecular analysis of 180 Caucasian, multiplex families. Am J Hum.Genet. 1996;59(5):1134–1148. [PMC free article] [PubMed] [Google Scholar]
- 15.Franke A, Hampe J, Rosenstiel P, et al. Systematic association mapping identifies NELL1 as a novel IBD disease gene. PLoS.ONE. 2007;2(1):e691. doi: 10.1371/journal.pone.0000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kugathasan S, Baldassano RN, Bradfield JP, et al. Loci on 20q13 and 21q22 are associated with pediatric-onset inflammatory bowel disease. Nat.Genet. 2008;40(10):1211–1215. doi: 10.1038/ng.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aulchenko YS, Hoppenbrouwers IA, Ramagopalan SV, et al. Genetic variation in the KIF1B locus influences susceptibility to multiple sclerosis. Nat.Genet. 2008;40(12):1402–1403. doi: 10.1038/ng.251. [DOI] [PubMed] [Google Scholar]
- 18.Comabella M, Craig DW, Camina-Tato M, et al. Identification of a novel risk locus for multiple sclerosis at 13q31.3 by a pooled genome-wide scan of 500,000 single nucleotide polymorphisms. PLoS.ONE. 2008;3(10):e3490. doi: 10.1371/journal.pone.0003490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316(5829):1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 20.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445(7130):881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 21.Timpson NJ, Lindgren CM, Weedon MN, et al. Adiposity-related heterogeneity in patterns of type 2 diabetes susceptibility observed in genome-wide association data. Diabetes. 2009;58(2):505–510. doi: 10.2337/db08-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeggini E, Scott LJ, Saxena R, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat.Genet. 2008;40(5):638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat.Genet. 2009 doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant SF, Qu HQ, Bradfield JP, et al. Follow-up analysis of genome-wide association data identifies novel loci for type 1 diabetes. Diabetes. 2009;58(1):290–295. doi: 10.2337/db08-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant SF, Hakonarson H. Genome-wide association studies in type 1 diabetes. Curr.Diab.Rep. 2009;9(2):157–163. doi: 10.1007/s11892-009-0026-5. [DOI] [PubMed] [Google Scholar]
- 27.Hakonarson H, Qu HQ, Bradfield JP, et al. A novel susceptibility locus for type 1 diabetes on Chr12q13 identified by a genome-wide association study. Diabetes. 2008;57(4):1143–1146. doi: 10.2337/db07-1305. [DOI] [PubMed] [Google Scholar]
- 28.Smyth DJ, Cooper JD, Bailey R, et al. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat.Genet. 2006;38(6):617–619. doi: 10.1038/ng1800. [DOI] [PubMed] [Google Scholar]
- 29.Cooper JD, Smyth DJ, Smiles AM, et al. Meta-analysis of genome-wide association study data identifies additional type 1 diabetes risk loci. Nat.Genet. 2008;40(12):1399–1401. doi: 10.1038/ng.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Todd JA, Walker NM, Cooper JD, et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat.Genet. 2007;39(7):857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fung EY, Smyth DJ, Howson JM, et al. Analysis of 17 autoimmune disease-associated variants in type 1 diabetes identifies 6q23/TNFAIP3 as a susceptibility locus. Genes Immun. 2009;10(2):188–191. doi: 10.1038/gene.2008.99. [DOI] [PubMed] [Google Scholar]
- 32.Todd JA. Statistical false positive or true disease pathway? Nat.Genet. 2006;38(7):731–733. doi: 10.1038/ng0706-731. [DOI] [PubMed] [Google Scholar]
- 33.Concannon P, Chen WM, Julier C, et al. Genome-wide scan for linkage to type 1 diabetes in 2,496 multiplex families from the Type 1 Diabetes Genetics Consortium. Diabetes. 2009;58(4):1018–1022. doi: 10.2337/db08-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howson JM, Walker NM, Smyth DJ, et al. Analysis of 19 genes for association with type I diabetes in the Type I Diabetes Genetics Consortium families. Genes Immun. 2009;10(Suppl 1):S74–S84. doi: 10.1038/gene.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu HQ, Bradfield JP, Grant SF, et al. Remapping the type I diabetes association of the CTLA4 locus. Genes Immun. 2009;10(Suppl 1):S27–S32. doi: 10.1038/gene.2009.88. S27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueda H, Howson JM, Esposito L, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423(6939):506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 37.Wei Z, Wang K, Qu HQ, et al. From disease association to risk assessment: an optimistic view from genome-wide association studies on type 1 diabetes. PLoS.Genet. 2009;5(10):e1000678. doi: 10.1371/journal.pgen.1000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckenrode SE, Ruan Q, Yang P, et al. Gene expression profiles define a key checkpoint for type 1 diabetes in NOD mice. Diabetes. 2004;53(2):366–375. doi: 10.2337/diabetes.53.2.366. [DOI] [PubMed] [Google Scholar]
- 39.Kodama K, Butte AJ, Creusot RJ, et al. Tissue- and age-specific changes in gene expression during disease induction and progression in NOD mice. Clin.Immunol. 2008;129(2):195–201. doi: 10.1016/j.clim.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mi QS, Meagher C, Delovitch TL. CD1d-restricted NKT regulatory cells: functional genomic analyses provide new insights into the mechanisms of protection against Type 1 diabetes. Novartis.Found.Symp. 2003;252:146–160. [PubMed] [Google Scholar]
- 41.Irie J, Reck B, Wu Y, et al. Genome-wide microarray expression analysis of CD4+ T Cells from nonobese diabetic congenic mice identifies Cd55 (Daf1) and Acadl as candidate genes for type 1 diabetes. J.Immunol. 2008;180(2):1071–1079. doi: 10.4049/jimmunol.180.2.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Z, Herman AE, Matos M, et al. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J.Exp.Med. 2005;202(10):1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zucchelli S, Holler P, Yamagata T, et al. Defective central tolerance induction in NOD mice: genomics and genetics. Immunity. 2005;22(3):385–396. doi: 10.1016/j.immuni.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Matos M, Park R, Mathis D, et al. Progression to islet destruction in a cyclophosphamide-induced transgenic model: a microarray overview. Diabetes. 2004;53(9):2310–2321. doi: 10.2337/diabetes.53.9.2310. [DOI] [PubMed] [Google Scholar]
- 45.Ortis F, Naamane N, Flamez D, et al. Cytokines interleukin-1beta and tumor necrosis factor-alpha regulate different transcriptional and alternative splicing networks in primary beta-cells. Diabetes. 2010;59(2):358–374. doi: 10.2337/db09-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortis F, Pirot P, Naamane N, et al. Induction of nuclear factor-kappaB and its downstream genes by TNF-alpha and IL-1beta has a pro-apoptotic role in pancreatic beta cells. Diabetologia. 2008;51(7):1213–1225. doi: 10.1007/s00125-008-0999-7. [DOI] [PubMed] [Google Scholar]
- 47.Flamez D, Roland I, Berton A, et al. A genomic-based approach identifies FXYD domain containing ion transport regulator 2 (FXYD2)gammaa as a pancreatic beta cell-specific biomarker. Diabetologia. 2010;53(7):1372–1383. doi: 10.1007/s00125-010-1714-z. [DOI] [PubMed] [Google Scholar]
- 48.Kutlu B, Burdick D, Baxter D, et al. Detailed transcriptome atlas of the pancreatic beta cell. BMC.Med.Genomics. 2009;2(3) doi: 10.1186/1755-8794-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kutlu B, Cardozo AK, Darville MI, et al. Discovery of gene networks regulating cytokine-induced dysfunction and apoptosis in insulin-producing INS-1 cells. Diabetes. 2003;52(11):2701–2719. doi: 10.2337/diabetes.52.11.2701. [DOI] [PubMed] [Google Scholar]
- 50.Eizirik DL, Kutlu B, Rasschaert J, et al. Use of microarray analysis to unveil transcription factor and gene networks contributing to Beta cell dysfunction and apoptosis. Ann.N.Y.Acad.Sci. 2003;1005:55–74. doi: 10.1196/annals.1288.007. [DOI] [PubMed] [Google Scholar]
- 51.Collins CD, Purohit S, Podolsky RH, et al. The application of genomic and proteomic technologies in predictive, preventive and personalized medicine. Vascul.Pharmacol. 2006;45(5):258–267. doi: 10.1016/j.vph.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Elo LL, Mykkanen J, Nikula T, et al. Early suppression of immune response pathways characterizes children with prediabetes in genome-wide gene expression profiling. J.Autoimmun. 2010;35(1):70–76. doi: 10.1016/j.jaut.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Kaizer EC, Glaser CL, Chaussabel D, et al. Gene expression in peripheral blood mononuclear cells from children with diabetes. J.Clin.Endocrinol.Metab. 2007;92(9):3705–3711. doi: 10.1210/jc.2007-0979. [DOI] [PubMed] [Google Scholar]
- 54.Reynier F, Pachot A, Paye M, et al. Specific gene expression signature associated with development of autoimmune type-I diabetes using whole-blood microarray analysis. Genes Immun. 2010;11(3):269–278. doi: 10.1038/gene.2009.112. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Jia S, Geoffrey R, et al. Identification of a molecular signature in human type 1 diabetes mellitus using serum and functional genomics. J.Immunol. 2008;180(3):1929–1937. doi: 10.4049/jimmunol.180.3.1929. [DOI] [PubMed] [Google Scholar]
- 56.Padmos RC, Hillegers MH, Knijff EM, et al. A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. Arch.Gen.Psychiatry. 2008;65(4):395–407. doi: 10.1001/archpsyc.65.4.395. [DOI] [PubMed] [Google Scholar]
- 57.Beyan H, Drexhage RC, van der Heul NL, et al. Monocyte gene-expression profiles associated with childhood-onset type 1 diabetes and disease risk: a study of identical twins. Diabetes. 2010;59(7):1751–1755. doi: 10.2337/db09-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.The TEDDY Group The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr.Diabetes. 2007;8(5):286–298. doi: 10.1111/j.1399-5448.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 59.Zhi W, Purohit S, Carey C, et al. Proteomic Technologies for the Discovery of Type 1 Diabetes Biomarkers. Journal of Diabetes Science and Technology. 2010;4(4):993–1003. doi: 10.1177/193229681000400431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Randall SA, McKay MJ, Molloy MP. Evaluation of blood collection tubes using selected reaction monitoring MS: Implications for proteomic biomarker studies. Proteomics. 2010;10(10):2050–2056. doi: 10.1002/pmic.200900517. [DOI] [PubMed] [Google Scholar]
- 61.Borges CR, Rehder DS, Jarvis JW, et al. Full-length characterization of proteins in human populations. Clin.Chem. 2010;56(2):202–211. doi: 10.1373/clinchem.2009.134858. [DOI] [PubMed] [Google Scholar]
- 62.Wang H, Hanash S. Intact-protein based sample preparation strategies for proteome analysis in combination with mass spectrometry. Mass Spectrom.Rev. 2005;24(3):413–426. doi: 10.1002/mas.20018. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, Fang A, Riley CP, et al. Multi-dimensional liquid chromatography in proteomics--a review. Anal.Chim.Acta. 2010;664(2):101–113. doi: 10.1016/j.aca.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kakisaka T, Kondo T, Okano T, et al. Plasma proteomics of pancreatic cancer patients by multi-dimensional liquid chromatography and two-dimensional difference gel electrophoresis (2D-DIGE): up-regulation of leucine-rich alpha-2-glycoprotein in pancreatic cancer. J.Chromatogr.B Analyt.Technol.Biomed.Life Sci. 2007;852(1-2):257–267. doi: 10.1016/j.jchromb.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okano T, Kondo T, Kakisaka T, et al. Plasma proteomics of lung cancer by a linkage of multi-dimensional liquid chromatography and two-dimensional difference gel electrophoresis. Proteomics. 2006;6(13):3938–3948. doi: 10.1002/pmic.200500883. [DOI] [PubMed] [Google Scholar]
- 66.Immler D, Greven S, Reinemer P. Targeted proteomics in biomarker validation: detection and quantification of proteins using a multi-dimensional peptide separation strategy. Proteomics. 2006;6(10):2947–2958. doi: 10.1002/pmic.200500659. [DOI] [PubMed] [Google Scholar]
- 67.Wang H, Hanash S. Multi-dimensional liquid phase based separations in proteomics. J.Chromatogr.B Analyt.Technol.Biomed.Life Sci. 2003;787(1):11–18. doi: 10.1016/s1570-0232(02)00335-5. [DOI] [PubMed] [Google Scholar]
- 68.Chatterjee NK, Haley TM, Nejman C. Functional alterations in pancreatic beta cells as a factor in virus-induced hyperglycemia in mice. J.Biol.Chem. 1985;260(23):12786–12791. [PubMed] [Google Scholar]
- 69.Waanders LF, Chwalek K, Monetti M, et al. Quantitative proteomic analysis of single pancreatic islets. Proc.Natl.Acad.Sci.U.S.A. 2009;106(45):18902–18907. doi: 10.1073/pnas.0908351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sparre T, Bergholdt R, Nerup J, et al. Application of genomics and proteomics in Type 1 diabetes pathogenesis research. Expert.Rev.Mol.Diagn. 2003;3(6):743–757. doi: 10.1586/14737159.3.6.743. [DOI] [PubMed] [Google Scholar]
- 71.Sparre T, Larsen MR, Heding PE, et al. Unraveling the pathogenesis of type 1 diabetes with proteomics: present and future directions. Mol.Cell Proteomics. 2005;4(4):441–457. doi: 10.1074/mcp.R500002-MCP200. [DOI] [PubMed] [Google Scholar]
- 72.Suss C, Solimena M. Proteomic profiling of beta-cells using a classical approach - two-dimensional gel electrophoresis. Exp.Clin.Endocrinol.Diabetes. 2008;116(Suppl 1):S13–S20. doi: 10.1055/s-2008-1080898. S13-20. Epub;%2008 Sep 5. [DOI] [PubMed] [Google Scholar]
- 73.Winer S, Tsui H, Lau A, et al. Autoimmune islet destruction in spontaneous type 1 diabetes is not beta-cell exclusive. Nat.Med. 2003;9(2):198–205. doi: 10.1038/nm818. [DOI] [PubMed] [Google Scholar]
- 74.Hu L, Evers S, Lu ZH, et al. Two-dimensional protein database of human pancreas. Electrophoresis. 2004;25(3):512–518. doi: 10.1002/elps.200305683. [DOI] [PubMed] [Google Scholar]
- 75.Hohmeier HE, Newgard CB. Cell lines derived from pancreatic islets. Mol.Cell Endocrinol. 2004;228(1-2):121–128. doi: 10.1016/j.mce.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 76.Rossini AA, Handler ES, Mordes JP, et al. Human autoimmune diabetes mellitus: lessons from BB rats and NOD mice--Caveat emptor. Clin.Immunol.Immunopathol. 1995;74(1):2–9. doi: 10.1006/clin.1995.1002. [DOI] [PubMed] [Google Scholar]
- 77.Brunner Y, Schvartz D, Priego-Capote F, et al. Glucotoxicity and pancreatic proteomics. J.Proteomics. 2009;71(6):576–591. doi: 10.1016/j.jprot.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 78.Sundsten T, Ortsater H. Proteomics in diabetes research. Mol.Cell Endocrinol. 2009;297(1-2):93–103. doi: 10.1016/j.mce.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 79.Hedman M, Ludvigsson J, Faresjo MK. Nicotinamide reduces high secretion of IFN-gamma in high-risk relatives even though it does not prevent type 1 diabetes. J.Interferon Cytokine Res. 2006;26(4):207–213. doi: 10.1089/jir.2006.26.207. [DOI] [PubMed] [Google Scholar]
- 80.Hussain MJ, Peakman M, Gallati H, et al. Elevated serum levels of macrophage-derived cytokines precede and accompany the onset of IDDM. Diabetologia. 1996;39(1):60–69. doi: 10.1007/BF00400414. [DOI] [PubMed] [Google Scholar]
- 81.Karlsson MG, Lawesson SS, Ludvigsson J. Th1-like dominance in high-risk first-degree relatives of type I diabetic patients. Diabetologia. 2000;43(6):742–749. doi: 10.1007/s001250051372. [DOI] [PubMed] [Google Scholar]
- 82.Karlsson Faresjo MG, Ludvigsson J. Diminished Th1-like response to autoantigens in children with a high risk of developing type 1 diabetes. Scand.J.Immunol. 2005;61(2):173–179. doi: 10.1111/j.0300-9475.2005.01544.x. [DOI] [PubMed] [Google Scholar]
- 83.Karlsson Faresjo MG, Ernerudh J, Ludvigsson J. Cytokine profile in children during the first 3 months after the diagnosis of type 1 diabetes. Scand.J.Immunol. 2004;59(5):517–526. doi: 10.1111/j.0300-9475.2004.01420.x. [DOI] [PubMed] [Google Scholar]
- 84.Ryden A, Stechova K, Durilova M, et al. Switch from a dominant Th1-associated immune profile during the pre-diabetic phase in favour of a temporary increase of a Th3-associated and inflammatory immune profile at the onset of type 1 diabetes. Diabetes Metab Res.Rev. 2009;25(4):335–343. doi: 10.1002/dmrr.958. [DOI] [PubMed] [Google Scholar]
- 85.Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat.Rev.Immunol. 2007;7(6):443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 86.Fujio K, Okamura T, Yamamoto K. The Family of IL-10-secreting CD4+ T cells. Adv.Immunol. 2010;105:99–130. doi: 10.1016/S0065-2776(10)05004-2. 99-130. [DOI] [PubMed] [Google Scholar]
- 87.Rubtsov YP, Rasmussen JP, Chi EY, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 88.Metz TO, Qian WJ, Jacobs JM, et al. Application of proteomics in the discovery of candidate protein biomarkers in a diabetes autoantibody standardization program sample subset. J.Proteome.Res. 2008;7(2):698–707. doi: 10.1021/pr700606w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Purohit S, Podolsky R, Schatz D, et al. Assessing the utility of SELDI-TOF and model averaging for serum proteomic biomarker discovery. Proteomics. 2006;6(24):6405–6415. doi: 10.1002/pmic.200600420. [DOI] [PubMed] [Google Scholar]
- 90.Overgaard AJ, Hansen HG, Lajer M, et al. Plasma proteome analysis of patients with type 1 diabetes with diabetic nephropathy. Proteome.Sci. 2010;8(4) doi: 10.1186/1477-5956-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cooper JD, Walker NM, Smyth DJ, et al. Follow-up of 1715 SNPs from the Wellcome Trust Case Control Consortium genome-wide association study in type I diabetes families. Genes Immun. 2009;10(Suppl 1):S85–S94. doi: 10.1038/gene.2009.97. S85-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wallace C, Smyth DJ, Maisuria-Armer M, et al. The imprinted DLK1-MEG3 gene region on chromosome 14q32.2 alters susceptibility to type 1 diabetes. Nat.Genet. 2010;42(1):68–71. doi: 10.1038/ng.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen R, Yi EC, Donohoe S, et al. Pancreatic cancer proteome: the proteins that underlie invasion, metastasis, and immunologic escape. Gastroenterology. 2005;129(4):1187–1197. doi: 10.1053/j.gastro.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 94.Coute Y, Brunner Y, Schvartz D, et al. Early activation of the fatty acid metabolism pathway by chronic high glucose exposure in rat insulin secretory beta-cells. Proteomics. 2010;10(1):59–71. doi: 10.1002/pmic.200900080. [DOI] [PubMed] [Google Scholar]
- 95.Choi JS, Cho YK, Yoon SH, et al. Proteomic analysis of porcine pancreas development. BMB.Rep. 2009;42(10):661–666. doi: 10.5483/bmbrep.2009.42.10.661. [DOI] [PubMed] [Google Scholar]
- 96.Alberti A, Karamessinis P, Peroulis M, et al. ERp46 is reduced by high glucose and regulates insulin content in pancreatic beta-cells. Am.J.Physiol Endocrinol.Metab. 2009;297(3):E812–E821. doi: 10.1152/ajpendo.00053.2009. [DOI] [PubMed] [Google Scholar]
- 97.Song G, Cui Y, Zhong N, et al. Proteomic characterisation of pancreatic islet beta-cells stimulated with pancreatic carcinoma cell conditioned medium. J.Clin.Pathol. 2009;62(9):802–807. doi: 10.1136/jcp.2009.065391. [DOI] [PubMed] [Google Scholar]
- 98.Nyblom HK, Bugliani M, Fung E, et al. Apoptotic, regenerative, and immune-related signaling in human islets from type 2 diabetes individuals. J.Proteome.Res. 2009;8(12):5650–5656. doi: 10.1021/pr9006816. [DOI] [PubMed] [Google Scholar]
- 99.Antwi K, Hanavan PD, Myers CE, et al. Proteomic identification of an MHC-binding peptidome from pancreas and breast cancer cell lines. Mol.Immunol. 2009;46(15):2931–2937. doi: 10.1016/j.molimm.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 100.Lee HS, Jeong J, Lee KJ. Characterization of vesicles secreted from insulinoma NIT-1 cells. J.Proteome.Res. 2009;8(6):2851–2862. doi: 10.1021/pr900009y. [DOI] [PubMed] [Google Scholar]
- 101.Jin J, Park J, Kim K, et al. Detection of differential proteomes of human beta-cells during islet-like differentiation using iTRAQ labeling. J.Proteome.Res. 2009;8(3):1393–1403. doi: 10.1021/pr800765t. [DOI] [PubMed] [Google Scholar]
- 102.Jeffrey KD, Alejandro EU, Luciani DS, et al. Carboxypeptidase E mediates palmitate-induced beta-cell ER stress and apoptosis. Proc.Natl.Acad.Sci.U.S.A. 2008;105(24):8452–8457. doi: 10.1073/pnas.0711232105. [DOI] [PMC free article] [PubMed] [Google Scholar]