Abstract
BACKGROUND
Intensive glycemic control has been shown to positively impact outcomes in an intensive care setting. Whether this practice is beneficial after liver transplantation (LT) is not known.
METHODS
A retrospective review of patients undergoing LT from 2/02-07/04 was conducted to analyze the association between peri-operative hyperglycemia and outcomes after LT. Covariates included pre-existing diabetes, mean glucose three months pre-LT, need for insulin drip post-LT, mean total glucose during the post-LT hospitalization, age, gender, type of transplant, and MELD score. Outcomes within one year of LT included rejection, infection, re-hospitalization, prolonged ventilation, and patient/graft survival.
RESULTS
113 LT and 31 liver-kidney recipients were included. By multivariate logistic regression adjusting for covariates, the rejection rate was significantly lower for patients with postoperative glucose levels < 200 mg/dL (n=114) vs. > 200 mg/dL (n=30) (OR 0.055, 95%CI [0.0154, 0.200], p<0.001). The need for prolonged ventilation was more common in patients with glucose < 200 vs. > 200 mg/dL (OR 4.30, 95%CI [1.284, 14.388], p=0.018). While other outcomes, infection, re-hospitalization, patient/graft survival, were not different among the glucose control groups, rejection was associated with increased re-hospitalizations and infections.
CONCLUSION
Our data demonstrate an association between immediate post-transplant glycemic control and the development of subsequent rejection. Prospective trials investigating the effects of perioperative glycemic control on outcomes and morbidity after LT are warranted.
Introduction
Hyperglycemia has been shown to be associated with adverse outcomes in several populations, including patients with cerebral vascular accidents and myocardial infarctions and following coronary artery bypass graft surgery (1-3). Strict glucose control (80-110 mg/dl) with intensive insulin therapy has been shown to reduce the overall incidence of mortality, acute renal failure, need for prolonged ventilation and length of stay in the surgical intensive care unit (ICU) setting (4). However, the mortality benefit is not as clear in the medical ICU (5,6).
Patients with peri- and postoperative hyperglycemia following kidney transplantation have higher rates of rejection and other related complications (7,8). Postoperative hyperglycemia is common after liver transplantation and is related to the use of high dose corticosteroids, immunosuppressive medications, and increased hepatic glucose production and insulin resistance (9). However, the effects of hyperglycemia on outcomes following liver transplantation have not been studied. The aim of this study is to analyze the association between peri-operative hyperglycemia and outcomes within one year after liver transplantation.
Methods
This study is a retrospective chart review of patients undergoing liver or liver/kidney transplantation at a single, tertiary care transplant center from February 2002 to July 2005. Initial inclusion criteria included all liver and liver-kidney transplant recipients. Exclusion criteria included patients who had any previous solid organ transplant, had other invasive surgeries done at time of transplant, those with incomplete medical records and those who followed up at other institutions post transplantation. Data were collected from the hospital medical records and transplant database. Multiple pre-transplant variables were recorded including age, gender, MELD score, average blood glucose three months prior to transplant, and prior diagnosis of diabetes.
Data was collected from the pre-transplant, peri-transplant and post-transplant period. The pre-transplant period was defined as three months prior to the date of transplant. The peri-transplant period was defined as the time of transplant to the first discharge from the hospital, and the post-transplant period was defined as the time of transplant up to and including 12 months following the transplant. Glucose data was collected from the peri-transplant period. Data on both primary and secondary outcomes were collected from the post-transplant period. The primary endpoints included graft rejection, re-hospitalization, and infection within the post-transplant period. Secondary endpoints included prolonged ventilation immediately post-transplant and graft survival up to 12 months post-transplant. Rejection was defined using either clinical criteria or biopsy proven criteria according to the Banff schema (10). Clinical rejection was defined by the presence of liver transaminase or alkaline phosphatase elevation greater than two times the upper limit of normal, which normalized following pulse dose corticosteroid therapy (methylprednisone 500 mg/day for 3 days.)
All glucose levels following liver transplantation, up to and including the day of the first discharge from the hospital were reviewed and recorded. Both serum glucose chemistry values as well as glucose measurements from bedside point-of care reflectance meter tests were included, as long as there was no discrepancy between glucoses completed on both testing modes at the same time period. Glucose levels were recorded from all time periods of both day and night, and presumed to have included both fasting and non-fasting values. These values were then averaged together to determine the mean glucose level of the hospitalization. Mean average glucose levels during the peri-transplant period were used, as a majority of patients did not have hemoglobin A1C values or fasting blood glucose values available for analysis. The use of an insulin drip, and corticosteroid dosing were recorded. The types of immunosuppression regimens and the differences between them were also recorded. Steroids and tacrolimus were the primary immunosuppressive agents used. Only 6 patients ever received cyclosporine and none received sirolimus. Mycophenolate mofetil was taken by only 37 patients. All blood glucose levels measured and then averaged were from the peri-transplant period alone, while all rejection events were recorded during the post-transplant period, as defined above.
Statistical Analysis
Different patient characteristics were compared between the patients with post-transplant glucose level averages <200 mg/dL and ≥200 mg/dL. This cutoff of 200 mg/dL was chosen because it had been found to be significant for kidney transplant rejection previously (11,12) as well as for cardiovascular outcomes (3). In addition, a quartile analysis was performed. The group differences in continuous and binary variables were assessed using t-tests and chi-square tests, respectively. Furthermore, a multiple logistic regression was used to examine the adjusted association between the outcome measures and covariates of interest. Covariates included pre-existing diabetes, mean glucose three months pre-transplant, need for insulin drip post-transplant, age, gender, type of transplant (liver or liver-kidney), and MELD score. A p value less than 0.05 was regarded as statistically significant.
The generalized additive model (GAM) was employed to evaluate the detailed association between the continuous glucose level and the outcomes variable of interest. The GAM model, a nonparametric approach, allowed the evaluation of the covariate effect of interest without the need of parametric modeling.
Results
In the period studied February of 2002 to July 2005, 308 patients underwent liver or liver/kidney transplantation. 144 subjects met the entry criteria and were included in the study. Three subjects were not included in the study because they had a CABG at the time of surgery, 10 were excluded because they had undergone a previous liver transplant, one was excluded because of ischemic bowel at the time of transplantation, and the others were excluded due to lack of follow up at the home institution or incomplete medical records for the year following transplant. Of our 144 subjects, 113 had liver transplants alone and 31 had combined liver-kidney transplants. Patient characteristics are summarized in Table 1. Overall, 50 (35%) of patients had a prior history of diabetes and there were 63 patients (44%) with rejection episodes, 84 patients (58%) with infections, 41 (28%) patients with prolonged ventilation, and 92 (64%) patients requiring rehospitalization. Of the 31 patients with liver-kidney transplants, two patients had suspected kidney rejection and were biopsied, however pathology showed no evidence of rejection in both patients. One year patient and hepatic graft survival were 90% and 88%, respectively.
Table 1.
Baseline Characteristics of patients undergoing Liver Transplantation
| Total Patients | N (%) or mean ± SD |
|---|---|
| Liver Transplant | 113 (78) |
| Liver-Kidney Transplant | 31 (22) |
| Mean Age | 53.9±11.1 |
| Sex | |
| Male | 98 (68) |
| Female | 46 (32) |
| Pre-transplant Diabetes | 50 (35) |
| MELD Score | 20.7±9.8 |
| Etiology of Liver Disease | |
| HCV | 56 (39) |
| Alcoholic | 22 (15) |
| PSC | 20 (14) |
| HBV | 13 (9) |
| Other | 33 (23) |
| Immunosupression at Discharge | |
| Tacrolimus | 104 (72) |
| Tacrolimus and MMF | 34 (24) |
| Tacrolimus and Cyclosporine | 3 (2) |
| Cyclosporine and MMF | 3 (2) |
MELD – Model of End-Stage Liver Disease; HCV – Hepatitis C Virus; PSC – Primary Sclerosing Cholangitis; HBV – Hepatitis B Virus; MMF – Mycophenolate Mofetil
Table 2 summarizes the patient characteristics according to whether their peri-transplant glucose levels were above or below 200 mg/dL. In the multivariate logistic regression analysis, the rejection rate was lower for patients with postoperative glucose levels < 200 mg/dL compared to > 200 mg/dL (OR 0.055, 95%CI [0.0154, 0.200], p<0.001) following adjustment for age, type of transplant, gender, MELD score, use of insulin drip, and the pre-transplant 3-month average glucose level. There was a greater proportion of patients with preexisting diabetes in the > 200 mg/dL group (60%, n=18) compared to the < 200 mg/dL group (28%, n=32) (p=0.002). There were no significant differences between the groups in the average doses of prednisone, the proportions of patients receiving tacrolimus, or the doses of tacrolimus. Prolonged ventilation also occurred more frequently for the patients with glucose levels less than 200 mg/dL, after similar adjustments of covariates (OR 4.30, 95%CI [1.284, 14.388], p=0.018). There were no significant differences in patient or graft survival, re-hospitalization or infection rates.
Table 2.
Peri-transplant Patient Characteristics and Outcomes Stratified by Blood Glucose Level
| Mean Glucose < 200 mg/dL n=114 N (%) or mean± SD |
Mean Glucose > 200 mg/dL n=30 N (%) or mean± SD |
Total n=144 N (%) or mean± SD |
p value | |
|---|---|---|---|---|
| Age | 53.7 ± 10.7 | 54.5 ± 12.5 | 53.9 ± 11.1 | p = ns |
| Gender (% female) | 37 (32.5) | 11 (36.7) | 46 (31.9) | p = ns |
| MELD score | 20.7 ± 9.8 | 20.6 ± 10.3 | 20.7 ± 9.8 | p = ns |
| Liver-Kidney Transplant | 26 (22.8) | 5 (16.7) | 31 (21.5) | p = ns |
| On Insulin Drip | 53 (46.5) | 20 (66.7) | 73 (50.6) | p = ns |
| Average Blood Glucose (Pre Liver Transplant) |
117.9 ± 35.4 | 156.6 ± 59.8 | 129.1 ± 43.9 | p < 0.001 |
| Average Blood Glucose (Post Liver Transplant) |
154.9 ± 22.3 | 232.8 ± 21.4 | 159.1 ± 31.8 | p < 0.001 |
| Diabetic Status | 32 (28%) | 18 (60%) | 50 (35) | p = 0.002 |
| Rejection | 40 (35.1) | 23 (76.7) | 63 (43.8) | p <0.001 |
| Re-Hospitalization | 71 (62.3) | 21 (70.0) | 92 (63.8) | p = ns |
| Infection | 68 (59.6) | 16 (53.3) | 84 (58.3) | p = ns |
| Graft survival | 98 (86.0) | 28 (93.3) | 126 (87.5) | p = ns |
| Patient Survival | 108 (94.7) | 28 (93.3) | 136 (94.4) | p = ns |
| Prolonged ventilation | 37 (32.5) | 4 (13.3) | 41 (28.5) | p = 0.047 |
| Average Prednisone Dose (mg/d) |
184.4 + 146.4 | 237.8 + 140.5 | 195.5 +146.0 | p=ns |
| Proportion using Tacrolimus (%) |
95.6% | 100% | 96.5% | p=ns |
| Tacrolimus Average Levels |
8.51 + 3.82 | 9.55 + 5.38 | 8.74 + 4.62 | p=ns |
MELD – Model of End-Stage Liver Disease
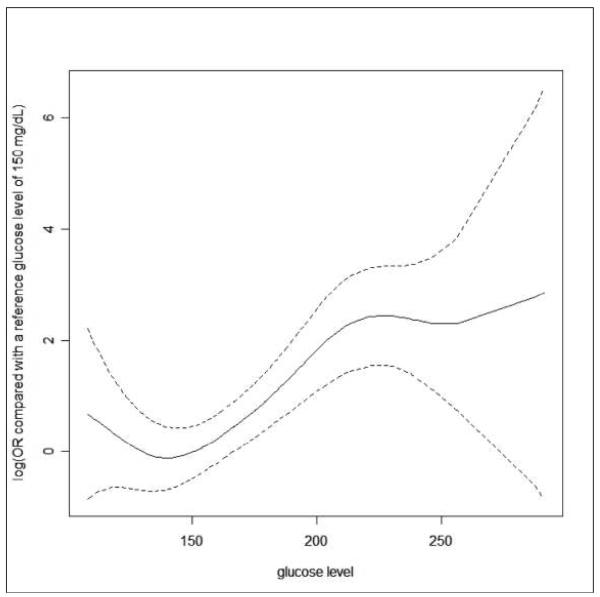
Table 3 summarizes the rejection rates in all patients stratified by mean blood glucose quartile values. Patients in quartile group IV (blood glucose >190 mg/dl) had higher rejection rates (OR 9.09, p < 0.001) in comparison with quartile group I, (blood glucose < 142.9 mg/dL) even following adjustment for age, type of transplant, gender, MELD score, and placement on insulin drip (OR 9.875, p < 0.001). Figure 1 characterizes the correlations between the post-transplant glucose level and the probability of rejection as a continuous function.
Table 3.
Odds Ratio of Rejection
| Rejection Rate Odds Ratio |
Rejection Rate p value |
|
|---|---|---|
| Blood Glucose < 200 vs. > = 200 | 0.164 | p < 0.001 |
| With adjustment for age, type of transplant, gender, MELD score, placement on insulin drip |
0.115 | p < 0.001 |
| Quartile Group II (Blood Glucose 142.9-163.1) vs. Reference Quartile Group I (Blood Glucose < 142.9) |
1.749 | p = 0.295 |
| With adjustment for age, type of transplant, gender, MELD score, placement on insulin drip |
1.083 | p = .891 |
| Quartile Group III (Blood Glucose 163.1-191) vs. Reference Quartile Group I |
3.130 | p = 0.029 |
| With adjustment for age, type of transplant, gender, MELD score, placement on insulin drip |
2.223 | p = 0.153 |
| Quartile Group IV (Blood Glucose > 191.1) vs. Reference Quartile Group I |
9.098 | p < 0.001 |
| With adjustment for age, type of transplant, gender, MELD score, placement on insulin drip |
9.875 | p < 0.001 |
Figure 1.
The relationship between the post-LT glucose level and the risk of rejection within one year post-LT. The solid line is the estimated log(OR) of the risk of rejection compared with the reference group of a glucose level of 150 mg/dl. The dotted line is the point-wise 95% confidence interval for the estimated log(OR).
Table 4 summarizes the patient characteristics with or without rejection following transplantation. The mean pre-transplant and post-transplant glucose measurements trended higher in the patients who had rejection in the post-transplant period, but the differences between the groups were not statistically significant. There was a greater proportion of patients with preexisting diabetes in the rejection group (44%, n=28) compared to the no rejection group (27%, n=22) (p=0.002). There were no significant differences between the groups in the average doses of prednisone, the proportions of patients receiving tacrolimus, or the doses of tacrolimus. However, patients with post-transplant rejection had a higher average number of infections (1.89 vs. 1.19; p < 0.001) and higher average number of re-hospitalization days (11.12 vs. 5.75; p <0.001.) compared to those without rejection.
Table 4.
Baseline Characteristics and Post Transplant Outcomes Stratified by Rejection
| 144 total patients | No Rejection (n=81) N (%) or mean± SD |
Rejection (n=63) N (%) or mean± SD |
p Value |
|---|---|---|---|
| Age | 52.4±11.6 | 55.8±10.2 | p = ns |
| Gender | |||
| Male (%) | 54 (67) | 42 (67) | p = ns |
| Female (%) | 27 (33) | 21 (33) | p = ns |
| MELD | 21.4±10.2 | 19.7±9.3 | p = ns |
| Etiology | p = ns | ||
| HCV (%) | 25 (31) | 31 (49) | |
| Alcoholic (%) | 14 (17) | 8 (13) | |
| PSC (%) | 11 (14) | 9 (14) | |
| HBV (%) | 8 (10) | 5 (8) | |
| Other cause (%) | 23 (28) | 10 (16) | |
| Type of transplant | |||
| Liver (%) | 59 (73) | 54 (86) | p = ns |
| Liver-Kidney (%) | 22 (27) | 9 (14) | p = ns |
| Pre-transplant Diabetes | 22 (27%) | 28 (44%) | p = 0.0354 |
| Average Glucose Pre-Transplant |
129.1±43.9 | 146.0±45.0 | p = ns |
| Average Glucose Post-Transplant |
159.2±31.8 | 186.5±41.4 | p = ns |
| Average # of Infections | 1.19 | 1.89 | p<0.001 |
| Average Rehospitalization Days |
5.75 | 11.12 | p<0.001 |
| Average Prednisone Dose | 179.9 ± 156.8 | 215.6 ± 130.1 | p=ns |
| Proportions using Tacrolimus |
92.6% | 100% | p=ns |
| Tacrolimus Average Levels (ng/mL) |
8.16 ± 3.73 | 9.42 ± 4.65 | p=ns |
MELD – Model of End-Stage Liver Disease; HCV – Hepatitis C Virus; PSC – Primary Sclerosing Cholangitis; HBV – Hepatitis B Virus
Discussion
Our data demonstrate a significant association between immediate post-transplant glycemic control and the development of subsequent rejection. Rejection rates were significantly higher in those with an average glucose level > 200 even following adjustment for confounding variables. This association has been demonstrated after kidney transplantation, but, to our knowledge, has not been shown after liver or other solid organ transplantation (7,8, 11,12). While graft survival was not different between those with and without rejection, patients that developed rejection had more related morbidity, i.e. significantly higher rates of infection and re-hospitalization days. Prolonged ventilation was found to be significantly more common in those patients whose glucose levels were less than 200 mg/dl, for reasons that are unclear. As could be expected, the group with glucose levels < 200 mg/dL had a higher proportion with preexisting diabetes. In addition, the group undergoing rejection also had a prior proportion with diabetes, as has been reported previously (16). There were no differences in prednisone dosage, tacrolimus usage or tacrolimus dosage between the >200 or < 200 mg/dl groups or the rejection or no rejection groups. However, we did not observe any significant evidence of association between glucose control and other post-transplant outcomes, including infection, re-hospitalization, and overall graft survival.
There has been recent evidence that intraoperative glycemic control during transplant is correlated with outcomes, such as graft survival (17). Another study showed that use of intensive insulin therapy post-operatively has been shown to reduce rates of liver allograft rejection (18). While there is some evidence that rejection may actually be associated with increased graft survival in non-HCV infected patients, rejection in our study was related to higher rates of infection and number of re-hospitalization days (19). Thus, control of hyperglycemia may directly reduce the rate of rejection and other related complications.
While the direct mechanism has not been elucidated, we hypothesize that the observed association between rejection and glycemic control maybe related to the inflammatory response associated with hyperglycemia. The role of hyperglycemia in induction of oxidative stress and ultimately endothelial dysfunction has been described (13). Endothelitis on liver biopsy is a characteristic histological finding of acute rejection in liver transplant recipients (14). Speculatively, hyperglycemia during the peri-transplant period may be associated with endothelial inflammation within the allograft, which may be a precursor to acute rejection (15).
This study has several limitations. As this was a retrospective review, the total number of glucose measurements obtained varied from patient to patient. The number of glucose measurements recorded and the therapy rendered was determined by the primary transplant team. The glucose levels were not obtained in all patients in a systematic fashion, such that elevated levels often led to more monitoring and glucose measurements in those patients. However, all glucose levels over the entire hospital stay were averaged into one mean glucose value, including fasting and non-fasting, laboratory chemistry and glucometer values. Therefore, the regularity by which glucose tests were performed becomes less critical to the analysis.
Another limitation was the fact that biopsy confirmation was not required at our center for treating a presumed clinical rejection. Biopsy-confirmed rejection was present in only 19 of the 63 patients. The overall incidence of acute (clinical and histological) rejection in our cohort was relatively high and possibly inflated due to “over-diagnosed” acute rejection. However, our strict criteria for response after pulse corticosteroid therapy, i.e. complete normalization of liver test abnormalities, supports the diagnosis of acute rejection. Other recent papers have used less stringent criteria in defining acute rejection. (20) Our mean glucose level obtained did not include glucose levels following pulse corticosteroid therapy. Hyperglycemia predated the occurrence of rejection in those patients.
In summary, hyperglycemia following transplantation was associated with an increased risk of rejection, which was tied to a higher rate of infection and length of re-hospitalization. These outcomes not only have clinical importance for the individual patient but also have significant economic implications. However, these associations determined retrospectively do not directly prove cause and effect. Therefore, prospective trials investigating the effects of standard versus strict glycemic control on outcomes after liver transplantation are warranted.
Footnotes
No disclosures/conflicts to report for any of the authors
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baird T, Parsons M, Phanh T, Butcher KS, Desmond PM, Tress BM, et al. Persistent post-stroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34(9):2208–14. doi: 10.1161/01.STR.0000085087.41330.FF. [DOI] [PubMed] [Google Scholar]
- 2.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systemic review. Lancet. 2000;355(9206):773–8. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 3.Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, et al. Continuous insulin infusion reduces mortality in patients with diabetes, undergoing coronary bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–18. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 4.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckz F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 5.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–61. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 6.Treggiari MM, Karir V, Yanez ND, Weiss NS, Daniel S, Deem SA. Intensive insulin therapy and mortality in critically ill patients. Crit Care. 2008;12(1):R29. doi: 10.1186/cc6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas MC, Mathew TH, Russ GR, Rao MM, Moran J. Early peri-operative glycemic control and renal allograft rejection in patients without diabetes. BMC Nephrology. 2000;1:1–6. doi: 10.1186/1471-2369-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tutone VK, Mark PB, Revanur V, Traynor J, Buist LJ, Geddes CC, et al. Random blood glucose measurements and survival in non-diabetic renal transplant recipients. Transplantation. 2004:3006–3011. doi: 10.1016/j.transproceed.2004.10.067. [DOI] [PubMed] [Google Scholar]
- 9.Delgado-Borrego A, Liu YS, Jordan SH, Agrawal S, Zhang H, Christofi M, et al. Prospective study of liver transplant recipients with HCV infection: evidence for a causal relationship between HCV and insulin resistance. Liver Transpl. 2008;14(2):193–201. doi: 10.1002/lt.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25(3):658–63. doi: 10.1002/hep.510250328. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 11.Thomas MC, Mathew TH, Russ GR, Rao MM, Moran J. Early peri-operative glycemic control and allograft rejection in patients with diabetes mellitus: a pilot study. Transplantation. 2001;72:1321–1324. doi: 10.1097/00007890-200110150-00024. [DOI] [PubMed] [Google Scholar]
- 12.Ganji MR, Charkhchian M, Hakemi M, Nederi GH, Solymanian T, Saddadi F, et al. Association of hyperglycemia on allograft function in the early period after renal transplantation. Transplantation Proc. 2007;39:852–854. doi: 10.1016/j.transproceed.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 13.Ceriello A. Oxidative stress and diabetes-associated complications. Endocr Pract. 2006;12(Suppl 1):60–2. doi: 10.4158/EP.12.S1.60. [DOI] [PubMed] [Google Scholar]
- 14.Steinhoff G, Behrend M, Haverich A. Signs of endothelial inflammation in human heart allografts. Eur Heart J. 1991;12(Suppl D):141–3. doi: 10.1093/eurheartj/12.suppl_d.141. [DOI] [PubMed] [Google Scholar]
- 15.Melter M, McMahon G, Fang J, Ganz P, Briscoe DM. Current understanding of chemokine involvement in allograft transplantation. Pediatr Transplant. 1999;3(1):10–21. doi: 10.1034/j.1399-3046.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- 16.John PR, Thulovath PJ. Outcome of liver transplantation in patients with diabetes mellitus: a case-control study. Hepatology. 2001;34:889–895. doi: 10.1053/jhep.2001.29134. [DOI] [PubMed] [Google Scholar]
- 17.Ammori JB, Sigakis M, Englesbe MJ, O’Reilly M, Pelletier SJ. Effect of intraoperative hyperglycemia during liver transplantation. J Surg Res. 2007;140(2):227–33. doi: 10.1016/j.jss.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Hsaiky LM, Bajjoka IE, Patel D, Abouljoud MS. Postoperative Use of Intense Insulin Therapy in Liver Transplant Recipients [abstract] Am J Transplant. 2008;8(S2):307. [Google Scholar]
- 19.Charlton M, Seaberg E. Impact of immunosuppression and acute rejection on recurrence of hepatitis C: results of the National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Liver Transpl Surg. 1999 Jul;5(4 Suppl 1):S107–14. doi: 10.1053/JTLS005s00107. [DOI] [PubMed] [Google Scholar]
- 20.Shaked A, Ghobrial RM, Merion RM, Shearon TH, Emond JC, Fair JH, Fisher RA, et al. A2ALL Study Group Incidence and severity of acute cellular rejection in recipients undergoing adult living donor or deceased donor liver transplantation. Am J Transplant. 2009 Feb;9(2):301–8. doi: 10.1111/j.1600-6143.2008.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]