Abstract
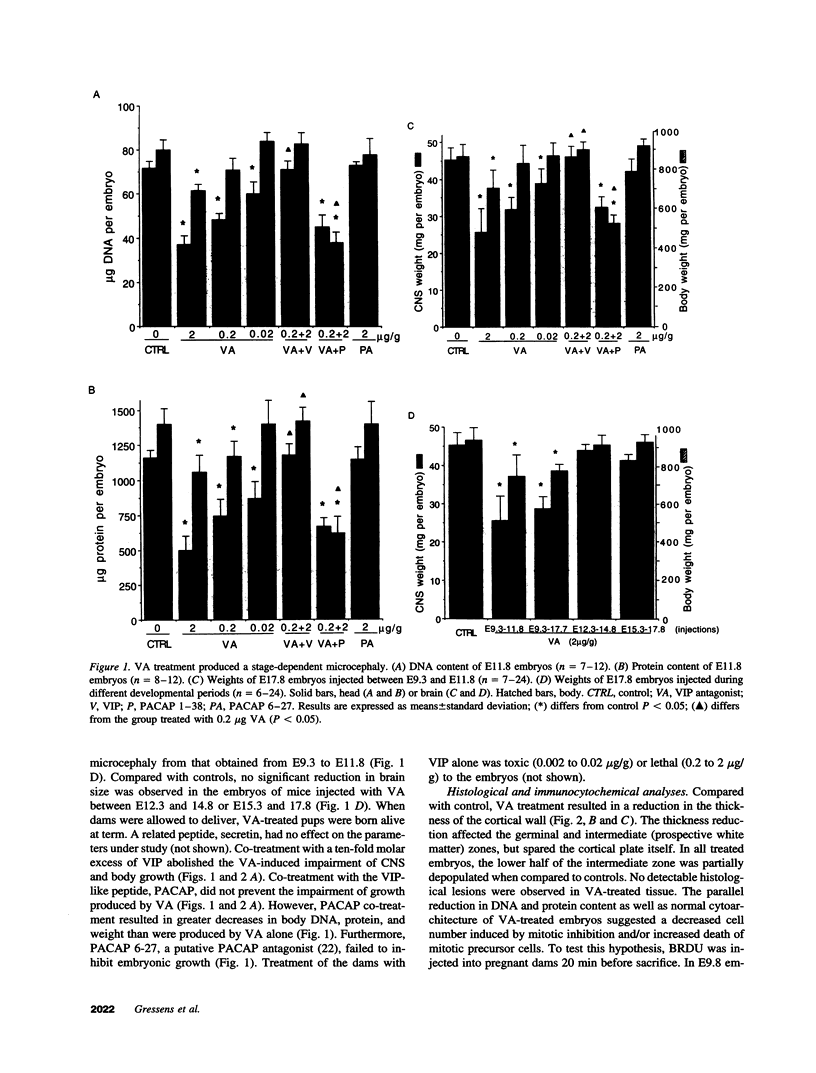
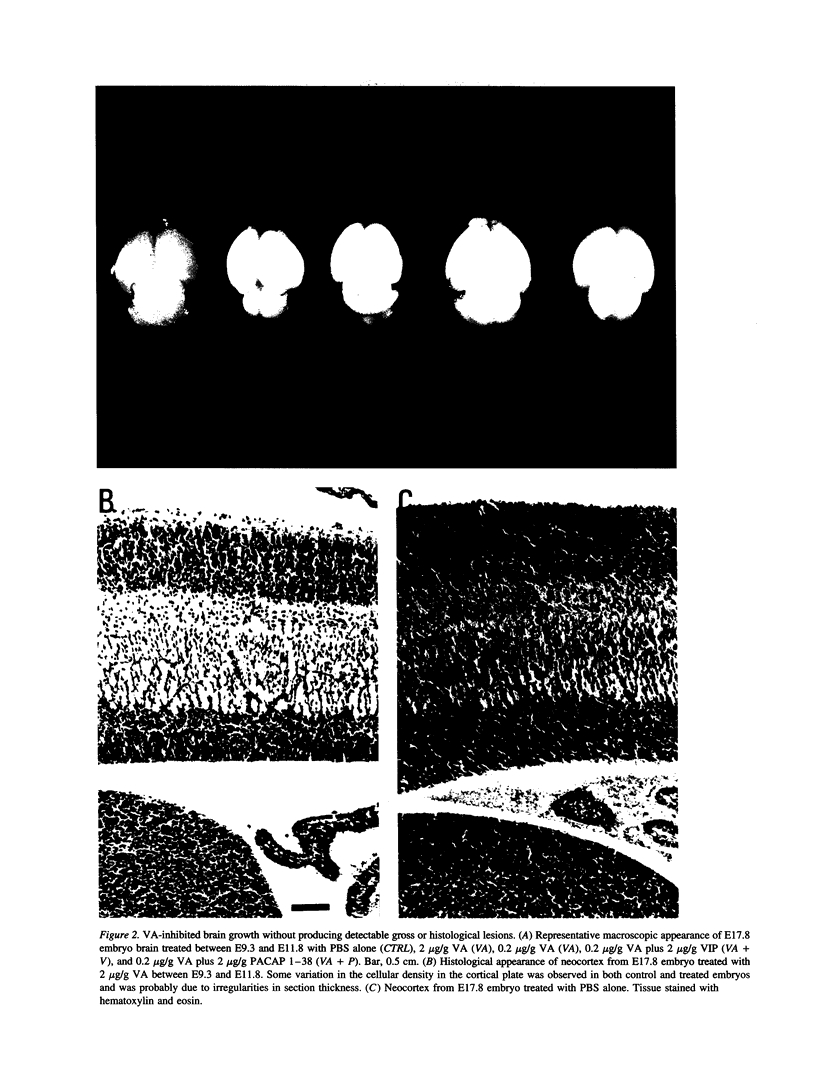
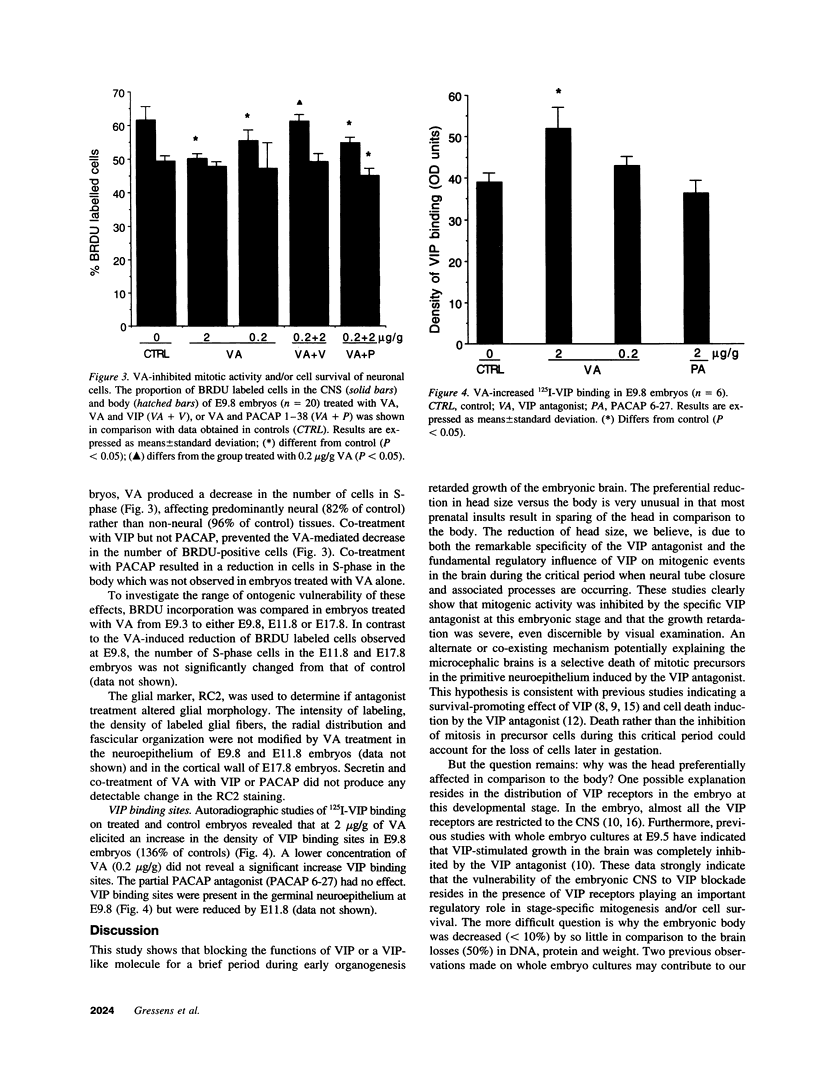
Vasoactive intestinal peptide (VIP) has potent growth-related actions that influence cell mitosis, neuronal survival, and neurodifferentiation in cell culture. VIP can also produce dramatic growth in postimplantation mouse embryos in vitro, characterized by large increases in cell number. The goal of the present study was to assess the role of VIP on early nervous system development in vivo. Pregnant mice were treated with a specific antagonist to VIP. Prenatal administration of the antagonist early in development (E9-E11) produced severe microcephaly characterized by decreased embryonic brain weight with reduced DNA and protein content. The retardation of growth was disproportionally manifested in the brain compared with the body and was prevented by co-treatment with VIP. Identical treatment with the antagonist later in gestation had no detectable effect on embryonic growth. VIP receptors, which were restricted to the central nervous system during this stage of embryonic development, were increased in the neuroepithelium of antagonist-treated embryos while the number of cells in S-phase was significantly decreased. Thus, VIP regulates brain growth in vivo and inhibition of its action provides new insight into a molecular mechanism for microcephaly.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell K. W., Webster W. S. The contribution of primary and secondary neuronal degeneration to prenatally-induced micrencephaly. Neurotoxicol Teratol. 1988 Jan-Feb;10(1):65–73. doi: 10.1016/0892-0362(88)90068-2. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfi S., Dorigotti L., Abbracchio M. P., Balduini W., Coen E., Ragusa C., Cattabeni F. Methylazoxymethanol microencephaly in rats: neurochemical characterization and behavioral studies with the nootropic oxiracetam. Pharmacol Res Commun. 1984 Jan;16(1):67–83. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brenneman D. E., Eiden L. E. Vasoactive intestinal peptide and electrical activity influence neuronal survival. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1159–1162. doi: 10.1073/pnas.83.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman D. E., Neale E. A., Foster G. A., d'Autremont S. W., Westbrook G. L. Nonneuronal cells mediate neurotrophic action of vasoactive intestinal peptide. J Cell Biol. 1987 Jun;104(6):1603–1610. doi: 10.1083/jcb.104.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman D. E., Nicol T., Warren D., Bowers L. M. Vasoactive intestinal peptide: a neurotrophic releasing agent and an astroglial mitogen. J Neurosci Res. 1990 Mar;25(3):386–394. doi: 10.1002/jnr.490250316. [DOI] [PubMed] [Google Scholar]
- DeChiara T. M., Efstratiadis A., Robertson E. J. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990 May 3;345(6270):78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- Fatatis A., Holtzclaw L. A., Avidor R., Brenneman D. E., Russell J. T. Vasoactive intestinal peptide increases intracellular calcium in astroglia: synergism with alpha-adrenergic receptors. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2036–2040. doi: 10.1073/pnas.91.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Hayasaka S., Itoh M., Takeuchi Y. Development of neurons and synapses in ochratoxin A-induced microcephalic mice: a quantitative assessment of somatosensory cortex. Neurotoxicol Teratol. 1992 May-Jun;14(3):191–196. doi: 10.1016/0892-0362(92)90015-3. [DOI] [PubMed] [Google Scholar]
- Gadisseux J. F., Evrard P. Glial-neuronal relationship in the developing central nervous system. A histochemical-electron microscope study of radial glial cell particulate glycogen in normal and reeler mice and the human fetus. Dev Neurosci. 1985;7(1):12–32. doi: 10.1159/000112273. [DOI] [PubMed] [Google Scholar]
- Gadisseux J. F., Evrard P., Misson J. P., Caviness V. S. Dynamic structure of the radial glial fiber system of the developing murine cerebral wall. An immunocytochemical analysis. Brain Res Dev Brain Res. 1989 Nov 1;50(1):55–67. doi: 10.1016/0165-3806(89)90126-0. [DOI] [PubMed] [Google Scholar]
- Gozes I., McCune S. K., Jacobson L., Warren D., Moody T. W., Fridkin M., Brenneman D. E. An antagonist to vasoactive intestinal peptide affects cellular functions in the central nervous system. J Pharmacol Exp Ther. 1991 Jun;257(3):959–966. [PubMed] [Google Scholar]
- Gozes I., Meltzer E., Rubinrout S., Brenneman D. E., Fridkin M. Vasoactive intestinal peptide potentiates sexual behavior: inhibition by novel antagonist. Endocrinology. 1989 Dec;125(6):2945–2949. doi: 10.1210/endo-125-6-2945. [DOI] [PubMed] [Google Scholar]
- Gozes Y., Brenneman D. E., Fridkin M., Asofsky R., Gozes I. A VIP antagonist distinguishes VIP receptors on spinal cord cells and lymphocytes. Brain Res. 1991 Feb 1;540(1-2):319–321. doi: 10.1016/0006-8993(91)90528-4. [DOI] [PubMed] [Google Scholar]
- Gressens P., Gofflot F., Van Maele-Fabry G., Misson J. P., Gadisseux J. F., Evrard P., Picard J. J. Early neurogenesis and teratogenesis in whole mouse embryo cultures. Histochemical, immunocytological and ultrastructural study of the premigratory neuronal-glial units in normal mouse embryo and in mouse embryos influenced by cocaine and retinoic acid. J Neuropathol Exp Neurol. 1992 Mar;51(2):206–219. doi: 10.1097/00005072-199203000-00010. [DOI] [PubMed] [Google Scholar]
- Gressens P., Hill J. M., Gozes I., Fridkin M., Brenneman D. E. Growth factor function of vasoactive intestinal peptide in whole cultured mouse embryos. Nature. 1993 Mar 11;362(6416):155–158. doi: 10.1038/362155a0. [DOI] [PubMed] [Google Scholar]
- Gressens P., Richelme C., Kadhim H. J., Gadisseux J. F., Evrard P. The germinative zone produces the most cortical astrocytes after neuronal migration in the developing mammalian brain. Biol Neonate. 1992;61(1):4–24. doi: 10.1159/000243526. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Mizutani M. Early neurobehavioral disorders in the micrencephalic offspring induced by prenatal treatment with N-methyl-N-nitrosourea or methylazoxymethanol in the rat. J Vet Med Sci. 1991 Aug;53(4):643–649. doi: 10.1292/jvms.53.643. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Agoston D. V., Gressens P., McCune S. K. Distribution of VIP mRNA and two distinct VIP binding sites in the developing rat brain: relation to ontogenic events. J Comp Neurol. 1994 Apr 8;342(2):186–205. doi: 10.1002/cne.903420204. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Gozes I., Hill J. L., Fridkin M., Brenneman D. E. Vasoactive intestinal peptide antagonist retards the development of neonatal behaviors in the rat. Peptides. 1991 Jan-Feb;12(1):187–192. doi: 10.1016/0196-9781(91)90186-s. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Harris A., Hilton-Clarke D. I. Regional distribution of guanine nucleotide-sensitive and guanine nucleotide-insensitive vasoactive intestinal peptide receptors in rat brain. Neuroscience. 1992 Jun;48(4):925–932. doi: 10.1016/0306-4522(92)90280-f. [DOI] [PubMed] [Google Scholar]
- Ishihara T., Shigemoto R., Mori K., Takahashi K., Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992 Apr;8(4):811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- Mercugliano M., Hyman S. L., Batshaw M. L. Behavioral deficits in rats with minimal cortical hypoplasia induced by methylazoxymethanol acetate. Pediatrics. 1990 Mar;85(3 Pt 2):432–436. [PubMed] [Google Scholar]
- Miyata A., Jiang L., Dahl R. D., Kitada C., Kubo K., Fujino M., Minamino N., Arimura A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem Biophys Res Commun. 1990 Jul 31;170(2):643–648. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- Moody T. W., Zia F., Draoui M., Brenneman D. E., Fridkin M., Davidson A., Gozes I. A vasoactive intestinal peptide antagonist inhibits non-small cell lung cancer growth. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4345–4349. doi: 10.1073/pnas.90.10.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- Opitz J. M., Holt M. C. Microcephaly: general considerations and aids to nosology. J Craniofac Genet Dev Biol. 1990;10(2):175–204. [PubMed] [Google Scholar]
- Pincus D. W., DiCicco-Bloom E. M., Black I. B. Vasoactive intestinal peptide regulates mitosis, differentiation and survival of cultured sympathetic neuroblasts. Nature. 1990 Feb 8;343(6258):564–567. doi: 10.1038/343564a0. [DOI] [PubMed] [Google Scholar]
- Pisegna J. R., Wank S. A. Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type I receptor. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6345–6349. doi: 10.1073/pnas.90.13.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberecht P., Woussen-Colle M. C., De Neef P., Gourlet P., Buscail L., Vandermeers A., Vandermeers-Piret M. C., Christophe J. The two forms of the pituitary adenylate cyclase activating polypeptide (PACAP (1-27) and PACAP (1-38)) interact with distinct receptors on rat pancreatic AR 4-2J cell membranes. FEBS Lett. 1991 Jul 29;286(1-2):133–136. doi: 10.1016/0014-5793(91)80958-6. [DOI] [PubMed] [Google Scholar]
- Spengler D., Waeber C., Pantaloni C., Holsboer F., Bockaert J., Seeburg P. H., Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993 Sep 9;365(6442):170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Sreedharan S. P., Patel D. R., Huang J. X., Goetzl E. J. Cloning and functional expression of a human neuroendocrine vasoactive intestinal peptide receptor. Biochem Biophys Res Commun. 1993 Jun 15;193(2):546–553. doi: 10.1006/bbrc.1993.1658. [DOI] [PubMed] [Google Scholar]