Abstract
NF-κB/p65 is constitutively activated in pancreatic cancers where it plays critical role in the transcriptional activation of multiple cell survival genes. We have previously demonstrated the apoptosis-inducing effects of BITC in pancreatic cancer cells. We hypothesized that inhibition of NF-κB/p65 could be the mechanism of BITC-induced apoptosis. Therefore, the effect of BITC on NF-κB/p65 was evaluated in BxPC-3, Capan-2 and normal HPDE-6 cells by western blotting, transcriptional and DNA-binding activity and by immunohistochemistry in the xenografted tumors. Our results reveal a remarkable decrease in the phosphorylation of NF-κB/p65 at Ser536 in both BxPC-3 and Capan-2 cells by BITC treatment. The expression of NF-kB/p65 was down-regulated significantly in BxPC-3 cells whereas it remained unchanged in Capan-2 cells. BITC treatment caused significant decrease in NF-κB transcriptional and DNA-binding activity in both BxPC-3 and Capan-2 cells. A drastic decrease was observed in the expression and reporter activity of cyclin D1 in both the cell lines. Moreover, BITC also caused significant decrease in the expression and activity of HDAC1 and HDAC3 in BxPC-3 and HDAC3 in Capan-2 cells. Overexpression of HDAC1 or HDAC3 abrogated the effects of BITC. BITC treatment did not caused any change in HDAC expression in normal HPDE-6 cells. Immunohistochemical analysis of tumors from BITC-treated mice showed significantly reduced staining for NF-kB, cyclin D1, HDAC-1/3, compared to control. Our results suggest that inhibition of HDAC1/3 by BITC as a plausible mechanism of NF-κB inactivation resulting in the in vitro and in vivo growth suppression of pancreatic cancer cells.
Keywords: Benzyl isothiocyanate, NF-kB, HDAC-3, HDAC-1, Cyclin D1, pancreatic cancer chemoprevention
Introduction
Number of studies supports the fact that food phytochemicals including isothiocyanates (ITCs) belonging to organosulfur group of compounds protect against cancer (1-2). ITCs occur as glucosinolates in cruciferous vegetables of Brassica species. Effectiveness of ITCs as chemoprotective agents against chemical carcinogenesis in experimental animals have been well documented (3). Besides inhibiting phase I enzymes which are required for the bioactivation of carcinogens and increase carcinogen excretion or inducing detoxification by phase II enzyme including glutathione (GSH) S-transferase in various model systems (4-5), ITCs are also known to induce caspase mediated apoptosis through multiple signaling pathways (6-7).
In cancer cells’ including pancreatic, breast, colon, and prostate, NF-κB is constitutively active which protects the cells from apoptosis and in some cases stimulates their growth (8-9). NF-κB is involved in the maintenance of normal cellular functions including cell cycle progression, cellular motility, cell to cell communication and cell lineage development (8-12). However, aberrant regulation of NF-κB has been observed in tumorigenesis, malignant transformation, metastasis and angiogenesis (8-9). Modulation of NF-κB activity is therefore extremely critical in regulating the fate of cancer cells (9). Thus many anti-cancer drugs seek to inhibit NF-κB activation for inhibiting tumor growth or sensitizing tumor cells to chemotherapy (13-14). NF-κB typically resides in cytoplasm bound to its inhibitory protein Iκ-Bα (15). Upon cellular stimulation, Iκ-Bα proteins are phosphorylated by IKK liberating NF-κB which translocate to nucleus and gets involved in the transcription of various genes (10-12). There are reports which suggest that NF-κB may shuttle between cytoplasm and nucleus in non-stimulated cells as well (16-17). IKKα and β the upstream kinases modulate the anti-apoptotic response and also cell growth by regulating the activation of NF-κB (18). IKK activation in turn is regulated by its upstream NF-κB activating kinase (NAK), which activates IKKβ through direct phosphorylation (19). Besides phosphorylation, several other modifications including ubiquitination, acetylation, sumoylation and nitrosylation also regulate NF-κB (20). Furthermore, NF-κB activity is also regulated by transcriptional co-activators or corepressors which include histone acetyltransferases (HATs) and HDACs (21-23). HDAC1, HDAC2 and HDAC3 have been reported to interact with NF-κB, however, their roles in the regulation of NF-κB activity are controversial (21-23). Acetylation of p65 either suppresses or activates NF-κB transcription depending on the biological context of the cell (24-26). Five lysine residues in the DNA binding domain of NF-κB/p65 (lysine 122, 123, 218, 221 and 310) play vital role in regulating its transcriptional activity and intra-nuclear mobility (27). Acetylation of lysine 218, 221 and 310 cause activation of NF-κB/p65 by inhibiting its IK-Bα binding and thereby decreasing nuclear export of this complex, whereas acetylation at lysine 122 and 123 suppresses transcriptional activity of NF-κB by reducing its DNA binding (27). HDAC inhibitors also lead to growth arrest by inducing p21WAF1 and down regulate transcription of cyclin D1 by inhibiting NF-κB DNA binding (28-29).
Previous studies from our laboratory showed that BITC mediated cell cycle arrest and apoptosis in pancreatic cancer cells was associated with the modulation of cell cycle regulatory proteins including induction of p21WAF1 and down-regulation of cyclin D1 (30-31). Since cyclin D1 is an important transcriptional target of NF-κB, we hypothesized that apoptosis in human pancreatic cancer cells by BITC could be due to inactivation of NF-κB.
In present study, we provide evidence that BITC specifically inhibits the expression and activity of HDAC1 and HDAC3 in BxPC-3 and HDAC3 in Capan-2 pancreatic cancer cells without causing any effect on HPDE-6 normal pancreatic ductal epithelial cells. We found that BITC inhibits NF-κB transcriptional activity and p65 DNA binding capacity in both BxPC-3 and Capan-2 cell lines which exhibit differential p65 phosphorylation. Over-expression of HDAC1 and HDAC3 in BxPC-3 and HDAC3 in Capan-2 cells significantly blocked the effects of BITC with respect to p65 DNA binding, NF-κB transcriptional activity and cyclin D1 reporter activity leading to the attenuation of BITC mediated apoptosis. Further, BxPC-3 tumor xenografts from BITC treated mice revealed reduced expression of NF-kB, cyclin D1, HDAC1 and HDAC3. Taken together, our findings suggest that BITC could be a novel HDAC inhibitor that regulates pancreatic cancer cell survival by inhibiting HDAC1 and HDAC3.
EXPERIMENTAL PROCEDURES
Chemicals and Plasmids
The antibodies against HDAC1, HDAC2, HDAC3, HDAC4, HDAC5, HDAC6, HDAC7, cleaved caspase-3, cleaved PARP, IKKα, IkBα, IkBα Ser32/36, p-NF-κB/p65 Ser536/Ser276, p300/CBP, p21WAF1 and cyclin D1 were purchased from Cell Signaling Technology (Danvers, MA), HDAC8, NF-κB/p65 and IKKγ were procured from Santa Cruz Biotechnology (Santa Cruz, CA) and BITC and β-actin from Sigma-Aldrich, (St Louis, MO). Chemicals for cell culture such as penicillin/ streptomycin antibiotic mixture (PSN), OPTI-MEM-I reduced serum medium were purchased from GIBCO BRL (Carlsbad, CA). Vorinostat (SAHA) was obtained from Cayman Chemicals (Ann Arbor, MI) and pure NF-kB protein from Panomics (Fremont, CA). Heat inactivated fetal bovine serum and RPMI and McCoy’s medium were purchased from Mediatech Cellgro (Herndon, VA). Electrophoresis reagents were procured from Amresco (Solon, OH).
The Flag-HDAC expression constructs were kindly provided by Dr. Edward Seto, H. Lee Moffitt Cancer Center, Tampa, FL (32), whereas Luciferase reporter plasmids encoding the full-length human cyclin D1 promoter (–1745CD1LUC) was a kind gift from Dr. Richard Pestell, Thomas Jefferson University, Philadelphia, PA (33). pRL-Renilla luciferase vector (control) construct was generously provided by Dr. Erguang Lee, The Scripps Research Institute, La Jolla, CA, while NF-κB luciferase reporter gene construct was a kind gift from Dr. Glen D. Rosen, Stanford University, Stanford, CA (34).
Cell Culture
Human pancreatic cancer cell line BxPC-3 and Capan-2 were obtained from American Type Culture Collection (Rockville, MD). Monolayer cultures of BxPC-3 cells were maintained in RPMI medium supplemented with 10% fetal bovine serum, PSN antibiotic mixture (1% v/v) (Gibco BRL, Grand Island, NY), 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate and 20% glucose, while monolayer cultures of Capan-2 cells were maintained in McCoy’s medium supplemented with 10% fetal bovine serum and PSN antibiotic mixture (1% v/v) at 37°C in a humidified chamber of 95% air and 5% CO2. Normal human pancreatic duct epithelial cells (HPDE-6) were a generous gift from Dr. Ming-Sound Tsao, University of Toronto, Canada. HPDE-6 cells were cultured in Keratinocyte-SFM serum free medium supplemented with 4mM L-glutamine and adjusted to contain 0.2ng/ml EGF, 30μg/ml BPE and 1% (v/v) PSN as described by us previously (7, 35).
Cell Extract Preparation and Immunobloting
Briefly, 1.0 × 106 human pancreatic cells BxPC-3, Capan-2 or HPDE-6 were treated with increasing concentrations of BITC for 24h or with 10μM BITC for varying time intervals. In another experiment, BxPC-3 cells were treated with 0-80μM SAHA for 24h. Cell pellets were collected after treatment and washed with ice cold PBS. For Western blot analysis, whole cell extracts were prepared using lysis buffer (8M Urea, 4% CHAPS, 40mM Tris-HCl containing, 10mM Na-glycerophosphate, 5mM Na-pyrophosphate, 50mM NaF, 1mM orthovanadate, 1mM DTT, 0.1mM PMSF, 2μg/ml of protease inhibitors: chymostatin, pepstatin, antipain, and leupeptin). Lysates were sonicated for five short pulses of 5 secs each (Fisher Scientific Sonic Dismembrator Model 100 at 2 Watt output power). Extracts were centrifuged for 15 mins at 14,000 x g at 4°C and supernatants were aliquoted and stored at −70°C for future analysis.
Protein content was determined by Bradford’s reagent. Equal amounts of protein were loaded and separated by SDS-PAGE and electroblotted on to nitrocellulose membrane (Hybond ECL, Amersham Life Science, Birmingham, UK). Membranes were blocked for 1h at 4°C in Tris-buffered saline (TBS) containing 0.1% Tween-20 and 5% non fat dry milk at room temperature for 1h, followed by overnight incubation with primary antibody. The blots were then incubated with horseradish peroxidase conjugated secondary antibody (1:3000, Santa Cruz Biotechnology Inc.) for 45-min in blocking buffer. Immunoblots were developed by enhanced chemiluminescence reagent according to the manufacturer’s instructions (Amersham; Princeton, NJ). Nuclear extracts were prepared using Fraction-PREP™ Cell Fractionation kit (Biovision, Mountain View, CA) as per the manufacturer’s protocol.
DNA Binding
NF-κB/p65 binding assays were performed as per the instructions of manufacture’s protocol (TransAM ELISA kit). Basically, the DNA binding motif of NF-κB (5′-GGGACTTTCC-3′) was coated to a 96-well plate. Transcriptionally active nuclear NF-κB binds to DNA and is recognized by antibody against p65. Briefly, nuclear lysates of treated/nontreated and/or transfected BxPC-3 and Capan-2 cells were prepared using nuclear extract kit from Active Motif (Carlsbad, CA) for DNA binding assays. Five microgram of nuclear extract mixed with binding buffer was added to the precoated plate. After 1h incubation, wells were washed and plates were incubated with NF-κB/p65 antibody for additional 1h. Following incubation, plates were washed three times with wash buffer and HRP-conjugated anti-rabbit IgG (Molecular Probes, Eugene) was added to each well. Plates were read at 450nm after adding the developing reagent.
In another experiment, pure 50ng NF-kB protein in 10mM Tris-HCl, pH7.4 was incubated with or without 10-20μM BITC for 4h at 37°C and the DNA binding activity was performed using TransAM ELISA kit as described above.
EMSA was performed using commercially available kit from Panomics with slight modification. Ten microgram of nuclear protein extracted from control or BITC treated BxPC-3 cells was incubated in presence of poly(dI-dC) at room temperature. Biotin-labelled NF-κB binding site oligomer 5′-AGTTGAGGGGACTTTCCCAGGC was then added and protein-DNA complexes were separated using 5% nondenaturing polyacrylamide gels in Tris-Borate/EDTA buffer (0.1M Tris, 0.09M boric acid containing 1mM EDTA) at 4°C. Complexes were transferred to nylon membranes and transferred oligos were immobilized by UV crosslinking for 3 min. For detection of bound oligos, membranes were blocked using blocking buffer (Panomics EMSA Gel-Shift Kit) followed by the addition of Streptavidin-horse radish peroxidase and blots were developed by ECL according to the manufacturer’s instructions.
Transient transfection
About 0.3 × 106 or 5 × 103 BxPC-3 or Capan-2 cells were plated in 6 or 96 well plates and transiently transfected with 0.75 μg Flag, Flag-HDAC1 and Flag-HDAC3 plasmid DNA or co-transfected with NF-κB or Cyclin D1 luciferase reporter plasmid DNA using Fugene 6 transfection reagent for 24 h. pRL-Renilla luciferase reporter plasmid was used as control. Transfected or co-transfected cells were treated with DMSO or 10μM BITC for 24 h followed by Sulforhodamine (SRB) cell survival assay, dual luciferase assay (Promega, Madison, WI), DNA binding assay or western blotting.
Dual Luciferase Activity
Co-transfected cells were lysed using passive lysis buffer from the dual-luciferase reporter assay system kit (Promega, Madison, WI). Cells were ruptured by passive lysis buffer as per the manufacturer’s instructions and 20μl of cell lysate was added to a tube containing 100μl luciferase assay buffer. Luciferase activity was determined using 20/20n luminometer from Turner Biosystems (Sunnyvale, CA). The reaction was then stopped by adding 100μl Stop & Glo buffer and the samples were read to determine Renilla luciferase activity.
Cell Survival Assays
Effect of BITC on the survival of BxPC-3 and Capan-2 cells transfected with empty vector, Flag-HDAC1 or Flag-HDAC3 was determined by SRB assay as described by us previously (31, 36). Briefly, transfected cells were treated with BITC, SAHA or DMSO (control) for 24h followed by SRB assay. Plates were read at 570 nm using Bio Kinetics plate reader EL-800 from BioTek Instrument Inc, Winooski, VA.
Immunohistochemistry of control and BITC treated tumors
In our previous studies, we have demonstrated that oral feeding of 12μmol BITC for six weeks significantly suppressed the growth of BxPC-3 tumor xenografts in athymic nude mice (7). The tumor sections from control and BITC treated mice were taken and immunohistochemistry (IHC) for NF-kB, cyclin D1, HDAC1 and HDAC3 were performed as described previously (7). Briefly, paraffin-embedded tissue sections were deparaffinized and rehydrated by washing the sections in xylene, 100% ethanol, 95% ethanol and finally in dH2O. Antigens were unmasked by boiling the sections in 10mM sodium citrate buffer (pH 6.0) and incubating in 3% hydrogen peroxide for 10 minutes. Tumor sections were washed twice in PBS containing 0.1% Tween-20, blocked in 5% horse serum diluted in TBST for 1 hour at room temperature, and then incubated with anti–NF-kB, cyclin D1, HDAC-3 and HDAC-1 antibody (1:300 in TBST) overnight at 4°C. After removal of the primary antibody, sections were washed three times in wash buffer for 5 minutes each followed by incubation with 200 μL of HRP-conjugated secondary antibody diluted 1:5000 in blocking solution for 30 minutes. Subsequently, sections were washed with wash buffer and incubated with 200 μL of avidin-biotin conjugate (ABC) reagent containing avidin and biotinylated HRP for 30 minutes at room temperature using ABC staining kit according to the manufacturer’s instructions (Santa Cruz Biotechnology Inc, Santa Cruz, CA). Three drops of peroxidase substrate was added to each section and incubated until the desired color developed. The sections were counterstained with hematoxylin and mounted and analyzed under a phase-contrast Olympus microscope (Olympus America Inc).
Statistical Analysis
All the statistical calculations were performed using GraphPad Prism 4.0 software. For comparisons that involved multiple variables and observations, ANOVA was used followed by Bonferroni or Newman-Keuls post hoc multiple comparison tests. The Student’s t test was used to compare variations in control and treated groups only. All data are expressed as means ± SD. Differences were considered statistically significant when p value was less than 0.05.
RESULTS
Inactivation of NF-κB/p65 by BITC in pancreatic cancer cells
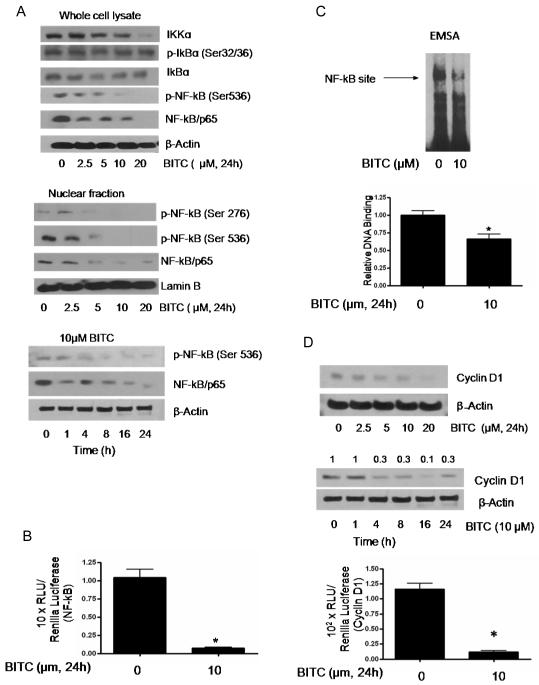
Previous studies from our laboratory demonstrated that BITC induces caspase-3 dependent apoptosis in BxPC-3 and Capan-2 cells (7, 30-31, 36). However, the exact mechanism of BITC mediated apoptosis was not clear. Since NF-κB has been shown to be involved in apoptosis and cell survival, we wanted to see whether BITC induced apoptosis in our model is mediated by modulation of NF-κB pathway. Therefore in order to unveil the molecular mechanism of BITC, we first determined the concentration dependent effect on the phosphorylation of NF-κB/p65 at Ser 536 in BxPC-3cells. BxPC-3 cells treated with 10 and 20 μM BITC for 24h resulted in about 90-99% decrease in the phosphorylation of NF-κB/p65 at Ser536 and Ser 276 (Fig 1A). Similarly, we observed a significant decrease in the basal level of NF-κB/p65 after BITC treatment in BxPC-3 cells (Fig 1A). Upon cellular stimulation, IkB kinase (IKKα) activates IkBα by phosphorylation at Ser32/36. Activated IkBα is ubiquitinated and degraded proteasomally liberating NF-kB to translocate to nucleus. Phosphorylation of NF-kB at Ser536 by IKK is also required for the transcriptional activity of NF-kB. The effect of BITC was thus evaluated on the expression and phosphorylation of IKK and IkBα. As shown in Fig 1A, BITC treatment reduced the expression level of IKKα without affecting the phosphorylation or protein expression of IkBα. Consistent with these observations, we observed drastic decrease in the phosphorylation and protein expression of NF-kB in the nuclear fraction of the cells treated with BITC for 24h as compared to control (Fig 1A). Time dependent experiment in BxPC-3 cells revealed a decrease in the phosphorylation or protein level of NF-κB/p65 starting from 8h of treatment with 10 μM BITC and persisted till the duration of the experiment (Fig 1A).
Figure 1. BITC treatment causes inhibition of NF-κB and cyclin D1 in BxPC-3 pancreatic cancer cells.
A, BxPC-3 cells were treated with 0-20 μM BITC for 24h or with 10μM BITC for different time intervals. Cells were lysed and 40 μg protein (whole cell or nuclear) was resolved by SDS-PAGE. Each blot was stripped and reprobed with β-actin for cytosolic and lamin B for nuclear proteins to ensure equal protein loading. Each experiment was repeated three times with similar results. B, The NF-kB luciferase assay was determined in control and BITC treated BxPC-3 cells using luminometer with the dual luciferase substrate system and luciferase activity was normalized with Renilla luciferase as internal control. C, EMSA was performed using nuclear protein obtained from BITC treated BxPC-3. In addition, NF-κB/p65 DNA binding by ELISA method was determined using 5 μg of nuclear protein from control and BITC treated cells and assayed for the presence of activated p65 using antibody specific for p65 following binding to NF-κB consensus sequence using TransAM NF-κB/p65 ELISA kit. D, BxPC-3 cells were treated with 0-20 μM BITC for 24h or treated with 10 μM BITC for varying time intervals and the whole cell lysates were subjected to SDS-PAGE. The membranes were probed with cyclin D1 antibody. Each blot was stripped and reprobed with β-actin to ensure equal protein loading. Each experiment was repeated three times with similar results. BxPC-3 cells were also transiently transfected with cyclin D1 promoter luciferase construct and Renilla luciferase plasmid as a control, followed by treatment with 10μM BITC or DMSO for 24 h. Cell lysates were subjected to luciferase activity and normalized with control Renilla luciferase. Data represents means ± SD of three independent experiments each performed in triplicates. *Statistically different when compared with control, P < 0.05.
BITC drastically decreases transcriptional activity of NF-κB
Phosphorylation of NF-kB/p65 at Ser 536 is required for the transcriptional activity of p65. Since our results showed decrease in p65 phosphorylation and protein level in BxPC-3 cells, we next determined the effect of BITC on the transcriptional activity of NF-κB. BxPC-3 cells were transiently transfected for 24h with NF-κB luciferase reporter construct and pRL-Renilla luciferase reporter plasmid as internal control, followed by treatment with 10 μM BITC for additional 24h. Our results demonstrate that BITC was able to drastically reduce the transcriptional activity of NF-κB as compared to control group in BxPC-3 cells (Fig 1B). For example, 10 μM BITC treatment for 24h reduced NF-κB transcriptional activity by 90% in BxPC-3 cells as compared to control (Fig 1B).
BITC suppresses NF-κB DNA binding capacity
Because we observed significant decrease in NF-κB transcriptional activity by BITC treatment, we subsequently investigated the effect of BITC on κB-DNA binding capacity of p65 in BxPC-3 cells. Significantly low p65 DNA binding was observed in BITC treated BxPC-3 cells as assessed by EMSA (Fig 1C, upper panel). These results were further confirmed by ELISA based p65 DNA binding assay. In agreement with EMSA results, decrease in DNA binding capacity of p65 was observed in response to BITC treatment (Fig 1C, middle panel). To further determine whether the decrease in DNA binding activity of NF-kB is due to direct modification of NF-kB by BITC, we performed in vitro assay where pure NF-kB protein was exposed to 10 and 20μM BITC at 37°C for 4h and DNA binding assay was performed. BITC treatment up to 20μM did not affect the DNA binding activity of NF-kB ruling out the physical interaction of BITC with NF-kB (data not shown). However, further structural studies are required to prove whether BITC at higher concentrations modifies NF-kB by physically binding.
BITC decreases cyclin D1 protein expression and cyclin D1 promoter reporter activity
Since our results showed significant decrease in NF-κB DNA binding, we next raised a question whether this change could affect its downstream transcriptional target cyclin D1 (30,37). As shown in Fig 1D, BITC treatment caused a substantial decrease in the expression of cyclin D1 in a concentration and time dependent manner in BxPC-3 cells. We further evaluated the effect of BITC on cyclin D1 promoter reporter activity and observed about 70-90% decrease in relative luciferase activity (Fig 1D, lower panel).
BITC is a HAT and HDAC inhibitor
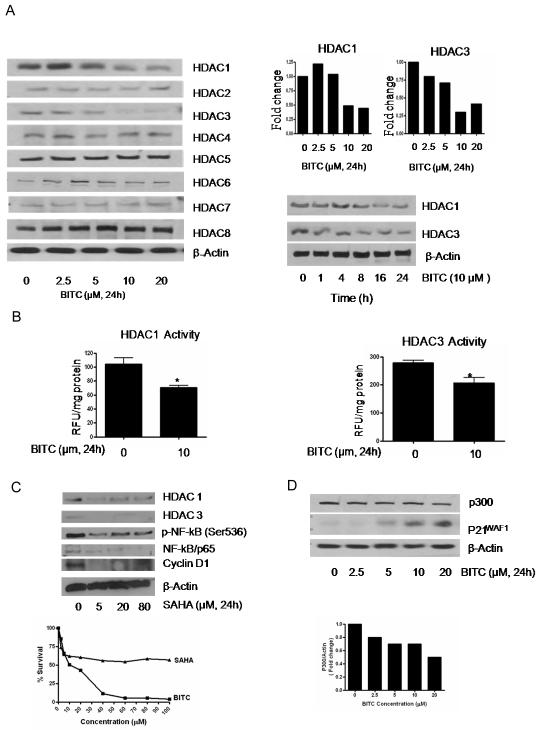
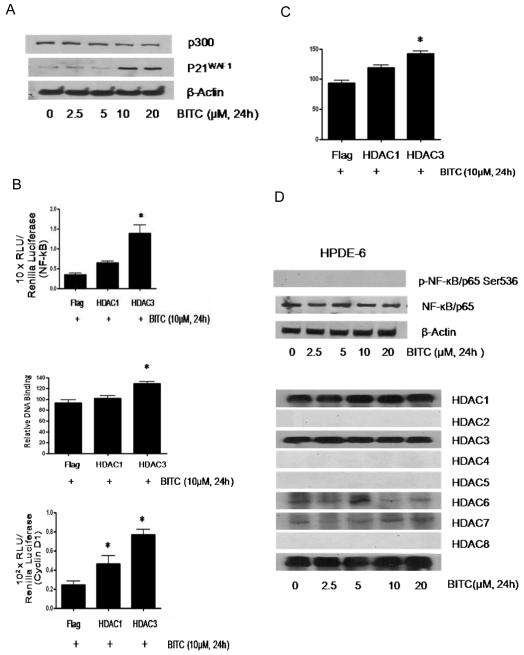
Histone acetyltransferases (HATs) and histone deacetylases (HDACs) are enzymes involved in acetyl modification of histone and non-histone proteins like NF-κB and act as critical gene silencer or activator (21-22). The effect of BITC was evaluated in pancreatic cancer cells on the expression and/or activity of HDACs and p300/CBP. Our results show a concentration and time dependent decrease in HDAC1 and HDAC3 expression in BITC treated BxPC-3 cells (Fig 2A). For example, treatment with 10-20 μM BITC resulted in about 55-60% decrease in HDAC1 and 65-70% decrease in HDAC3 expression in BxPC-3 cells (Fig 2A, upper panel). In a time dependent experiment in BxPC-3 cells, a decrease in HDAC1 expression was observed after 16 and 24h of BITC treatment (Fig 2A, lower panel). The expression of HDAC 2, 5,6,7,8 nevertheless did not changed in response to BITC treatment in BxPC-3 cells (Fig 2A, upper panel). We further confirmed our observations on HDAC inhibition by evaluating the effect of BITC on the deacetylase activity of HDACs. As shown in Fig 2B, we observed 30-35% decrease in deacetylase activity of both HDAC1 and HDAC3 in 10 μM BITC treated BxPC-3 cells. Further, we compared BITC-mediated inhibition of HDAC1/3 with a known HDAC inhibitor SAHA (Vorinostat). Although, 5μM SAHA treatment for 24h decreased the expression of HDAC1, HDAC3, NF-kB and cyclin D1, it failed to significantly suppress the survival of BxPC-3 cells as compared to BITC (Fig 2C). Our results also demonstrate that 10μM BITC was able to down regulate about 35% of p300/CBP expression in BxPC-3 cells (Fig 2D).
Figure 2. BITC causes decrease in HDAC1 and HDAC3 expression and activity level.
A, BxPC-3 cells were treated with different concentrations of BITC for 24h or treated with 10μM BITC for varying time intervals and the whole cell lysates were subjected to SDS-PAGE. Representative immunoblots show the effect of BITC treatment on the expression of HDAC1, HDAC2, HDAC3, HDAC4, HDAC5, HDAC6, HDAC7 and HDAC8. Each blot was stripped and reprobed with anti-β-actin to ensure equal protein loading. Each experiment was repeated three times with similar results. B, HDAC1 and HDAC3 activity was determined in the nuclear lysates of DMSO and BITC treated BxPC-3 cells using HDAC Fluorescent Activity Assay kit. *Statistically different when compared with control, P < 0.05. C, Effect of SAHA was determined in BxPC-3 cells and compared with the effects of BITC. Cells were exposed to 0-100μM SAHA or BITC for 24h and evaluated for NF-kB, cyclin D1 and HDAC1/3 expression by western blotting and cell survival by SRB assay. D, BxPC-3 cells were treated with 0-20 μM BITC for 24h. Whole cell lysates were subjected to SDS-PAGE and the membranes were probed with p300/CBP, p21WAF1 and β-actin antibody. Each experiment was repeated three times with similar results.
BITC induces p21WAF1 expression
As HDAC inhibitors such as trichostatin A and depsipeptide are known to induce p21WAF1 expression (38-39), we wanted to see whether HDAC inhibition by BITC in our model also cause an increase in p21WAF1expression. As shown in Fig 2D, treatment of BxPC-3 cells with 0-20 μM BITC for 24h lead to a substantial increase in p21WAF1expression.
Over expression of HDAC1 and HDAC3 increases NF-κB transcriptional activity and DNA binding
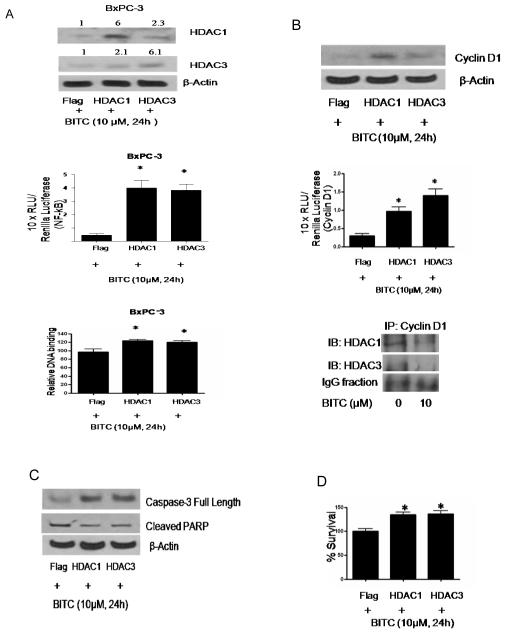
In order to establish the involvement of HDACs in BITC mediated regulation of NF-κB, cells were transfected with HDAC1 and HDAC3 expression plasmids and treated with 10μM BITC for 24h. As shown in Fig 3A, BxPC-3 cells transfected with HDAC1 or HDAC3 plasmid resulted in the increased expression of HDAC1 and HDAC3 protein by about 6 fold as compared with vector (Flag) transfected cells. Because BITC efficiently inhibits NF-κB transcriptional activity and its κB-DNA binding, we next determined the effect of HDAC1 and HDAC3 over expression on BITC mediated regulation of transcriptional activity and κB-DNA binding of p65. BxPC-3 cells were transfected with Flag, Flag-HDAC1, Flag-HDAC3 with/without NF-κB luciferase reporter plasmid and Renilla luciferase as control followed by treatment with 10 μM BITC for 24h. A 7-10 fold increase in NF-κB luciferase activity (Fig 3A, middle panel) and 20-30 percent increase in relative DNA binding of p65 (Fig 3A, lower panel) was observed in HDAC1 and HDAC3 overexpressing BxPC-3 cells, altogether neutralizing the effect of BITC.
Figure 3. HDAC1 and 3 overexpression blocks BITC mediated inactivation of NF-κB and cyclin D1 and induction of apoptosis.
A, BxPC-3 cells were transfected with Flag, Flag-HDAC1 or Flag-HDAC3 plasmid with/without NF-κB luciferase plasmid and Renilla luciferase as control for 24h followed by treatment with 10μM BITC for 24h. Whole cell lysate from transfected and/or treated cells were subjected to SDS-PAGE and the immunoblots were probed with HDAC1 and HDAC3 antibody. Equal loading was determined with β-actin antibody. The relative NF-κB luciferase activity was performed in BxPC-3 cells as described in Figure 1 and normalized with Renilla luciferase as a control. Data represents means ± SD of three independent experiments. Nuclear lysates from transfected and BITC treated BxPC-3 cells were used to determine relative DNA binding of p65 by ELISA kit as described above. Data represents means ± SD of three independent experiments. *Statistically different when compared with control, P < 0.05. B, BxPC-3 cells were transiently transfected with Flag, Flag-HDAC1 and Flag-HDAC3 plasmid DNA with or without cyclin D1 luciferase reporter plasmid and Renilla luciferase as internal control followed by treatment with 10μM BITC for 24 hours. Cell lysates were resolved using SDS-PAGE and the membrane was probed with cyclin D1 antibody. For equal loading the membrane was blotted with β-actin antibody. Relative cyclin D1 reporter luciferase activities was performed in transfected cells as described above and normalized against Renilla luciferase as a control. Cyclin D1 was further immunoprecipitated from control and BITC treated BxPC-3 cells and the lysate was immunoblotted for HDAC1 and HDAC3. Data represents means ± SD of three independent experiments. C, Cells were transiently transfected with Flag, Flag-HDAC1 and Flag-HDAC3 plasmids as described above and treated with10μM BITC or DMSO for 24h and analyzed for full length caspase-3 and cleaved PARP. Each experiment was repeated three times with similar results. D, HDAC overexpressing cells after BITC treatment were subjected to Sulforhodamine B cell survival assay. Values are means ± SD of three independent experiments. *Statistically different when compared with control, P < 0.05.
HDAC over expression increase cyclin D1 expression and promoter reporter activity in BITC treated cells
As BITC treatment lead to drastically reduced cyclin D1 expression and promoter activity in BxPC-3 cells after inhibiting NF-κB/p65 DNA binding, we next wanted to see if HDAC over expression can prevent BITC mediated degradation of cyclin D1. Interestingly, over expression of HDAC1 led to 3.5 fold increase in cyclin D1 and HDAC3 over expression caused about 2.4 fold increase in cyclin D1 expression in BxPC-3 cells following BITC treatment as compared to control cells (Fig 3B). We next co-transfected BxPC-3 cells with Flag, Flag-HDAC1 or Flag-HDAC3 along with cyclin D1 promoter reporter and Renilla luciferase reporter and subsequently treated the cells with 10 μM BITC for 24h. Our results reveal about 3-5 fold increase in cyclin D1 promoter reporter activity in HDAC1 and HDAC3 overexpressing BxPC-3 cells as compared to the cells transfected with empty vector (Fig 3B, lower panel). Although these results suggest a connection between HDAC1/3 and cyclin D1, we further confirmed this by immunoprecipitating cyclin D1 from control and BITC-treated BxPC-3 cells and immunoblotted with HDAC1 and HDAC3 antibody. As compared to control, expression of both HDAC1 and HDAC3 were reduced in response to BITC treatment in cyclinD1 immunoprecipitated samples, indicating an association of cyclin D1 with HDAC1 and HDAC3. Overall, our results establish HDAC1/3 as molecular target of BITC in BxPC-3 cells.
Over expression of HDACs protect BxPC-3 cells from BITC mediated apoptosis
So far, our results suggested that HDAC over expression abrogated the effect of BITC mediated inhibition of NF-κB and HDACs. We therefore next wanted to see whether HDAC inhibition is directly linked to BITC induced apoptosis. In order to do that, BxPC-3 cells were transfected with Flag, Flag-HDAC1 and Flag-HDAC3 expression plasmids and then treated with 10 μM BITC for 24h. It is interesting to point out that the concentrations at which BITC significantly inhibit HDAC expression and activity also induced apoptosis in both the cell lines (36). Over expression of HDAC1 and HDAC3 decreased the cleavage of caspase-3 and PARP in BITC treated BxPC-3 cells as compared to control cells indicating protection from BITC (Fig 3C).
HDACs over expression confers resistance to BITC in pancreatic cancer cells
Over expression of HDAC1 and HDAC3 in BxPC-3 cells offered about 35-40% protection against BITC treatment in terms of cell survival as compared to vector transfected cells (Fig 3D). These results suggest that over expression of HDAC1 and HDAC3 confers resistance to apoptosis and growth suppressive effects of BITC and establish a critical role of HDACs in the survival of pancreatic cancer cells.
Effect of BITC on NF-kB and HDACs in Capan-2 cells
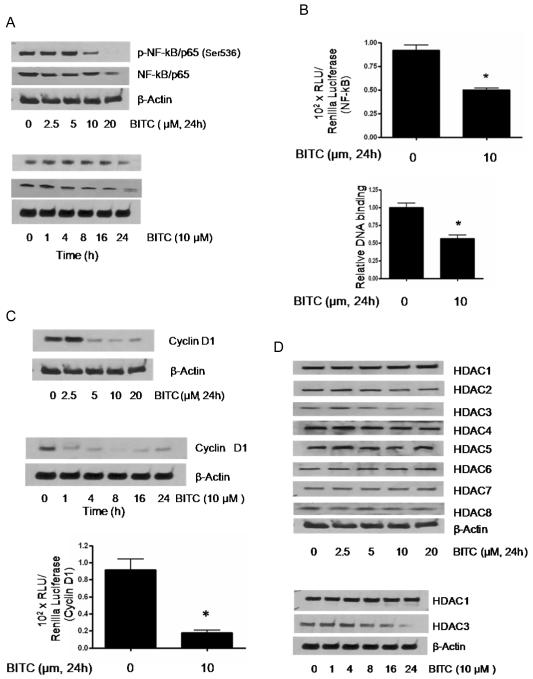
To rule out the cell specific effects of BITC, we evaluated the effect of BITC in Capan-2 pancreatic cancer cells and normal HPDE-6 cells. As compared to BxPC-3 cells, treatment of Capan-2 cells with BITC also resulted in the decrease in the phosphorylation of NF-kB at Ser536, however the protein level was modestly reduced at higher BITC concentration (Fig 4A). In a time-dependent experiment, BITC treatment caused changes in the phosphorylation and protein level only at 24h (Fig 4A, lower panel) as opposed to BxPC-3 cells where the effect of BITC was as early as 4h (Fig 1A). The transcriptional activity of NF-kB was determined in Capan-2 cells using NF-kB luciferase reporter construct and pRL-Renilla luciferase reporter plasmid as internal control, as described in BxPC-3 cells. After 24h of treatment of Capan-2 cells with 10μM BITC resulted in about 45% reduction in NF-kB transcriptional activity (Fig 4B). Similarly, we observed about 50% inhibition of the DNA binding capacity of NF-kB in Capan-2 cells in response to BITC treatment (Fig 4B, lower panel). The next step was to determine the effect of BITC on the expression and transcriptional level of cyclin D1, which is the downstream transcriptional target of NF-kB. As shown in Fig 4C, BITC treatment of Capan-2 cells resulted in the substantial reduction in the expression of cyclin D1 in a concentration and time-dependent manner. The effect of BITC on the expression of cyclin D1 was evident just after 1h of treatment (Fig 4C, middle panel). The transcriptional activity of cyclin D1 was also reduced drastically by BITC treatment in Capan-2 cells (Fig 4C, lower panel). Further, the effect of BITC was evaluated on the expression of various HDACs in Capan-2 cells (Fig 4D). Unlike BxPC-3 cells, BITC treatment was able to decrease only HDAC3 expression in Capan-2 cells and this effect was evident at 24h (Fig 4D). Nevertheless, BITC treatment significantly down-regulated the expression of p300/CBP in Capan-2 cells (Fig 5A). On the contrary, expression of p21 was significantly increased in this cell line by BITC treatment (Fig 5A).
Figure 4. Effect of BITC on NF-kB, cyclin D1 and HDACs in Capan-2 cells.
A, Capan-2 cells were treated with 0-20 μM BITC for 24h or with 10μM BITC for different time intervals. Proteins were resolved by SDS-PAGE and immunobloted with p-NF-κB/p65 (Ser536) and NF-κB/p65 antibodies. Each blot was stripped and reprobed with β-actin antibody to ensure equal protein loading. Each experiment was repeated three times with similar results. B, Control and BITC treated cells were transfected with NF-κB luciferase reporter plasmid as described in Fig 1. The luciferase assay was performed with the dual luciferase substrate system and luciferase activity was normalized with Renilla luciferase as internal control. In addition, NF-κB/p65 DNA binding was determined using TransAM NF-κB/p65 ELISA kit. Data represents means ± SD of three independent experiments each performed in triplicates. *Statistically different when compared with control, P < 0.05. C, Capan-2 cells were treated with 0-20 μM BITC for 24h or treated with 10 μM BITC for varying time intervals and the whole cell lysates were subjected to SDS-PAGE. The membranes were probed with cyclin D1antibodies. Control and BITC treated cells were also transiently transfected with cyclin D1 promoter luciferase construct and subjected to luciferase activity as described in Fig 1. Data represents means ± SD of three independent experiments each performed in triplicates. *Statistically different when compared with control, P < 0.05. D, Capan-2 cells were treated with different concentrations of BITC for 24h or treated with 10μM BITC for varying time intervals, and whole cell lysates were subjected to SDS-PAGE. Representative immunoblots show the effect of BITC on the expression of HDAC1, HDAC2, HDAC3, HDAC4, HDAC5, HDAC6, HDAC7 and HDAC8. Each blot was stripped and reprobed with anti-β-actin to ensure equal protein loading. Each experiment was repeated three times with similar results.
Figure 5. HDAC3 overexpression protect the cells from BITC-mediated changes in Capan-2 cells and HPDE-6 cells are unaffected by BITC treatment.
A, Capan-2 cells were treated with 0-20 μM BITC for 24h and whole cell lysate was subjected to SDS-PAGE and the membranes were probed with p300/CBP, p21WAF1 and β-actin antibody. B, NF-κB luciferase activity, DNA binding and cyclin D1 luciferase activity was performed in control and BITC treated Capan-2 cells transfected with Flag, Flag-HDAC1 or Flag-HDAC3 plasmid DNA with/without NF-κB luciferase plasmid and Renilla luciferase as described in Figure 3. Data represents means ± SD of three independent experiments. *Statistically different when compared with control, P < 0.05. C, HDAC overexpressing cells treated with BITC were subjected to Sulforhodamine B cell survival assay. Values are means ± SD of three independent experiments. *Statistically different when compared with control, P < 0.05. D, HPDE-6 cells were treated with different concentrations of BITC for 24h and whole cell lysates were subjected to SDS-PAGE. Representative immunoblots show the effect of BITC treatment on the expression of phospho-NF-κB/p65 Ser536, NF-κB/p65, HDAC1, HDAC2, HDAC3, HDAC4, HDAC5, HDAC6, HDAC7 and HDAC8. Each blot was stripped and reprobed with anti-β-actin to ensure equal protein loading. Each experiment was repeated three times with similar results.
In order to confirm the role of HDAC3 in BITC-mediated NF-kB and cyclinD1 inhibition leading to decreased cell survival, Capan-2 cells were transfected with HDAC plasmids as explained in detail in method section followed by treatment with BITC. A significant increase in NF-kB luciferase activity and DNA binding of p65 was observed in HDAC3 overexpressing BITC-treated Capan-2 cells but not much change was observed by HDAC1 overexpression (Fig 5B). Similarly, a 3-fold increase in cyclin D1 promoter reporter activity in HDAC3 overexpressing Capan-2 cells was observed (Fig 5B, lower panel). Further, HDAC3 overexpression offered significant cell survival advantage in BITC-treated Capan-2 cells (Fig 5C). Taken together, these results clearly indicate the role of HDAC3 but not HDAC1 in the survival of Capan-2 cells.
BITC failed to alter NF-kB or HDAC expression in normal HPDE cells
We next wanted to see if BITC treatment can modulate the expression of NF-kB and HDAC expression in normal HPDE cells. Consistent with the notion that NF-kB is activated in transformed or malignant cells but not in normal cells, we did not observed any constitutive phosphorylation of NF-kB in HPDE cells (Fig 5D, upper panel). Further BITC treatment did not changed the constitutive p65 expression of NF-kB in HPDE cells (Fig 5D). We did not observed any constitutive expression of HDAC2, 4, 5 or 6 in HPDE cells and BITC treatment failed to cause any change in the expression of HDAC1,3,6 or 7 (Fig 5D). We have already shown in our previous publication that HPDE cells were resistant to the deleterious effects of BITC (36).
Oral BITC administration suppresses the growth of pancreatic tumor xenograft by down-regulating NF-kB, cyclin D1HDAC1 and HDAC3 in vivo
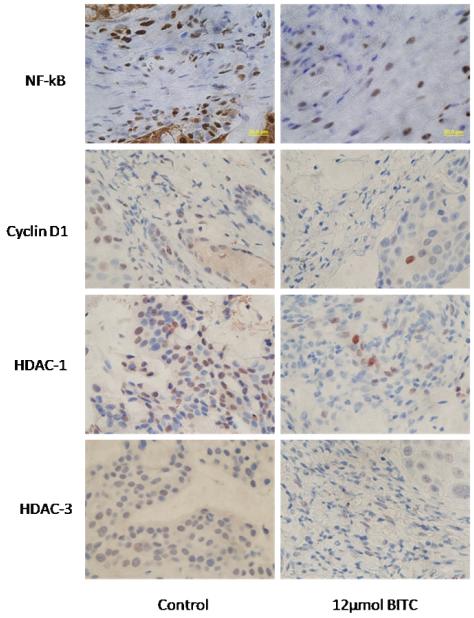
In our previously published study, we have demonstrated that oral administration of 12μmol (90mg/Kg Bwt) BITC in PBS, 5 days per week for six weeks significantly suppressed the growth of BxPC-3 pancreatic tumor xenografts in athymic nude mice (7). To further establish whether NF-kB and HDAC1,3 plays any role in the growth of pancreatic tumor, the tumors from control and BITC-treated mice were analyzed by immunohistochemistry for the expression of NF-kB, cyclin D1, HDAC1 and HDAC3. As shown in Fig 6, reduced staining of NF-kB, cyclin D1, HDAC1 and HDAC3 was observed in the tumor sections from BITC-treated mice as compared to tumor sections from control mice. Based on these observations, it can be concluded that the reduced tumor growth in BITC-fed mice was due to reduced expression of NF-kB, cyclin D1, HDAC1 and HDAC3.
Figure 6. BITC treatment suppress the expression of NF-kB, cyclin D1, HDAC1 and HDAC3 in vivo.
Tumor sections from control and 12μmol BITC treated mice were analyzed for the expression of NF-kB, cyclin D1, HDAC1 and HDAC3 by immunohistochemistry. Each section was analyzed under the microscope at 200 x magnification.
DISCUSSION
In this study, we explored the mechanism of BITC mediated regulation of NF-κB in pancreatic cancer cells. We used BxPC-3 and Capan-2 human pancreatic cancer cell lines and compared their sensitivity towards BITC in normal pancreatic cell line HPDE-6. Interestingly we observed that BITC treatment lead to significant inhibition in the activation of NF-κB/p65 by suppressing the phosphorylation of p65 at Ser536 in BxPC-3 and Capan-2 cells. The expression of NF-kB/p65 was significantly reduced in BxPC-3 but not in Capan-2 cells by BITC treatment. Phosphorylation of NF-kB at Ser 536 by IkB kinase (IKKα) is required for the transcriptional activity of NF-kB. IKKα also phosphorylate IkBα at Ser 32/36 resulting in liberating NF-kB which then translocate to nucleus. Our results show reduced levels of IKK after BITC treatment without affecting IkBα indicating that the reduced phosphorylation of NF-kB is due to reduced IKKα. On the contrary, BITC treatment did not show any effect on NF-κB activation or expression in normal HPDE-6 cells. In fact, we did not observe any basal level of NF-κB phosphorylation in HPDE-6 cells. These results are in agreement with previous studies which suggest that NF-κB is activated in tumor cells but not in normal cells (40-41). Following activation NF-κB translocates to the nucleus where it binds to specific elements (κB-sites) within the promoters of responsive genes to activate their transcription (10-12). We did observed a decrease in nuclear translocation of NF-κB/p65 in BxPC-3 cells. Further, BITC treatment caused a drastic decrease in NF-κB/p65 transcriptional activity and κB -DNA binding in both BxPC-3 and Capan-2 cells treated with BITC. We also observed that BITC treatment significantly reduced expression and promoter reporter activity of cyclin D1, a NF-κB-responsive gene, in both BxPC-3 and Capan-2 cells. NF-κB mediated cyclin D1 regulation has been reported earlier (29, 37). It is important to point out that expression of cyclin D1 has been inversely correlated with the decreased median survival of patients with pancreatic cancer (42).
Many signaling pathways and transcriptional factors are regulated by HDACs (22, 25, 29). Several studies have reported the inhibition of NF-κB transcriptional activity by HDAC inhibitors, although the mechanism appeared different in various cell lineages (43). On the other hand, activation of NF-κB by HDAC inhibitors has also been described in the literature (44-45). Thus HDACs are not only transcriptional activators but also transcriptional repressors. Previous studies suggested that NF-κB/p65 not only associates with but its transcriptional activity is also regulated by HDAC1, HDAC2 and HDAC3 (21-22). Based on our results, we suggest that BITC inactivates NF-κB in BxPC-3 and Capan-2 cells by dephosphorylation leading to its turn off in BxPC-3 cells by modulating the action of coactivators and/or corepressors such as HATs and HDACs. p300/ CBP play a major role in acetylation of NF-κB/p65 at lysine 122 and 123 residues by its intrinsic HAT activity (20). Our results show that BITC significantly repress the expression of p300/CBP in Capan-2 cells, however its effect in BxPC-3 cells was of lower magnitude. We further observed that BITC treatment decrease the expression and activity of HDAC1 and HDAC3 in BxPC-3 cells and HDAC3 but not HDAC1 in Capan-2 cells. Differential inhibition of HDAC1/3 by BITC could be cell line specific as both the cell lines differ from each other significantly. BxPC-3 cells have wild type K-Ras and mutated p53 whereas Capan-2 cells harbor wild type p53 and mutated K-Ras. HDAC1/3 inhibition by BITC in our model provides some indirect evidence that increased acetylation of lysine residues terminates NF-κB/p65 transcriptional activity. Acetylation of lysine 122 and 123 is known to suppress the transcriptional activity of NF-κB by reducing its DNA binding (46-47).
Nonetheless, more studies are required to link the involvement of lysine residues and their acetylation in BITC-mediated inhibition of NF-kB activity. Regulation of NF-κB activity by HDAC inhibition has been shown by other anti-cancer agents (29, 43). Vorinostat (SAHA) is a well established HDAC inhibitor and is currently used for the treatment of cutaneous T cell lymphoma. Although, SAHA reduced HDAC1/3, NF-kB and cyclin D1 expression and reduced the viability of BxPC-3 cells, BITC treatment at similar concentrations had much pronounced effect and reduced the survival of BxPC-3 cells by almost 95%, indicating the involvement of other pathways. Since HDAC inhibition is also associated with the induction of the cell cycle inhibitor p21WAF1, its effect on normal cell proliferation and differentiation could be related to the anti-neoplastic effects of HDAC inhibitors (38-39). In agreement, our results show the induction of p21WAF1 by BITC treatment. Our results thus indicate that BITC-mediated differential post translational modification of NF-κB by HDACs adversely affects the growth of pancreatic cancer cells.
Overexpression of either HDAC1 or HDAC3 nevertheless increased NF-κB transcriptional activity, its κB-DNA binding capacity, cyclin D1 expression and promoter reporter activity in BITC treated pancreatic cancer cells. Studies have demonstrated direct correlation between cyclin D1 expression and HDAC1 activity (29). Cyclin D1−/− MEF’s showed reduced HDAC1 activity, whereas its reconstitution resulted in increased HDAC1 activity. Furthermore co-immunoprecipitation studies in Cyclin D1+/+ MEF’s revealed a direct association of boHDAC1 and HDAC3 with cyclin D1 (48). A recent study also revealed NF-κB mediated transcriptional regulation of cyclin D1 and control of cell growth and differentiation in 10T1/2 fibroblasts (37). Studies are in progress to study the association of cyclin D1 with HDACs and NF-κB in pancreatic cancer cells using cyclin D1−/− MEF’s. Furthermore, overexpression of HDAC1 and HDAC3 led to a significant decrease in BITC mediated caspase-3 and PARP cleavage. Our in vivo data also clearly indicate that oral administration of 12μmol BITC significantly suppress the growth of BxPC-3 tumor xenografts (7), and that the tumor growth suppression was associated with the reduced expression of NF-kB, cyclin D1, HDAC1 and HDAC3, complementing our in vitro observations.
As the pharmacokinetics of BITC in humans has not been documented, it is difficult to predict how much cruciferous vegetable would need to be consumed to achieve serum concentration of 10μM BITC. However, a very recent study suggested that orally feeding male Sprague-Dawley rats with 10 or 100 μmol PEITC/Kg (an analog of BITC), resulted in the rapid absorption and reached peak concentration of 9.2 ± 0.6 and 42.1 ± 11.4 μM PEITC respectively in the plasma after 0.44 ± 0.1 and 2.0 ± 1 hour of PEITC feeding respectively, suggesting that micromolar concentrations may be achieved in vivo (49). In another pharmacokinetics study, four human volunteers were fed with a single dose of myrosinase hydrolyzed extract of 3-day old broccoli sprouts (containing about 200 μmol of total isothiocyanates), peak concentration of 0.94-2.27 μM isothicyanates reached in the plasma, serum and erythrocytes at 1 hour after broccoli extract ingestion (50). Nevertheless, a detailed pharmacokinetic study on BITC is required and is the focus of our laboratory.
Present in vitro and in vivo findings indicate that: (a) BITC regulates NF-κB at multiple levels in pancreatic cancer cells, (b) the importance of HDAC1 and HDAC3 in regulating pancreatic cancer cell growth and differentiation, and (c) HDACs as the molecular target of BITC, in human pancreatic cancer cells. Taken together, our results suggests that BITC mediated inhibition of HDAC1 and HDAC3 as a probable mechanism of NF-κB inactivation leading to decreased expression of cyclin D1 thus resulting in the in vitro and in vivo growth suppression of pancreatic cancer cells.
Acknowledgements
This investigation was supported in part by USPHS RO1 grants CA106953 and CA129038 (to S.K.S.) awarded by the National Cancer Institute and funding from Texas Tech University Health Sciences Center, School of Pharmacy (to S.K.S.). An instrument grant from Turner Biosystems Inc. CA (to R.P.S) is also acknowledged. The authors wish to thank Dr. Ming-Sound Tsao, University of Toronto, Canada for providing HPDE-6 cells, Dr. Edward Seto, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL for providing Flag-HDAC expression constructs, Dr. Richard Pestell, Thomas Jefferson University, Philadelphia, PA for providing Luciferase reporter plasmid encoding the full-length human cyclin D1 promoter luciferase gene construct. Dr. Erguang Lee, The Scripps Research Institute, La Jolla, California for providing pRL- Renilla luciferase vector construct (control) and Dr. Glen D. Rosen, Stanford University, Stanford, CA for providing NF-κB luciferase reporter gene construct.
Financial Support: Supported in part by RO1 grants CA106953 and CA129038 (to S.K.S) awarded by the National Cancer Institute. The funds from Texas Tech University Health Sciences Center, School of Pharmacy (S.K.S) is also acknowledged.
Abbreviations
- BITC
benzyl isothiocyanate
- HPDE-6
human pancreatic ductal epithelial
- HDAC
histone deacetylase
- SRB
Sulforhodamine B
Footnotes
Conflict of Interest: All the authors listed in this study declare no conflict of interest.
REFERENCES
- 1.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–48. [PubMed] [Google Scholar]
- 2.Beecher CW. Cancer preventive properties of varieties of Brassica oleracea: a review. Am. J. Clin. Nutr. 1994;59:1166S–1170S. doi: 10.1093/ajcn/59.5.1166S. [DOI] [PubMed] [Google Scholar]
- 3.Stoner GD, Morse MA. Isothiocyanates and plant polyphenols as inhibitors of lung and esophageal cancer. Cancer Lett. 1997;114:113–119. doi: 10.1016/s0304-3835(97)04639-9. [DOI] [PubMed] [Google Scholar]
- 4.Guo Z, Smith TJ, Wang E Eklind K, Chung FL, Yang CS. Structure-activity relationships of arylalkyl isothiocyanates for the inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone metabolism and the modulation of xenobiotic-metabolizing enzymes in rats and mice. Carcinogenesis. 1993;14:1167–1173. doi: 10.1093/carcin/14.6.1167. [DOI] [PubMed] [Google Scholar]
- 5.Fahey JW, Talalay P. Antioxidant functions of sulforaphane: a potent inducer of Phase II detoxication enzymes. Food Chem. Toxicol. 1999;37:973–979. doi: 10.1016/s0278-6915(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 6.Yu R, Mandlekar S, Harvey KJ, Ucker DS, Kong ANT. Chemopreventive isothiocyanates induce apoptosis and caspase-3-like protease activity. Cancer Res. 1998;58:402–408. [PubMed] [Google Scholar]
- 7.Sahu RP, Srivastava SK. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. J Natl Cancer Inst. 2009;101:176–93. doi: 10.1093/jnci/djn470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle SL, O’ Neill LAJ. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem. Pharmacol. 2007;72:1102–1113. doi: 10.1016/j.bcp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006;13:759–72. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- 10.Friedman R, Hughes AL. Molecular evolution of the NF-kappaB signaling system. Immunogenetics. 2002;53:964–974. doi: 10.1007/s00251-001-0399-3. [DOI] [PubMed] [Google Scholar]
- 11.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 12.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 13.Tyagi A, Singh RP, Ramasamy K, Raina K, Redente EF, Dwyer-Nield LD, Radcliffe RA, Malkinson AM, Agarwal R. Growth inhibition and regression of lung tumors by silibinin: modulation of angiogenesis by macrophage-associated cytokines and nuclear factor-kappaB and signal transducers and activators of transcription 3. Cancer Prev Res (Phila Pa) 2009;2:74–83. doi: 10.1158/1940-6207.CAPR-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabi T, Shukla S, Gupta S. Betulinic acid suppresses constitutive and TNFalpha-induced NF-kappaB activation and induces apoptosis in human prostate carcinoma PC-3 cells. Mol Carcinog. 2008;47:964–73. doi: 10.1002/mc.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 16.Liu TZ, HU CCA, Chen YH, Stern A, Cheng JT. Differentiation status modulates transcription factor NF-kappaB activity in unstimulated human hepatocellular carcinoma cell lines. Cancer Letts. 2000;151:49–56. doi: 10.1016/s0304-3835(99)00404-8. [DOI] [PubMed] [Google Scholar]
- 17.Gelbmann CM, Leeb SN, Vogl D, Maendel M, Herfarth H, Scholmerich J, Falk W, Rogler G. Inducible CD40 expression mediates NFkappaB activation and cytokine secretion in human colonic fibroblasts. Gut. 2003;52:1448–1456. doi: 10.1136/gut.52.10.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 19.Tojima Y, Fujimoto A, Delhase M, Chen Y, Hatakeyama S, Nakayama KI, Kaneko Y, Nimura Y, Motoyama N, Ikeda K, Karin M, Nakanishi M. NAK is an IkappaB kinase-activating kinase. Nature. 2000;404:778–782. doi: 10.1038/35008109. [DOI] [PubMed] [Google Scholar]
- 20.Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene. 2006;25:6717–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 21.Ashburner BP, Westerheide SD, Baldwin AS., Jr. The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol. 2001;21:7065–77. doi: 10.1128/MCB.21.20.7065-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002;9:625–36. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- 23.Campbell KJ, Rocha S, Perkins ND. Active repression of antiapoptotic gene expression by RelA(p65) NF-kappa B. Mol Cell. 2004;13:853–65. doi: 10.1016/s1097-2765(04)00131-5. [DOI] [PubMed] [Google Scholar]
- 24.Chen FE, Huang DB, Chen YQ, Ghosh G. Crystal structure of p50/p65 heterodimer of transcription factor NF-kappaB bound to DNA. Nature. 1998;391:410–3. doi: 10.1038/34956. [DOI] [PubMed] [Google Scholar]
- 25.Kiernan R, Brès V, Ng RW, Coudart MP, El Messaoudi S, Sardet C, Jin DY, Emiliani S, Benkirane M. Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. J Biol Chem. 2003;278:2758–66. doi: 10.1074/jbc.M209572200. [DOI] [PubMed] [Google Scholar]
- 26.Chen Lf, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–7. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 27.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002;21:6539–48. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blagosklonny MV, Robey R, Sackett DL, Du L, Traganos F, Darzynkiewicz Z, Fojo T, Bates SE. Histone deacetylase inhibitors all induce p21 but differentially cause tubulin acetylation, mitotic arrest, and cytotoxicity. Mol Cancer Ther. 2002;1:937–41. [PubMed] [Google Scholar]
- 29.Hu J, Colburn NH. Histone deacetylase inhibition down-regulates cyclin D1 transcription by inhibiting nuclear factor-kappaB/p65 DNA binding. Mol Cancer Res. 2005;3:100–9. doi: 10.1158/1541-7786.MCR-04-0070. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava SK, Singh SV. Cell cycle arrest, apoptosis induction and inhibition of nuclear factor kappa B activation in anti-proliferative activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis. 2004;25:1701–9. doi: 10.1093/carcin/bgh179. [DOI] [PubMed] [Google Scholar]
- 31.Zhang R, Loganathan S, Humphreys I, Srivastava SK. Benzyl isothiocyanate-induced DNA damage causes G2/M cell cycle arrest and apoptosis in human pancreatic cancer cells. J Nutr. 2006;136:2728–34. doi: 10.1093/jn/136.11.2728. [DOI] [PubMed] [Google Scholar]
- 32.Yang WM, Yao YL, Sun JM, Davie JR, Seto E. Isolation and characterization of cDNAs corresponding to an additional member of the human histone deacetylase gene family. J Biol Chem. 1997;272:28001–7. doi: 10.1074/jbc.272.44.28001. [DOI] [PubMed] [Google Scholar]
- 33.Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell RG. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–97. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 34.Lee KY, Chang W, Qiu D, Kao PN, Rosen GD. PG490 (triptolide) cooperates with tumor necrosis factor-alpha to induce apoptosis in tumor cells. J Biol Chem. 1999;274:13451–5. doi: 10.1074/jbc.274.19.13451. [DOI] [PubMed] [Google Scholar]
- 35.Sahu RP, Batra S, Srivastava SK. Activation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. Br J Can. 2009;100:1425–33. doi: 10.1038/sj.bjc.6605039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahu RP, Zhang R, Batra S, Shi Y, Srivastava SK. Benzyl isothiocyanate mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of MAPK in human pancreatic cancer cells. Carcinogenesis. 2009;30:1744–53. doi: 10.1093/carcin/bgp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–99. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajgolikar G, Chan KK, Wang HC. Effects of a novel antitumor depsipeptide, FR901228, on human breast cancer cells. Breast Cancer Res Treat. 1998;51:29–38. doi: 10.1023/a:1006091014092. [DOI] [PubMed] [Google Scholar]
- 39.Huang L, Sowa Y, Sakai T, Pardee AB. Activation of the p21WAF1/CIP1 promoter independent of p53 by the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) through the Sp1 sites. Oncogene. 2000;19:5712–9. doi: 10.1038/sj.onc.1203963. [DOI] [PubMed] [Google Scholar]
- 40.Hafeez BB, Siddiqui IA, Asim M, Malik A, Afaq F, Adhami VM, Saleem M, Din M, Mukhtar H. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: involvement of nuclear factor-kappaB signaling. Cancer Res. 2008 Oct 15;68(20):8564–72. doi: 10.1158/0008-5472.CAN-08-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawhney M, Rohatgi N, Kaur J, Shishodia S, Sethi G, Gupta SD, Deo SV, Shukla NK, Aggarwal BB, Ralhan R. Expression of NF-kappaB parallels COX-2 expression in oral precancer and cancer: association with smokeless tobacco. Int J Cancer. 2007;120:2545–56. doi: 10.1002/ijc.22657. [DOI] [PubMed] [Google Scholar]
- 42.Kornmann M, Ishiwata T, Itakura J, Tangvoranuntakul P, Beger HG, Korc M. Increased cyclin D1 in human pancreatic cancer is associated with decreased postoperative survival. Oncology. 1998;55:363–9. doi: 10.1159/000011879. [DOI] [PubMed] [Google Scholar]
- 43.D’Acquisto F, May MJ, Ghosh S. Inhibition of nuclear factor kappa B (NF-B): an emerging theme in anti-inflammatory therapies. Mol Interv. 2002;2:22–35. doi: 10.1124/mi.2.1.22. [DOI] [PubMed] [Google Scholar]
- 44.Denlinger CE, Rundall BK, Jones DR. Proteasome inhibition sensitizes non-small cell lung cancer to histone deacetylase inhibitor-induced apoptosis through the generation of reactive oxygen species. J Thorac Cardiovasc Surg. 2004;128:740–8. doi: 10.1016/j.jtcvs.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Denlinger CE, Keller MD, Mayo MW, Broad RM, Jones DR. Combined proteasome and histone deacetylase inhibition in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2004;127:1078–86. doi: 10.1016/s0022-5223(03)01321-7. [DOI] [PubMed] [Google Scholar]
- 46.Chen LF, Greene WC. Regulation of distinct biological activities of the NF-kappaB transcription factor complex by acetylation. J Mol Med. 2003;81:549–57. doi: 10.1007/s00109-003-0469-0. [DOI] [PubMed] [Google Scholar]
- 47.Greene WC, Chen LF. Regulation of NF-kappaB action by reversible acetylation. Novartis Found Symp. 2004;259:208–17. [PubMed] [Google Scholar]
- 48.Fu M, Rao M, Bouras T, Wang C, Wu K, Zhang X, Li Z, Yao TP, Pestell RG. Cyclin D1 inhibits peroxisome proliferator-activated receptor gamma-mediated adipogenesis through histone deacetylase recruitment. J Biol Chem. 2005;280:16934–41. doi: 10.1074/jbc.M500403200. [DOI] [PubMed] [Google Scholar]
- 49.Ji Y, Kuo Y, Morris ME. Pharmacokinetics of dietary phenethylisothiocyanate in rats. Pharm Res. 2005;22:1658–66. doi: 10.1007/s11095-005-7097-z. [DOI] [PubMed] [Google Scholar]
- 50.Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine:pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta. 2002;316:4. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]