Abstract
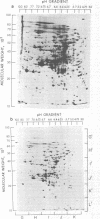
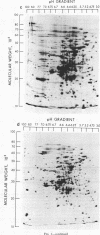
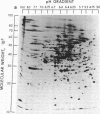
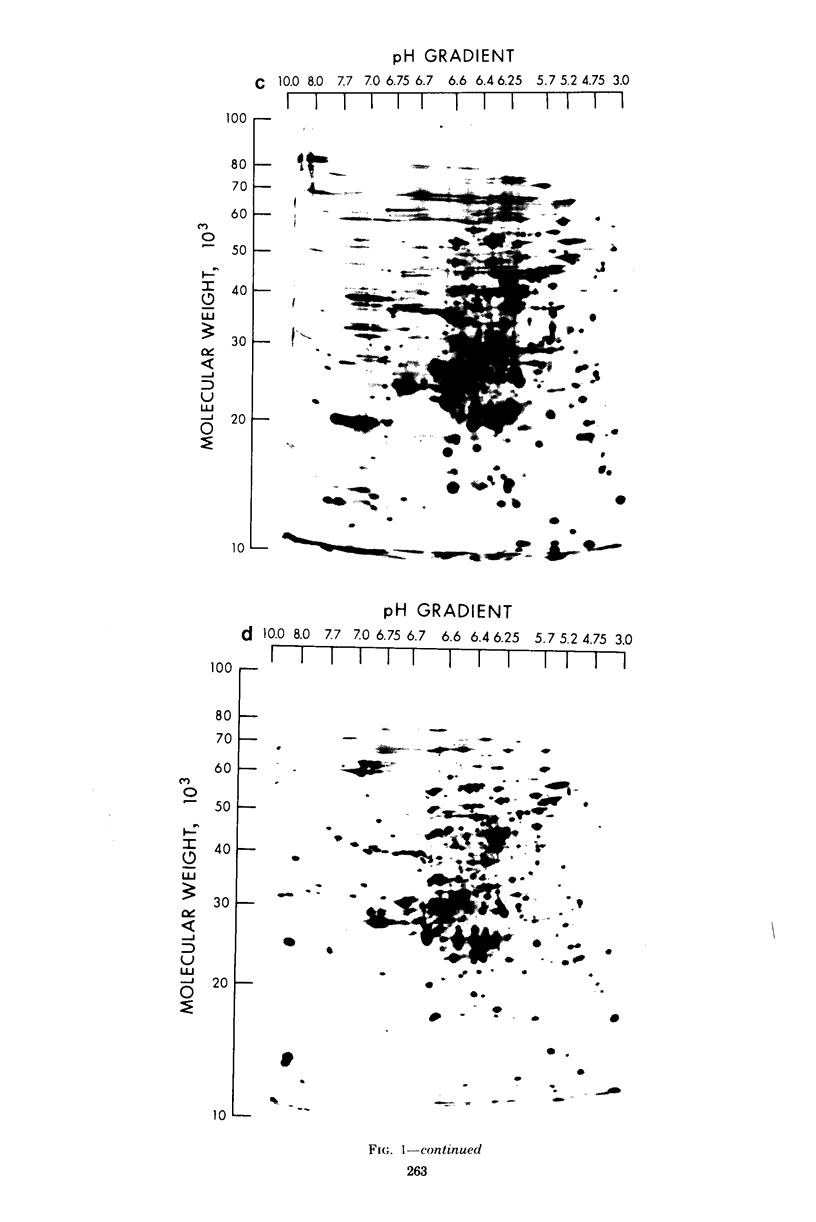
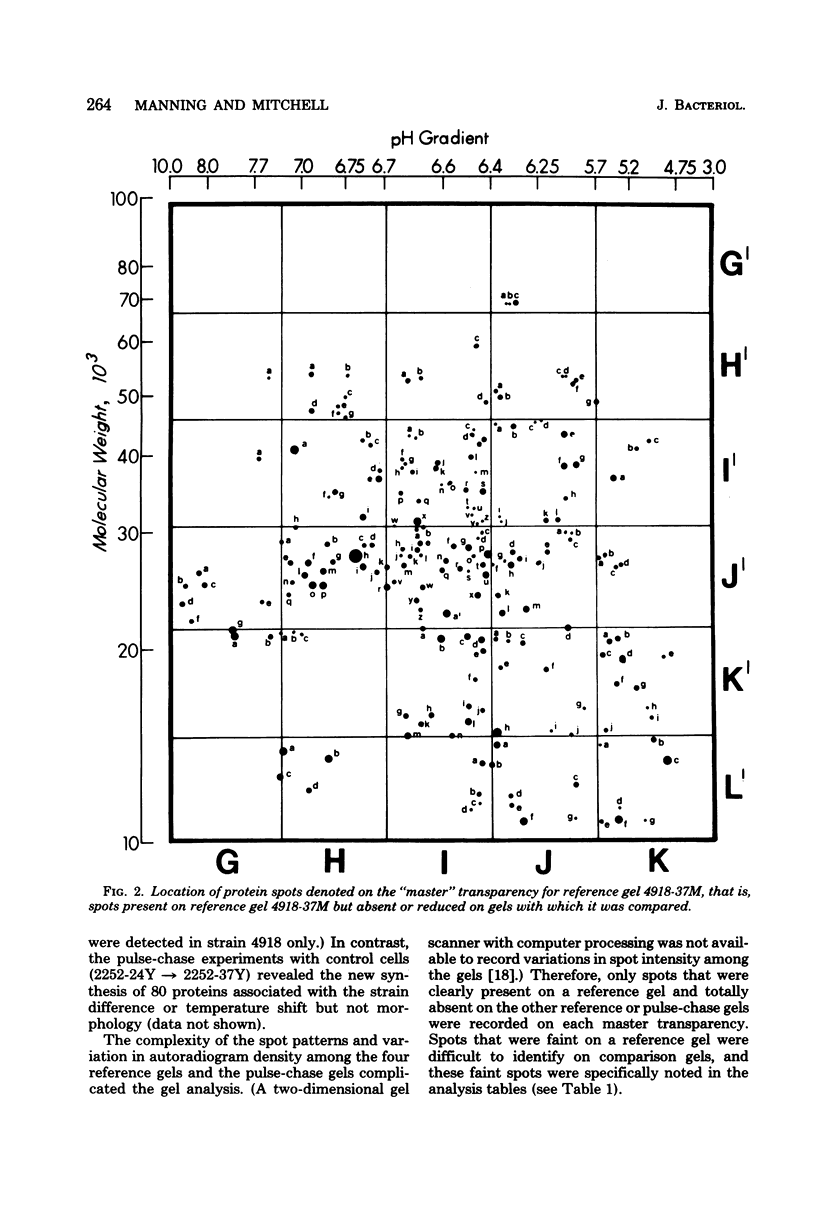
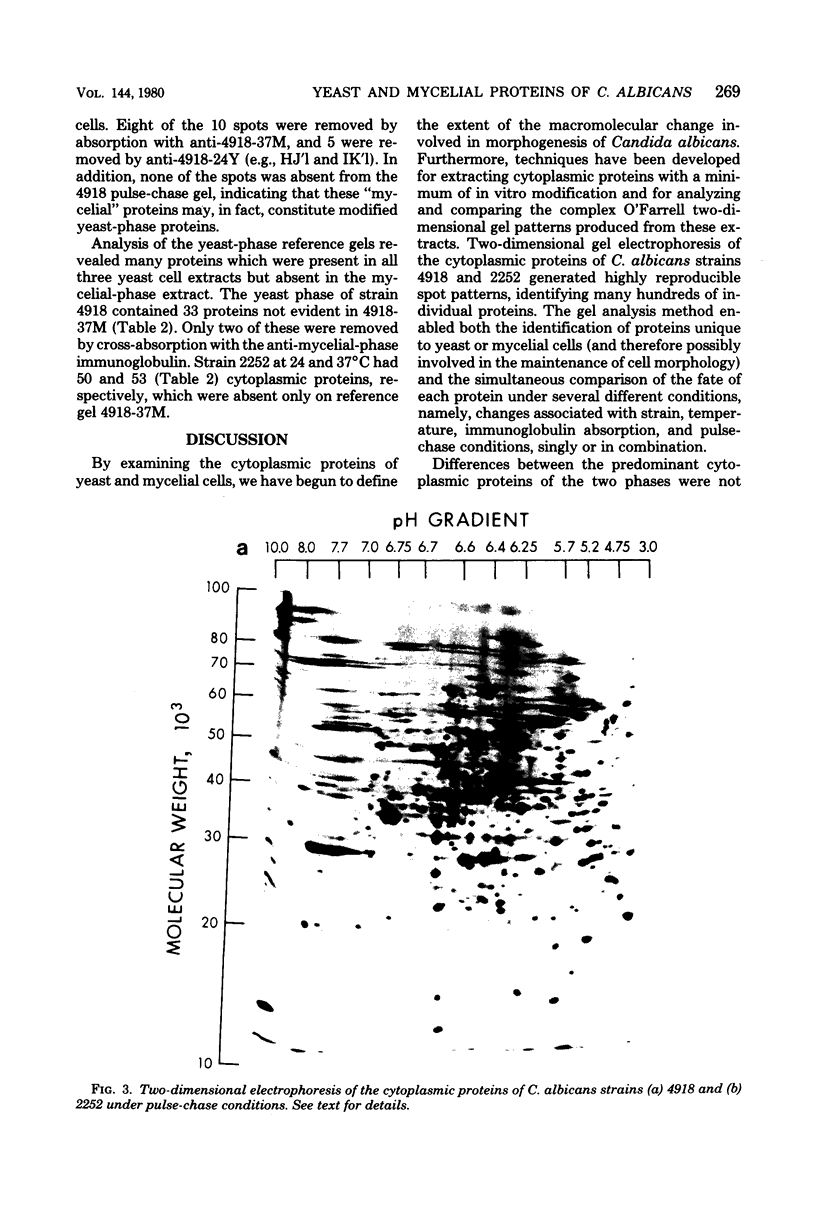
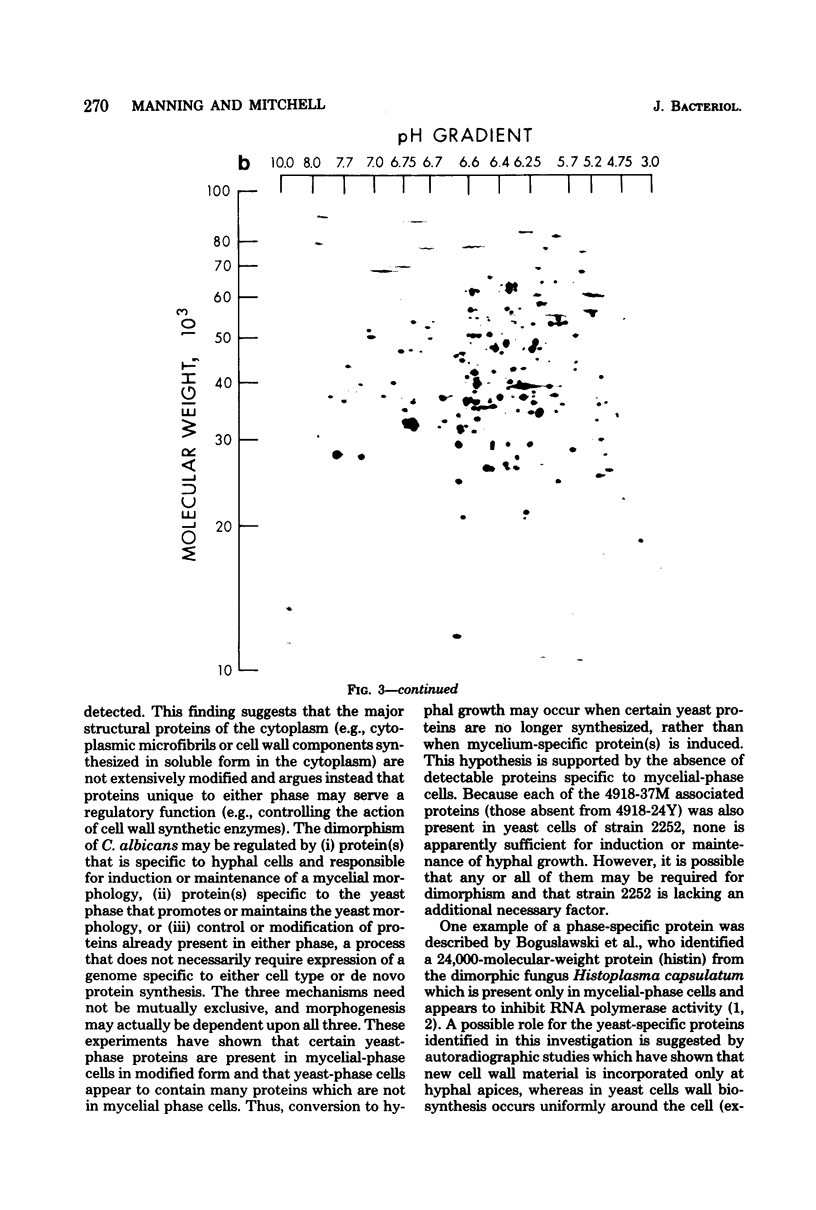
The extent of change in cytoplasmic proteins which accompanies yeast-to-mycelium morphogenesis of Candida albicans was analyzed by two-dimensional gel electrophoresis. Pure cultures of yeasts and true hyphae (i.e., without concomitant production of pseudohyphae) were grown in a synthetic low-sulfate medium. The two strains selected for this study were strain 4918, which produces pure mycelial cultures in low-sulfate medium at 37 degrees C and yeast cells at 24 degrees C, and strain 2252, which produces yeast cells exclusively at both 24 and 37 degrees C in low-sulfate medium. The proteins of both strains were labeled at both temperatures with [35S]sulfate, cytoplasmic fractions were prepared by mechanical disruption and ultracentrifugation, and the labeled proteins were analyzed by two-dimensional electrophoresis. Highly reproducible protein spot patterns were obtained which defined hundreds of proteins in each extract. Ten protein spots were identified on the two-dimensional gels of the 4918 mycelial-phase extract which were not present in the 4918 yeast-phase extract. These proteins appeared to be modifications of preexisting yeast-phase proteins rather than proteins synthesized de novo in the mycelial cells because 5 were absorbed by rabbit anti-yeast-phase immunoglobulin and each of the 10 was also present in extracts of strain 2252 grown at 24 and 37 degrees C, indicating that they were neither unique to filamentous cells nor sufficient for induction or maintenance of the mycelial morphology. Thirty-three proteins were identified in the 4918 yeast-phase extract which were not present in the 4918 mycelial-phase extract. Pulse-chase experiments revealed the synthesis of new proteins during yeast-to-mycelial conversion, but none of these was unique to mycelial cells. No differences in the major cytoplasmic proteins of any of the yeast- or mycelial-phase extracts were identified. This finding suggests that the major structural proteins of the cytoplasm are not extensively modified and argues instead that proteins unique to either phase may serve a regulatory function.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boguslawski G., Kobayashi G. S., Schlessinger D., Medoff G. Characterization of an inhibitor of ribonucleic acid polymerase from the mycelial phase of Histoplasma capsulatum. J Bacteriol. 1975 May;122(2):532–537. doi: 10.1128/jb.122.2.532-537.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguslawski G., Medoff G., Schlessinger D., Kobayashi G. S. Histin, an RNA polymerase inhibitor isolated from Histoplasma capsulatum. Biochem Biophys Res Commun. 1975 May 19;64(2):625–632. doi: 10.1016/0006-291x(75)90367-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Braun P. C., Calderone R. A. Chitin synthesis in Candida albicans: comparison of yeast and hyphal forms. J Bacteriol. 1978 Mar;133(3):1472–1477. doi: 10.1128/jb.133.3.1472-1477.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E. Molecular aspects of yeast morphogenesis. Annu Rev Microbiol. 1975;29:191–214. doi: 10.1146/annurev.mi.29.100175.001203. [DOI] [PubMed] [Google Scholar]
- Chattaway F. W., Bishop R., Holmes M. R., Odds F. C., Barlow A. J. Enzyme activities associated with carbohydrate synthesis and breakdown in the yeast and mycelial forms of Candida albicans. J Gen Microbiol. 1973 Mar;75(1):97–109. doi: 10.1099/00221287-75-1-97. [DOI] [PubMed] [Google Scholar]
- Chattaway F. W., Holmes M. R., Barlow A. J. Cell wall composition of the mycelial and blastospore forms of Candida albicans. J Gen Microbiol. 1968 May;51(3):367–376. doi: 10.1099/00221287-51-3-367. [DOI] [PubMed] [Google Scholar]
- Dabrowa N., Howard D. H., Landau J. W., Shechter Y. Synthesis of nueic acids and proteins in the dimorphic forms of Candida albicans. Sabouraudia. 1970 Nov;8(3):163–169. doi: 10.1080/00362177085190831. [DOI] [PubMed] [Google Scholar]
- Evans E. G., Richardson M. D., Odds F. C., Holland K. T. Relevance of antigenicity of Candida albicans growth phases to diagnosis of systemic candidiasis. Br Med J. 1973 Oct 13;4(5884):86–87. doi: 10.1136/bmj.4.5884.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRESHAM G. A., WHITTLE C. H. Studies of the invasive, mycelial form of Candida albicans. Sabouraudia. 1961 Jan;1:30–33. doi: 10.1080/00362176285190081. [DOI] [PubMed] [Google Scholar]
- Hazen K. C., Cutler J. E. Autoregulation of germ tube formation by Candida albicans. Infect Immun. 1979 Jun;24(3):661–666. doi: 10.1128/iai.24.3.661-666.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry A. T. Inhibition of catalase activity of Candida albicans by serum. Sabouraudia. 1972 Jul;10(2):193–204. [PubMed] [Google Scholar]
- Joshi K. R., Gavin J. B., Wheeler E. E. A scanning electron microscopic study of the morphogenesis of Candida albicans in vitro. Sabouraudia. 1973 Nov;11(3):263–266. doi: 10.1080/00362177385190531. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Land G. A., McDonald W. C., Stjernholm R. L., Friedman L. Factors affecting filamentation in Candida albicans: changes in respiratory activity of Candida albicans during filamentation. Infect Immun. 1975 Jul;12(1):119–127. doi: 10.1128/iai.12.1.119-127.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land G. A., McDonald W. C., Stjernholm R. L., Friedman T. L. Factors affecting filamentation in Candida albicans: relationship of the uptake and distribution of proline to morphogenesis. Infect Immun. 1975 May;11(5):1014–1023. doi: 10.1128/iai.11.5.1014-1023.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lemkin P., Merril C., Lipkin L., Van Keuren M., Oertel W., Shapiro B., Wade M., Schultz M., Smith E. Software aids for the analysis of 2D gel electrophoresis images. Comput Biomed Res. 1979 Dec;12(6):517–544. doi: 10.1016/0010-4809(79)90036-3. [DOI] [PubMed] [Google Scholar]
- Lingappa B. T., Prasad M., Lingappa Y., Hunt D. F., Biemann K. Phenethyl alcohol and tryptophol: autoantibiotics produced by the fungus Candida albicans. Science. 1969 Jan 10;163(3863):192–194. doi: 10.1126/science.163.3863.192. [DOI] [PubMed] [Google Scholar]
- Mackenzie D. W. Morphogenesis of Candida albicans in vivo. Sabouraudia. 1964 Jun;3(3):225–232. [PubMed] [Google Scholar]
- Manning M., Mitchell T. G. Strain variation and morphogenesis of yeast- and mycelial-phase Candida albicans in low-sulfate, synthetic medium. J Bacteriol. 1980 May;142(2):714–719. doi: 10.1128/jb.142.2.714-719.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott M. S. Enzymic activity of purified plasma membranes from the yeast and mycelial forms of Candida albicans. J Gen Microbiol. 1975 Aug;89(2):345–352. doi: 10.1099/00221287-89-2-345. [DOI] [PubMed] [Google Scholar]
- Marriott M. S. Isolation and chemical characterization of plasma membranes from the yeast and mycelial forms of Candida albicans. J Gen Microbiol. 1975 Jan;86(1):115–132. doi: 10.1099/00221287-86-1-115. [DOI] [PubMed] [Google Scholar]
- Marriott M. S. Mannan-protein location and biosynthesis in plasma membranes from the yeast form of Candida albicans. J Gen Microbiol. 1977 Nov;103(1):51–59. doi: 10.1099/00221287-103-1-51. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ogletree F. F., Abdelal A. T., Ahearn D. G. Germ-tube formation by atypical strains of Candida albicans. Antonie Van Leeuwenhoek. 1978;44(1):15–24. doi: 10.1007/BF00400073. [DOI] [PubMed] [Google Scholar]
- Pringle J. R. Methods for avoiding proteolytic artefacts in studies of enzymes and other proteins from yeasts. Methods Cell Biol. 1975;12:149–184. doi: 10.1016/s0091-679x(08)60956-5. [DOI] [PubMed] [Google Scholar]
- Scherwitz C., Martin R., Ueberberg H. Ultrastructural investigations of the formation of Candida albicans germ tubes and septa. Sabouraudia. 1978 Jun;16(2):115–124. [PubMed] [Google Scholar]
- Yamaguchi H. Control of dimorphism in Candida albicans by zinc: effect on cell morphology and composition. J Gen Microbiol. 1975 Feb;86(2):370–372. doi: 10.1099/00221287-86-2-370. [DOI] [PubMed] [Google Scholar]